

Table of SynGAP1 Isoform α2 (UniProt Q96PV0-1) Missense Variants.
| c.dna | Variant | SGM Consensus | Domain | ClinVar | gnomAD | ESM1b | AlphaMissense | REVEL | FoldX | Rosetta | Foldetta | PremPS | PROVEAN | PolyPhen-2 HumDiv | PolyPhen-2 HumVar | FATHMM | SIFT | PAM | Physical | SASA | Normalized B-factor backbone | Normalized B-factor sidechain | SynGAP Structural Annotation | DOI | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Status | Review | Subm. | ID | Allele count | Allele freq. | LLR score | Prediction | Pathogenicity | Class | Optimized | Score | Prediction | Average ΔΔG | Prediction | StdDev | ΔΔG | Prediction | ΔΔG | Prediction | ΔΔG | Prediction | Score | Prediction | pph2_prob | Prediction | pph2_prob | Prediction | Nervous System Score | Prediction | Prediction | Status | Conservation | Sequences | PAM250 | PAM120 | Hydropathy Δ | MW Δ | Average | Δ | Δ | StdDev | Δ | StdDev | Secondary | Tertiary bonds | Inside out | GAP-Ras interface | At membrane | No effect | MD Alert | Verdict | Description | |||||
| c.3520G>A | E1174K 2D  AIThe SynGAP1 missense variant E1174K is listed in ClinVar with an uncertain significance (ClinVar ID 1905754.0) and is present in gnomAD (variant ID 6‑33444555‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification, matching the reported SGM‑Consensus result. AlphaMissense‑Optimized is uncertain, and no Foldetta stability assessment is available. Taken together, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | 6-33444555-G-A | 2 | 1.24e-6 | -4.345 | Likely Benign | 0.898 | Likely Pathogenic | Ambiguous | 0.442 | Likely Benign | -1.59 | Neutral | 0.962 | Probably Damaging | 0.367 | Benign | 5.52 | Benign | 0.03 | Affected | 4.32 | 2 | 0 | 1 | -0.4 | -0.94 | ||||||||||||||||||||||||||
| c.3529G>A | E1177K 2D  AISynGAP1 missense variant E1177K is listed in ClinVar with an Uncertain significance status and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from PROVEAN, SIFT, ESM1b, and FATHMM, while pathogenic calls come from REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments give AlphaMissense‑Optimized as Uncertain, SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Benign, and Foldetta stability analysis is unavailable. Overall, the balance of evidence leans toward a benign effect, which does not contradict the ClinVar designation of Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | -3.413 | Likely Benign | 0.944 | Likely Pathogenic | Ambiguous | 0.560 | Likely Pathogenic | -1.75 | Neutral | 0.905 | Possibly Damaging | 0.637 | Possibly Damaging | 5.44 | Benign | 0.11 | Tolerated | 4.32 | 2 | 0 | 1 | -0.4 | -0.94 | |||||||||||||||||||||||||||||
| c.3557C>T | S1186L 2D  AIThe SynGAP1 missense variant S1186L (ClinVar ID 930096.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33444592‑C‑T). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized reports an uncertain outcome. The high‑accuracy consensus (SGM Consensus) derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN yields a tie, leaving the result inconclusive. Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, has no available output for this variant. Overall, the majority of evidence points toward a pathogenic impact, and this assessment does not contradict the ClinVar Uncertain classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Coiled-coil | Uncertain | 1 | 6-33444592-C-T | -4.829 | Likely Benign | 0.923 | Likely Pathogenic | Ambiguous | 0.177 | Likely Benign | -2.58 | Deleterious | 0.998 | Probably Damaging | 0.992 | Probably Damaging | 2.65 | Benign | 0.04 | Affected | 3.82 | 4 | -3 | -2 | 4.6 | 26.08 | |||||||||||||||||||||||||||||
| c.3567G>C | E1189D 2D  AIThe SynGAP1 missense variant E1189D (gnomAD ID 6-33444602‑G‑C) is listed in ClinVar as Benign (ClinVar ID 833989.0). In silico predictors that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Predictors that indicate a pathogenic effect are PolyPhen‑2 HumDiv and PolyPhen‑2 HumVar. The high‑accuracy AlphaMissense‑Optimized tool classifies the variant as benign, and the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also favors a benign outcome. No Foldetta stability assessment is available for this residue. Overall, the majority of evidence points to a benign impact, and this conclusion aligns with the ClinVar designation, showing no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Likely Benign | 1 | 6-33444602-G-C | 3 | 1.86e-6 | -3.582 | Likely Benign | 0.461 | Ambiguous | Likely Benign | 0.359 | Likely Benign | -1.42 | Neutral | 0.992 | Probably Damaging | 0.989 | Probably Damaging | 5.30 | Benign | 0.25 | Tolerated | 3.82 | 4 | 3 | 2 | 0.0 | -14.03 | ||||||||||||||||||||||||||
| c.3572G>A | R1191Q 2D  AIThe SynGAP1 missense variant R1191Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33444607‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while polyPhen‑2 (HumDiv and HumVar) and AlphaMissense‑Default predict a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain”; the SGM‑Consensus (majority vote) remains “Likely Benign”; and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this is not contradictory to the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 2 | 6-33444607-G-A | 9 | 5.58e-6 | -1.069 | Likely Benign | 0.943 | Likely Pathogenic | Ambiguous | 0.343 | Likely Benign | -1.41 | Neutral | 0.998 | Probably Damaging | 0.992 | Probably Damaging | 2.68 | Benign | 0.08 | Tolerated | 3.82 | 4 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||
| c.3574C>G | L1192V 2D  AIThe SynGAP1 missense variant L1192V is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. The high‑accuracy AlphaMissense‑Optimized score is benign, and the SGM‑Consensus also indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Thus, based on current predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | -4.132 | Likely Benign | 0.471 | Ambiguous | Likely Benign | 0.041 | Likely Benign | -0.89 | Neutral | 0.779 | Possibly Damaging | 0.527 | Possibly Damaging | 2.69 | Benign | 0.16 | Tolerated | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||
| c.3595G>A | E1199K 2D  AIThe SynGAP1 missense variant E1199K (ClinVar ID 1026146.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33446587‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM, whereas tools that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta results are not available. Overall, the majority of evidence points toward a pathogenic impact, which does not contradict the ClinVar Uncertain classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Coiled-coil | Uncertain | 1 | 6-33446587-G-A | 1 | 6.20e-7 | -10.853 | Likely Pathogenic | 0.954 | Likely Pathogenic | Ambiguous | 0.171 | Likely Benign | -2.26 | Neutral | 1.000 | Probably Damaging | 0.995 | Probably Damaging | 2.52 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | 1 | -0.4 | -0.94 | |||||||||||||||||||||||||||
| c.3607C>G | H1203D 2D  AIThe SynGAP1 missense variant H1203D is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only polyPhen‑2 HumDiv predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as “Likely Benign”; a Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact. This conclusion does not contradict the ClinVar designation, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | -6.729 | Likely Benign | 0.525 | Ambiguous | Likely Benign | 0.403 | Likely Benign | -1.89 | Neutral | 0.473 | Possibly Damaging | 0.265 | Benign | 5.51 | Benign | 0.24 | Tolerated | 3.77 | 5 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||
| c.3607C>T | H1203Y 2D  AIThe SynGAP1 missense variant H1203Y is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (ID 6‑33446599‑C‑T). Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all score the variant as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a benign prediction. Foldetta results are unavailable. Overall, the evidence strongly supports a benign impact for H1203Y, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | 6-33446599-C-T | 2 | 1.24e-6 | -6.834 | Likely Benign | 0.149 | Likely Benign | Likely Benign | 0.233 | Likely Benign | -1.52 | Neutral | 0.006 | Benign | 0.011 | Benign | 5.55 | Benign | 0.10 | Tolerated | 3.77 | 5 | 2 | 0 | 1.9 | 26.03 | ||||||||||||||||||||||||||
| c.3614T>C | L1205P 2D  AIThe SynGAP1 missense variant L1205P is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. All available in silico predictors classify the change as pathogenic: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts a benign effect. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized is pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Pathogenic.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the computational evidence strongly indicates that the variant is pathogenic, which contradicts the current ClinVar designation of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 1 | -16.878 | Likely Pathogenic | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.536 | Likely Pathogenic | -5.91 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.45 | Pathogenic | 0.00 | Affected | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||
| c.3631A>G | M1211V 2D  AIThe SynGAP1 missense variant M1211V is listed in ClinVar as Benign (ClinVar ID 3674914.0) and is present in gnomAD (ID 6‑33446623‑A‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar classification and not contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Benign | 1 | 6-33446623-A-G | 3 | 1.86e-6 | -2.101 | Likely Benign | 0.258 | Likely Benign | Likely Benign | 0.412 | Likely Benign | -0.29 | Neutral | 0.932 | Possibly Damaging | 0.949 | Probably Damaging | 5.43 | Benign | 0.72 | Tolerated | 3.77 | 5 | 1 | 2 | 2.3 | -32.06 | ||||||||||||||||||||||||||
| c.3632T>A | M1211K 2D  AIThe SynGAP1 missense variant M1211K is listed in ClinVar (ID 834052.0) as benign and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from FATHMM and AlphaMissense‑Optimized, while the remaining seven tools—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default—classify the change as pathogenic. High‑accuracy assessments further clarify the picture: AlphaMissense‑Optimized predicts a benign effect, whereas the SGM Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely pathogenic outcome; Foldetta data are unavailable. Overall, the preponderance of evidence from standard predictors and the SGM Consensus supports a pathogenic interpretation, which contradicts the benign classification reported in ClinVar. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Likely Benign | 1 | -9.013 | Likely Pathogenic | 0.662 | Likely Pathogenic | Likely Benign | 0.595 | Likely Pathogenic | -2.95 | Deleterious | 0.987 | Probably Damaging | 0.979 | Probably Damaging | 5.59 | Benign | 0.01 | Affected | 3.77 | 5 | 0 | -1 | -5.8 | -3.02 | |||||||||||||||||||||||||||||
| c.3633G>A | M1211I 2D  AIThe SynGAP1 missense variant M1211I is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6-33446625-G-A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are AlphaMissense‑Default, polyPhen‑2 HumDiv, and polyPhen‑2 HumVar. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy predictions therefore point to a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus also indicates benign. Based on the aggregate predictions, the variant is most likely benign, which does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | 6-33446625-G-A | 3 | 1.86e-6 | -1.537 | Likely Benign | 0.764 | Likely Pathogenic | Likely Benign | 0.298 | Likely Benign | -0.42 | Neutral | 0.969 | Probably Damaging | 0.968 | Probably Damaging | 5.40 | Benign | 1.00 | Tolerated | 3.77 | 5 | 1 | 2 | 2.6 | -18.03 | ||||||||||||||||||||||||||
| c.3635C>T | S1212F 2D  AIThe SynGAP1 missense variant S1212F is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) score—predict a pathogenic impact. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports it as likely pathogenic. No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence from multiple independent predictors indicates that the variant is most likely pathogenic, which is consistent with its ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Conflicting | 2 | -14.445 | Likely Pathogenic | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.271 | Likely Benign | -4.52 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.03 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||
| c.3638A>C | N1213T 2D  AIThe SynGAP1 missense variant N1213T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33446630‑A‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support benignity: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Conflicting | 2 | 6-33446630-A-C | 46 | 2.85e-5 | -5.428 | Likely Benign | 0.266 | Likely Benign | Likely Benign | 0.097 | Likely Benign | -1.08 | Neutral | 0.959 | Probably Damaging | 0.721 | Possibly Damaging | 2.74 | Benign | 1.00 | Tolerated | 3.77 | 5 | 0 | 0 | 2.8 | -13.00 | ||||||||||||||||||||||||||
| c.3638A>G | N1213S 2D  AIThe SynGAP1 missense variant N1213S is listed in ClinVar as Benign (ClinVar ID 708250.0) and is present in the gnomAD database (gnomAD ID 6‑33446630‑A‑G). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). In contrast, PolyPhen‑2 (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar classification and indicating no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Benign | 1 | 6-33446630-A-G | 13 | 8.05e-6 | -4.086 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.094 | Likely Benign | -0.56 | Neutral | 0.906 | Possibly Damaging | 0.551 | Possibly Damaging | 2.82 | Benign | 0.68 | Tolerated | 3.77 | 5 | 1 | 1 | 2.7 | -27.03 | 10.1016/j.ajhg.2020.11.011 | |||||||||||||||||||||||||
| c.3640C>T | R1214W 2D  AIThe SynGAP1 missense variant R1214W is listed in ClinVar with an uncertain significance (ClinVar ID 1476244.0) and is present in the gnomAD database (gnomAD ID 6‑33446632‑C‑T). Functional prediction tools cluster into two groups: benign predictions come from REVEL and AlphaMissense‑Optimized, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further refine the picture: AlphaMissense‑Optimized indicates a benign effect, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—labels the variant as likely pathogenic. No Foldetta stability analysis is available for this residue. Overall, the majority of evidence points toward a pathogenic impact, and this conclusion does not conflict with the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 1 | 6-33446632-C-T | 2 | 1.24e-6 | -8.799 | Likely Pathogenic | 0.710 | Likely Pathogenic | Likely Benign | 0.143 | Likely Benign | -4.95 | Deleterious | 1.000 | Probably Damaging | 0.983 | Probably Damaging | 2.45 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 2 | -3 | 3.6 | 30.03 | ||||||||||||||||||||||||||
| c.3653A>T | E1218V 2D  AISynGAP1 missense variant E1218V is listed in ClinVar with an uncertain significance (ClinVar ID 1015602.0) and is not reported in gnomAD. Functional prediction tools show a split opinion: benign predictions come from REVEL and ESM1b, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. When the high‑accuracy consensus is considered, AlphaMissense‑Optimized is uncertain, but the SGM‑Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—labels the variant as likely pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this change. Overall, the majority of evidence points toward a pathogenic effect, which is consistent with the ClinVar designation of uncertain significance rather than a benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 2 | -5.647 | Likely Benign | 0.936 | Likely Pathogenic | Ambiguous | 0.418 | Likely Benign | -5.68 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.21 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||
| c.3655T>C | Y1219H 2D  AIThe SynGAP1 missense variant Y1219H is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Pathogenic)—all predict a pathogenic impact. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy tools indicates that the variant is most likely pathogenic, which does not contradict its current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 1 | -9.511 | Likely Pathogenic | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.363 | Likely Benign | -3.62 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.15 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | 2 | -1.9 | -26.03 | |||||||||||||||||||||||||||||
| c.3661C>T | R1221W 2D  AIThe SynGAP1 missense variant R1221W is listed in ClinVar with an uncertain significance (ClinVar ID 1050818.0) and is present in the gnomAD database (gnomAD ID 6‑33446653‑C‑T). Functional prediction tools show a split assessment: benign predictions come from REVEL and FATHMM, whereas pathogenic predictions are reported by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. High‑accuracy analyses further refine the picture: AlphaMissense‑Optimized classifies the variant as benign, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—deems it likely pathogenic. No Foldetta stability assessment is available for this residue. Overall, the majority of computational evidence points toward a pathogenic effect, which is consistent with the ClinVar designation of uncertain significance rather than a definitive benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Conflicting | 3 | 6-33446653-C-T | 1 | 6.20e-7 | -10.938 | Likely Pathogenic | 0.651 | Likely Pathogenic | Likely Benign | 0.174 | Likely Benign | -4.57 | Deleterious | 1.000 | Probably Damaging | 0.987 | Probably Damaging | 2.50 | Benign | 0.01 | Affected | 3.77 | 5 | 2 | -3 | 3.6 | 30.03 | ||||||||||||||||||||||||||
| c.3662G>A | R1221Q 2D  AIThe SynGAP1 missense variant R1221Q is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (ID 6‑33446654‑G‑A). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support this: AlphaMissense‑Optimized predicts benign, SGM Consensus is likely benign, while Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the preponderance of evidence points to a benign impact, which is consistent with the ClinVar “Uncertain” classification and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Conflicting | 2 | 6-33446654-G-A | 4 | 2.48e-6 | -5.491 | Likely Benign | 0.115 | Likely Benign | Likely Benign | 0.078 | Likely Benign | -1.46 | Neutral | 0.836 | Possibly Damaging | 0.153 | Benign | 2.56 | Benign | 0.12 | Tolerated | 3.77 | 5 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||
| c.3686A>C | Q1229P 2D  AIThe SynGAP1 missense variant Q1229P is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL and SIFT, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is “Likely Pathogenic.” No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 1 | -10.397 | Likely Pathogenic | 0.980 | Likely Pathogenic | Likely Pathogenic | 0.422 | Likely Benign | -3.69 | Deleterious | 0.998 | Probably Damaging | 0.995 | Probably Damaging | 1.75 | Pathogenic | 0.12 | Tolerated | 3.77 | 5 | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||
| c.36C>G | S12R 2D  AIThe SynGAP1 missense variant S12R is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33420300‑C‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors a benign classification; Foldetta’s protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33420300-C-G | 4 | 2.59e-6 | -4.033 | Likely Benign | 0.500 | Ambiguous | Likely Benign | 0.097 | Likely Benign | -0.30 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.09 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||
| c.3705G>A | M1235I 2D  AIThe SynGAP1 missense variant M1235I is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that classify the variant as benign include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic effect. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as “Likely Benign”; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | -4.312 | Likely Benign | 0.310 | Likely Benign | Likely Benign | 0.027 | Likely Benign | -1.44 | Neutral | 0.139 | Benign | 0.056 | Benign | 2.69 | Benign | 0.04 | Affected | 3.77 | 5 | 1 | 2 | 2.6 | -18.03 | |||||||||||||||||||||||||||||
| c.371C>T | A124V 2D  AIThe SynGAP1 A124V missense variant is listed in ClinVar (ID 1040523.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33432236‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of computational evidence indicates a benign effect, and this consensus does not contradict the ClinVar “Uncertain” designation. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 2 | 6-33432236-C-T | 9 | 5.58e-6 | -4.259 | Likely Benign | 0.138 | Likely Benign | Likely Benign | 0.073 | Likely Benign | -1.52 | Neutral | 0.173 | Benign | 0.009 | Benign | 4.07 | Benign | 0.03 | Affected | 3.61 | 5 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.3721C>A | L1241M 2D  AISynGAP1 missense variant L1241M is listed in ClinVar with an Uncertain significance and is not reported in gnomAD. Functional prediction tools show a split verdict: benign calls come from REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic calls are made by polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is unresolved (2 benign vs. 2 pathogenic). Foldetta, a protein‑folding stability predictor that combines FoldX‑MD and Rosetta outputs, has no available result for this variant. Consequently, the high‑accuracy tools do not converge on a single interpretation. Overall, the predictions are balanced between benign and pathogenic, leaving the variant’s effect uncertain, which aligns with its ClinVar designation of Uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Coiled-coil | Uncertain | 1 | -5.881 | Likely Benign | 0.782 | Likely Pathogenic | Likely Benign | 0.167 | Likely Benign | -1.43 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.65 | Pathogenic | 0.00 | Affected | 4 | 2 | -1.9 | 18.03 | ||||||||||||||||||||||||||||||||
| c.3731G>A | S1244N 2D  AIThe SynGAP1 missense variant S1244N is listed in ClinVar with an “Uncertain” status (ClinVar ID 931075.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and AlphaMissense‑Optimized, whereas PolyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Default all predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—indicates pathogenicity. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the balance of evidence leans toward a pathogenic effect, which does not contradict the ClinVar designation of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 1 | -9.008 | Likely Pathogenic | 0.751 | Likely Pathogenic | Likely Benign | 0.154 | Likely Benign | -1.87 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.10 | Pathogenic | 0.15 | Tolerated | 3.77 | 5 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||
| c.373C>T | P125S 2D  AIThe SynGAP1 missense variant P125S is listed in ClinVar (ID 837156.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic predictions come from PROVEAN, polyPhen‑2 HumDiv, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the preponderance of evidence points to a benign impact, and this conclusion does not contradict the ClinVar designation, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -3.769 | Likely Benign | 0.238 | Likely Benign | Likely Benign | 0.121 | Likely Benign | -3.57 | Deleterious | 0.580 | Possibly Damaging | 0.140 | Benign | 2.86 | Benign | 0.02 | Affected | 3.61 | 5 | 1 | -1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||
| c.3773A>G | Q1258R 2D  AIThe SynGAP1 missense variant Q1258R is listed in ClinVar with an uncertain significance (ClinVar ID 3359527.0) and is not observed in gnomAD. Functional prediction tools largely agree on a deleterious effect: pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default, while only REVEL predicts a benign outcome. The high‑accuracy predictors give the following results: AlphaMissense‑Optimized is uncertain; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely pathogenic classification; Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, has no available output for this variant. Based on the preponderance of pathogenic predictions and the SGM Consensus, the variant is most likely pathogenic, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 1 | -10.971 | Likely Pathogenic | 0.931 | Likely Pathogenic | Ambiguous | 0.316 | Likely Benign | -3.19 | Deleterious | 0.994 | Probably Damaging | 0.988 | Probably Damaging | 2.00 | Pathogenic | 0.00 | Affected | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||
| c.3788T>C | I1263T 2D 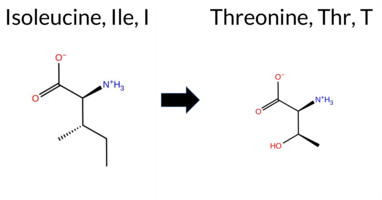 AIThe SynGAP1 missense variant I1263T is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (ID 6‑33446780‑T‑C). Functional prediction tools largely agree on a deleterious effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report pathogenicity, while only ESM1b predicts a benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely pathogenic classification. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods points to a pathogenic effect, which aligns with the ClinVar designation of uncertainty but does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 1 | 6-33446780-T-C | 2 | 1.24e-6 | -6.564 | Likely Benign | 0.962 | Likely Pathogenic | Likely Pathogenic | 0.529 | Likely Pathogenic | -4.15 | Deleterious | 0.946 | Possibly Damaging | 0.673 | Possibly Damaging | 1.81 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | -1 | -5.2 | -12.05 | ||||||||||||||||||||||||||
| c.3794G>C | R1265T 2D 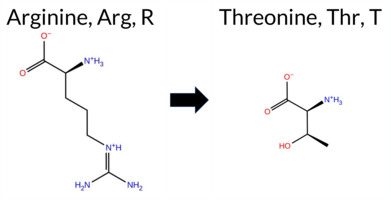 AIThe SynGAP1 missense variant R1265T is reported in ClinVar as Pathogenic (ClinVar ID 522047.0) and is not found in gnomAD. Functional prediction tools uniformly indicate a deleterious effect: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as pathogenic. No tool in the dataset predicts a benign outcome. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts pathogenicity, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a Likely Pathogenic verdict. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of all available predictions supports a pathogenic classification, which is consistent with the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Likely Pathogenic | 1 | -10.129 | Likely Pathogenic | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.529 | Likely Pathogenic | -4.97 | Deleterious | 0.997 | Probably Damaging | 0.994 | Probably Damaging | 2.29 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -1 | -1 | 3.8 | -55.08 | |||||||||||||||||||||||||||||
| c.379C>T | R127W 2D  AISynGAP1 missense variant R127W is listed in ClinVar with an Uncertain significance status and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM, while pathogenic calls come from PROVEAN, polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is Uncertain, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive, and Foldetta stability analysis is unavailable. Consequently, the evidence does not favor a clear benign or pathogenic outcome; the predictions are balanced and align with the ClinVar designation of Uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | -4.776 | Likely Benign | 0.806 | Likely Pathogenic | Ambiguous | 0.118 | Likely Benign | -2.98 | Deleterious | 0.989 | Probably Damaging | 0.420 | Benign | 3.88 | Benign | 0.00 | Affected | 2 | -3 | 3.6 | 30.03 | |||||||||||||||||||||||||||||||||
| c.37A>G | I13V 2D 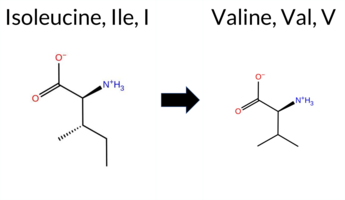 AIThe SynGAP1 I13V missense variant is listed in ClinVar (ID 3364831.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for I13V, and this conclusion does not conflict with the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.497 | Likely Benign | 0.105 | Likely Benign | Likely Benign | 0.110 | Likely Benign | 0.01 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.25 | Benign | 0.00 | Affected | 4 | 3 | -0.3 | -14.03 | ||||||||||||||||||||||||||||||||
| c.3806T>A | V1269E 2D  AIThe SynGAP1 missense change V1269E is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that flag the variant as benign include only REVEL, whereas the remaining predictors—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently classify it as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a “Likely Pathogenic” outcome. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic and the SGM‑Consensus as likely pathogenic; the Foldetta stability analysis is unavailable. Overall, the preponderance of evidence points to a pathogenic effect, which does not contradict the ClinVar designation of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Coiled-coil | Uncertain | 1 | -11.418 | Likely Pathogenic | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.403 | Likely Benign | -5.05 | Deleterious | 0.999 | Probably Damaging | 0.995 | Probably Damaging | 2.09 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -2 | -2 | -7.7 | 29.98 | |||||||||||||||||||||||||||||
| c.380G>A | R127Q 2D  AIThe SynGAP1 missense variant R127Q (ClinVar ID 2898917.0) is listed as ClinVar status Uncertain and is present in gnomAD (6‑33432245‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta stability analysis is unavailable. Overall, the majority of computational evidence supports a benign effect, which is consistent with the ClinVar Uncertain status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33432245-G-A | 6 | 3.72e-6 | -1.711 | Likely Benign | 0.320 | Likely Benign | Likely Benign | 0.037 | Likely Benign | -1.04 | Neutral | 0.006 | Benign | 0.001 | Benign | 4.04 | Benign | 0.02 | Affected | 3.74 | 4 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.3820C>T | R1274C 2D  AIThe SynGAP1 missense variant R1274C is listed in ClinVar with an “Uncertain” significance and is present in gnomAD (ID 6‑33447868‑C‑T). Prediction tools that agree on benign impact include REVEL, ESM1b, and AlphaMissense‑Optimized, while those that predict pathogenicity are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). AlphaMissense‑Default remains uncertain, and Foldetta results are unavailable. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus as pathogenic, and no Foldetta data to weigh in. Overall, the majority of evaluated tools (seven pathogenic vs. three benign) suggest a pathogenic effect. This consensus does not contradict the ClinVar “Uncertain” status, which remains inconclusive. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33447868-C-T | -6.467 | Likely Benign | 0.439 | Ambiguous | Likely Benign | 0.170 | Likely Benign | -5.22 | Deleterious | 1.000 | Probably Damaging | 0.996 | Probably Damaging | 2.46 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -4 | -3 | 7.0 | -53.05 | ||||||||||||||||||||||||||||||
| c.3821G>A | R1274H 2D  AIThe SynGAP1 missense variant R1274H (ClinVar ID 2803246.0) is classified as Benign in ClinVar and is present in gnomAD (ID 6‑33447869‑G‑A). Functional prediction tools show mixed results: benign calls come from REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments indicate that AlphaMissense‑Optimized predicts a benign effect, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie and is therefore inconclusive. No Foldetta stability prediction is available for this residue. Overall, the majority of conventional tools predict pathogenicity, and the high‑accuracy AlphaMissense‑Optimized result is benign, leaving the evidence mixed. Based on the preponderance of pathogenic predictions, the variant is most likely pathogenic, contradicting its ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 1 | 6-33447869-G-A | 4 | 2.58e-6 | -5.259 | Likely Benign | 0.256 | Likely Benign | Likely Benign | 0.149 | Likely Benign | -3.20 | Deleterious | 1.000 | Probably Damaging | 0.995 | Probably Damaging | 2.49 | Pathogenic | 0.01 | Affected | 3.77 | 5 | 0 | 2 | 1.3 | -19.05 | ||||||||||||||||||||||||||||
| c.3824G>A | R1275Q 2D  AIThe SynGAP1 missense variant R1275Q is listed in ClinVar with an uncertain significance (ClinVar ID 1720188.0) and is present in gnomAD (6‑33447872‑G‑A). Consensus from multiple in‑silico predictors shows a split: benign calls from REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, whereas pathogenic calls come from polyPhen‑2 HumDiv and SIFT. High‑accuracy tools reinforce the benign trend: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports likely benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this assessment does not conflict with the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33447872-G-A | 2 | 1.29e-6 | -4.928 | Likely Benign | 0.121 | Likely Benign | Likely Benign | 0.103 | Likely Benign | -1.72 | Neutral | 0.898 | Possibly Damaging | 0.147 | Benign | 2.59 | Benign | 0.03 | Affected | 3.77 | 5 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.3824G>T | R1275L 2D  AIThe SynGAP1 missense variant R1275L is listed in ClinVar as benign and is present in gnomAD (ID 6‑33447872‑G‑T). Functional prediction tools show a split: benign calls come from REVEL, ESM1b, FATHMM, AlphaMissense‑Optimized, and polyPhen2_HumVar, while pathogenic calls come from PROVEAN, polyPhen2_HumDiv, and SIFT. Grouping by agreement, the benign‑predicted tools outnumber the pathogenic ones (5 vs 3). High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) resolves to benign, and Foldetta results are unavailable. Overall, the computational evidence leans toward a benign effect, consistent with the ClinVar classification and showing no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 1 | 6-33447872-G-T | 1 | 6.45e-7 | -6.052 | Likely Benign | 0.446 | Ambiguous | Likely Benign | 0.117 | Likely Benign | -4.04 | Deleterious | 0.800 | Possibly Damaging | 0.277 | Benign | 2.55 | Benign | 0.01 | Affected | 3.77 | 5 | -3 | -2 | 8.3 | -43.03 | ||||||||||||||||||||||||||||
| c.382C>A | P128T 2D  AIThe SynGAP1 missense variant P128T is listed in ClinVar with an “Uncertain” status (ClinVar ID 2801315.0) and is present in gnomAD (ID 6‑33432247‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33432247-C-A | 1 | 6.20e-7 | -4.217 | Likely Benign | 0.267 | Likely Benign | Likely Benign | 0.075 | Likely Benign | -0.96 | Neutral | 0.952 | Possibly Damaging | 0.500 | Possibly Damaging | 4.19 | Benign | 0.35 | Tolerated | 3.74 | 4 | -1 | 0 | 0.9 | 3.99 | |||||||||||||||||||||||||||
| c.3835G>A | A1279T 2D  AIThe SynGAP1 missense variant A1279T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33447883‑G‑A). All available in silico predictors report a benign effect: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized are benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” No tool predicts pathogenicity. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is benign; Foldetta results are not available. Overall, the computational evidence strongly supports a benign classification, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33447883-G-A | 2 | 1.29e-6 | -4.871 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.178 | Likely Benign | -0.30 | Neutral | 0.001 | Benign | 0.000 | Benign | 2.71 | Benign | 0.09 | Tolerated | 3.77 | 5 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.3846G>C | E1282D 2D  AIThe SynGAP1 missense variant E1282D is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6-33447894-G-C). All available in silico predictors classify the substitution as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool reports a pathogenic prediction. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) is likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no reported result for this variant, so its status is unavailable. Overall, the computational evidence overwhelmingly supports a benign effect, which is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33447894-G-C | 1 | 6.44e-7 | -3.879 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.104 | Likely Benign | -1.26 | Neutral | 0.112 | Benign | 0.036 | Benign | 2.70 | Benign | 0.39 | Tolerated | 3.77 | 5 | 3 | 2 | 0.0 | -14.03 | |||||||||||||||||||||||||||
| c.3848C>T | P1283L 2D  AIThe SynGAP1 missense variant P1283L is listed in ClinVar (ID 536994.0) as Benign and is present in gnomAD (gnomAD ID 6‑33447896‑C‑T). All evaluated in‑silico predictors classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool reports a pathogenic prediction. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts Benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the computational evidence overwhelmingly supports a benign effect, aligning with the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33447896-C-T | 32 | 2.06e-5 | -3.740 | Likely Benign | 0.093 | Likely Benign | Likely Benign | 0.047 | Likely Benign | -1.04 | Neutral | 0.005 | Benign | 0.003 | Benign | 2.76 | Benign | 0.06 | Tolerated | 3.77 | 5 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||
| c.3858A>T | E1286D 2D  AIThe SynGAP1 missense variant E1286D is listed in ClinVar (ID 469159.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33447906‑A‑T). All evaluated in‑silico predictors classify the substitution as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores. No tool in the set predicts pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so its status is unavailable. Overall, the computational evidence strongly supports a benign effect, and this conclusion does not contradict the current ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 4 | 6-33447906-A-T | 143 | 9.22e-5 | -4.010 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.036 | Likely Benign | 1.02 | Neutral | 0.001 | Benign | 0.004 | Benign | 2.96 | Benign | 1.00 | Tolerated | 3.77 | 5 | 3 | 2 | 0.0 | -14.03 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||
| c.3859C>A | P1287T 2D  AIThe SynGAP1 missense variant P1287T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33447907‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33447907-C-A | -3.940 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -0.22 | Neutral | 0.126 | Benign | 0.041 | Benign | 2.78 | Benign | 0.04 | Affected | 3.77 | 5 | -1 | 0 | 0.9 | 3.99 | |||||||||||||||||||||||||||||
| c.3860C>T | P1287L 2D  AIThe SynGAP1 missense variant P1287L is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33447908‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 2 | 6-33447908-C-T | -2.800 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.061 | Likely Benign | -1.66 | Neutral | 0.021 | Benign | 0.017 | Benign | 2.76 | Benign | 0.02 | Affected | 3.77 | 5 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||
| c.3862A>G | K1288E 2D 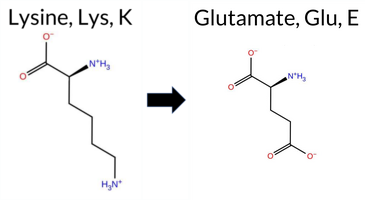 AISynGAP1 missense variant K1288E is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33447910‑A‑G). Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and AlphaMissense‑Optimized, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—labels the variant pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this change. Overall, the preponderance of evidence points to a pathogenic effect, which is consistent with the ClinVar designation of uncertain significance rather than a clear benign classification. Thus, the variant is most likely pathogenic, and this does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33447910-A-G | 5 | 3.22e-6 | -2.751 | Likely Benign | 0.407 | Ambiguous | Likely Benign | 0.185 | Likely Benign | -3.27 | Deleterious | 0.979 | Probably Damaging | 0.973 | Probably Damaging | 2.13 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 1 | 0 | 0.4 | 0.94 | ||||||||||||||||||||||||||||
| c.3902C>A | P1301H 2D  AIThe SynGAP1 missense variant P1301H is listed in ClinVar (ID 212356.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451776‑C‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The high‑accuracy consensus methods report a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” No Foldetta stability result is available. Overall, the majority of predictions, including the high‑accuracy consensus, support a benign classification, which does not contradict the ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 2 | 6-33451776-C-A | 5 | 3.10e-6 | -5.756 | Likely Benign | 0.104 | Likely Benign | Likely Benign | 0.232 | Likely Benign | -1.13 | Neutral | 0.642 | Possibly Damaging | 0.378 | Benign | 2.79 | Benign | 0.04 | Affected | 3.77 | 5 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||
| c.3902C>G | P1301R 2D  AIThe SynGAP1 missense variant P1301R is listed in ClinVar with an uncertain significance (ClinVar ID 2092739.0) and is present in gnomAD (ID 6‑33451776‑C‑G). Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report benign effects. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are unavailable. In summary, all available predictions agree on a benign impact, and this consensus does not contradict the ClinVar uncertain status. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451776-C-G | 15 | 9.30e-6 | -4.753 | Likely Benign | 0.162 | Likely Benign | Likely Benign | 0.076 | Likely Benign | -1.13 | Neutral | 0.077 | Benign | 0.059 | Benign | 2.81 | Benign | 0.10 | Tolerated | 3.77 | 5 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||
| c.3906G>C | L1302F 2D  AISynGAP1 missense variant L1302F is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments indicate AlphaMissense‑Optimized predicts benign, whereas the SGM consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta stability analysis is unavailable. Overall, the majority of predictions (five pathogenic versus four benign) lean toward a pathogenic effect. Thus, the variant is most likely pathogenic, a conclusion that contrasts with its current ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | -5.674 | Likely Benign | 0.148 | Likely Benign | Likely Benign | 0.211 | Likely Benign | -2.70 | Deleterious | 0.960 | Probably Damaging | 0.657 | Possibly Damaging | 1.53 | Pathogenic | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||
| c.3907G>A | G1303S 2D  AIThe SynGAP1 missense variant G1303S is listed in ClinVar (ID 1736068.0) with an “Uncertain” clinical significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Only polyPhen‑2 HumDiv predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; a Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.271 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.155 | Likely Benign | -0.19 | Neutral | 0.649 | Possibly Damaging | 0.433 | Benign | 2.84 | Benign | 0.18 | Tolerated | 1 | 0 | -0.4 | 30.03 | ||||||||||||||||||||||||||||||||
| c.3913A>G | T1305A 2D  AIThe SynGAP1 missense variant T1305A is listed in ClinVar (ID 411587.0) with an “Uncertain” clinical significance and is present in the gnomAD database (variant ID 6‑33451787‑A‑G). All evaluated in‑silico predictors classify the change as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool reports a pathogenic or likely pathogenic outcome. High‑accuracy assessments reinforce this benign prediction: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are unavailable. Overall, the computational evidence overwhelmingly supports a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 4 | 6-33451787-A-G | 30 | 1.86e-5 | -2.692 | Likely Benign | 0.055 | Likely Benign | Likely Benign | 0.069 | Likely Benign | 1.74 | Neutral | 0.000 | Benign | 0.001 | Benign | 3.24 | Benign | 1.00 | Tolerated | 3.77 | 5 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||
| c.391G>C | G131R 2D  AIThe SynGAP1 missense variant G131R is listed in ClinVar with an “Uncertain” significance and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and FATHMM, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive due to a 2‑to‑2 split and therefore does not contribute evidence. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑confidence tools predict a pathogenic effect, and the variant is most likely pathogenic based on current predictions, which does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | -6.564 | Likely Benign | 0.983 | Likely Pathogenic | Likely Pathogenic | 0.099 | Likely Benign | -3.82 | Deleterious | 0.983 | Probably Damaging | 0.656 | Possibly Damaging | 3.92 | Benign | 0.00 | Affected | 3.61 | 5 | -2 | -3 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||
| c.3920C>A | P1307Q 2D  AIThe SynGAP1 missense variant P1307Q is listed in ClinVar (ID 982827.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451794‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451794-C-A | -4.227 | Likely Benign | 0.114 | Likely Benign | Likely Benign | 0.192 | Likely Benign | -0.88 | Neutral | 0.988 | Probably Damaging | 0.765 | Possibly Damaging | 2.82 | Benign | 0.03 | Affected | 3.77 | 5 | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||||
| c.3920C>T | P1307L 2D  AIThe SynGAP1 missense variant P1307L is listed in ClinVar (ID 1991214.0) as benign and is present in gnomAD (variant ID 6‑33451794‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign,” and AlphaMissense‑Optimized also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions, including the high‑accuracy consensus, indicate a benign impact. This conclusion aligns with the ClinVar benign classification and does not contradict the reported clinical status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33451794-C-T | 11 | 6.82e-6 | -4.044 | Likely Benign | 0.144 | Likely Benign | Likely Benign | 0.292 | Likely Benign | -1.49 | Neutral | 0.779 | Possibly Damaging | 0.220 | Benign | 2.82 | Benign | 0.04 | Affected | 3.77 | 5 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||
| c.3922C>T | R1308C 2D  AIThe SynGAP1 missense variant R1308C is listed in ClinVar with an “Uncertain” significance and is present in the gnomAD database (ID 6‑33451796‑C‑T). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default remains uncertain. The high‑accuracy consensus (SGM Consensus) derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN yields a pathogenic verdict. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a pathogenic impact, and this assessment does not contradict the current ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Conflicting | 2 | 6-33451796-C-T | 4 | 2.48e-6 | -4.994 | Likely Benign | 0.421 | Ambiguous | Likely Benign | 0.352 | Likely Benign | -4.89 | Deleterious | 0.999 | Probably Damaging | 0.993 | Probably Damaging | 2.31 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -4 | -3 | 7.0 | -53.05 | ||||||||||||||||||||||||||||
| c.3923G>A | R1308H 2D  AIThe SynGAP1 missense variant R1308H (ClinVar ID 1996244.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33451797‑G‑A). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessment shows AlphaMissense‑Optimized predicts a benign outcome; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive, and Foldetta results are unavailable. Consequently, the overall computational evidence leans toward a pathogenic interpretation, but the presence of a single high‑accuracy benign prediction and the inconclusive SGM Consensus leave the variant’s effect uncertain. This computational assessment does not contradict the ClinVar status, which remains Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33451797-G-A | 3 | 1.86e-6 | -3.586 | Likely Benign | 0.201 | Likely Benign | Likely Benign | 0.319 | Likely Benign | -3.12 | Deleterious | 0.998 | Probably Damaging | 0.991 | Probably Damaging | 2.33 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||||||||
| c.3929C>T | T1310M 2D  AIThe SynGAP1 missense variant T1310M is listed in ClinVar (ID 2160201.0) as Benign and is present in gnomAD (gnomAD ID 6‑33451803‑C‑T). All evaluated in‑silico predictors report a benign outcome: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the computational evidence overwhelmingly supports a benign classification, which aligns with the ClinVar status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33451803-C-T | 17 | 1.05e-5 | -4.822 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.069 | Likely Benign | 2.19 | Neutral | 0.021 | Benign | 0.005 | Benign | 2.98 | Benign | 0.93 | Tolerated | 3.77 | 5 | -1 | -1 | 2.6 | 30.09 | |||||||||||||||||||||||||||
| c.3932T>C | L1311P 2D  AIThe SynGAP1 missense variant L1311P is listed in ClinVar (ID 833866.0) as Benign and is present in gnomAD (variant ID 6‑33451806‑T‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only polyPhen‑2 HumDiv predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a Likely Benign classification, and AlphaMissense‑Optimized also reports Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, is not available for this variant. Overall, the majority of computational evidence supports a benign effect, which is consistent with the ClinVar benign annotation and does not contradict the database status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | 6-33451806-T-C | 1 | 6.21e-7 | -1.831 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.123 | Likely Benign | -0.52 | Neutral | 0.579 | Possibly Damaging | 0.335 | Benign | 2.72 | Benign | 0.18 | Tolerated | 3.77 | 5 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||
| c.3941C>T | P1314L 2D  AIThe SynGAP1 missense variant P1314L is listed in ClinVar as a benign alteration (ClinVar ID 646689.0) and is present in the gnomAD database (gnomAD ID 6‑33451815‑C‑T). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, which is consistent with the ClinVar classification. Thus, the variant is most likely benign and does not contradict the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | 6-33451815-C-T | 2 | 1.24e-6 | -4.040 | Likely Benign | 0.118 | Likely Benign | Likely Benign | 0.049 | Likely Benign | -0.20 | Neutral | 0.421 | Benign | 0.066 | Benign | 4.19 | Benign | 0.05 | Affected | 3.77 | 5 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||
| c.3943T>C | W1315R 2D  AIThe SynGAP1 missense variant W1315R is listed in ClinVar (ID 1029092.0) with an “Uncertain” clinical significance and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus score (which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only AlphaMissense‑Default predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as benign; Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 0.205 | Likely Benign | 0.660 | Likely Pathogenic | Likely Benign | 0.114 | Likely Benign | 1.31 | Neutral | 0.000 | Benign | 0.001 | Benign | 4.37 | Benign | 0.91 | Tolerated | 3.77 | 5 | 2 | -3 | -3.6 | -30.03 | ||||||||||||||||||||||||||||||
| c.3949G>A | G1317S 2D  AIThe SynGAP1 missense variant G1317S is listed in ClinVar with an uncertain significance and is present in the gnomAD database. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized returns a benign prediction, and the SGM‑Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the computational evidence overwhelmingly points to a benign effect, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | 6-33451823-G-A | 1 | 6.26e-7 | -3.522 | Likely Benign | 0.145 | Likely Benign | Likely Benign | 0.092 | Likely Benign | -2.45 | Neutral | 0.127 | Benign | 0.045 | Benign | 4.08 | Benign | 0.00 | Affected | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.3956C>G | A1319G 2D  AIThe SynGAP1 missense variant A1319G is listed in ClinVar (ID 1690510.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451830‑C‑G). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this is consistent with the ClinVar “Uncertain” status rather than contradicting it. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33451830-C-G | -3.927 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.128 | Likely Benign | -0.74 | Neutral | 0.819 | Possibly Damaging | 0.581 | Possibly Damaging | 4.07 | Benign | 0.06 | Tolerated | 3.77 | 5 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||
| c.3958C>T | P1320S 2D  AIThe SynGAP1 missense variant P1320S is listed in ClinVar (ID 469160.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451832‑C‑T). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this is not in conflict with the ClinVar “Uncertain” status. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451832-C-T | 2 | 1.28e-6 | -4.928 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.097 | Likely Benign | -0.69 | Neutral | 0.980 | Probably Damaging | 0.968 | Probably Damaging | 4.25 | Benign | 0.00 | Affected | 3.77 | 5 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||
| c.3961C>T | P1321S 2D  AIThe SynGAP1 missense variant P1321S is listed in ClinVar (ID 1806027.0) with an “Uncertain” clinical significance and is present in the gnomAD database (gnomAD ID 6‑33451835‑C‑T). All evaluated in‑silico predictors classify the substitution as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool reports a pathogenic prediction. High‑accuracy assessments corroborate this benign trend: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so its status is unavailable. Overall, the computational evidence strongly supports a benign effect, which is consistent with the ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33451835-C-T | 10 | 6.46e-6 | -4.897 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.049 | Likely Benign | 0.68 | Neutral | 0.028 | Benign | 0.004 | Benign | 4.27 | Benign | 0.71 | Tolerated | 3.77 | 5 | 1 | -1 | 0.8 | -10.04 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||
| c.3962C>A | P1321Q 2D  AIThe SynGAP1 missense variant P1321Q is listed in ClinVar (ID 833687.0) as benign and is present in gnomAD (variant ID 6‑33451836‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only polyPhen‑2 HumDiv predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification, and AlphaMissense‑Optimized also reports benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence—including the high‑confidence SGM consensus and AlphaMissense‑Optimized—supports a benign impact. This conclusion aligns with the ClinVar benign status, with no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33451836-C-A | 1 | 6.58e-7 | -5.594 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.055 | Likely Benign | -0.74 | Neutral | 0.659 | Possibly Damaging | 0.034 | Benign | 4.24 | Benign | 0.09 | Tolerated | 3.77 | 5 | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||
| c.3964G>C | A1322P 2D  AIThe SynGAP1 missense variant A1322P is reported in ClinVar (ID 1169945.0) as benign and is present in gnomAD (variant ID 6‑33451838‑G‑C). Across the available in‑silico predictors, all tools uniformly classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign predictions. No tool in the dataset indicates a pathogenic effect. High‑accuracy assessments corroborate this consensus: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” verdict. The Foldetta protein‑folding stability analysis is not available for this variant. Overall, the computational evidence strongly supports a benign classification, which is consistent with the ClinVar status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33451838-G-C | -1.153 | Likely Benign | 0.063 | Likely Benign | Likely Benign | 0.090 | Likely Benign | 0.03 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.15 | Benign | 0.23 | Tolerated | 3.77 | 5 | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||
| c.3970C>T | P1324S 2D  AIThe SynGAP1 missense variant P1324S is listed in ClinVar as Benign (ClinVar ID 1137951.0) and is present in gnomAD (ID 6‑33451844‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) also benign; Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, which is consistent with its ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | 6-33451844-C-T | 5 | 3.26e-6 | -5.451 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.049 | Likely Benign | 0.35 | Neutral | 0.225 | Benign | 0.092 | Benign | 4.33 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||
| c.3974C>T | P1325L 2D  AIThe SynGAP1 missense variant P1325L is listed in ClinVar (ID 1720534.0) with an uncertain significance designation and is present in gnomAD (variant ID 6‑33451848‑C‑T). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect for P1325L, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451848-C-T | -5.256 | Likely Benign | 0.085 | Likely Benign | Likely Benign | 0.146 | Likely Benign | -1.05 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.05 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||
| c.3977C>A | P1326Q 2D  AIThe SynGAP1 missense variant P1326Q is listed in ClinVar (ID 2806103.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33451851‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion does not contradict the ClinVar “Uncertain” classification, which remains inconclusive. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451851-C-A | 1 | 6.40e-7 | -5.422 | Likely Benign | 0.128 | Likely Benign | Likely Benign | 0.138 | Likely Benign | -0.86 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 3.62 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -1.9 | 31.01 | |||||||||||||||||||||||||||
| c.3977C>G | P1326R 2D  AIThe SynGAP1 missense variant P1326R is listed in ClinVar (ID 2429486.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) also benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -5.097 | Likely Benign | 0.240 | Likely Benign | Likely Benign | 0.133 | Likely Benign | -0.82 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 3.62 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||
| c.3977C>T | P1326L 2D  AIThe SynGAP1 missense variant P1326L is listed in ClinVar (ID 1004879.0) with an “Uncertain” clinical significance and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as “Likely Benign,” and AlphaMissense‑Optimized also predicts benign. No Foldetta (FoldX‑MD/ Rosetta) stability result is available for this variant. Overall, the majority of evidence—including the high‑confidence SGM consensus and AlphaMissense‑Optimized prediction—supports a benign classification, which does not contradict the current ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -5.541 | Likely Benign | 0.115 | Likely Benign | Likely Benign | 0.117 | Likely Benign | -1.06 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 3.62 | Benign | 0.00 | Affected | 3.77 | 5 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||
| c.3979C>T | P1327S 2D  AIThe SynGAP1 missense variant P1327S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33451853‑C‑T). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451853-C-T | -4.744 | Likely Benign | 0.131 | Likely Benign | Likely Benign | 0.092 | Likely Benign | 0.28 | Neutral | 0.980 | Probably Damaging | 0.857 | Possibly Damaging | 4.25 | Benign | 0.71 | Tolerated | 3.77 | 5 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||
| c.3980C>T | P1327L 2D  AIThe SynGAP1 missense variant P1327L is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33451854‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The high‑accuracy AlphaMissense‑Optimized score is benign, and the SGM Consensus—derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also indicates a benign outcome. Foldetta results are not available for this variant. Overall, the majority of computational evidence supports a benign classification, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451854-C-T | 2 | 1.28e-6 | -5.264 | Likely Benign | 0.242 | Likely Benign | Likely Benign | 0.142 | Likely Benign | -1.24 | Neutral | 0.994 | Probably Damaging | 0.908 | Possibly Damaging | 4.12 | Benign | 0.10 | Tolerated | 3.77 | 5 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||
| c.3983G>A | R1328Q 2D  AIThe SynGAP1 missense variant R1328Q is listed in ClinVar (ID 1805359.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451857‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; a Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this is not in conflict with the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 3 | 6-33451857-G-A | 35 | 1.49e-4 | -2.921 | Likely Benign | 0.273 | Likely Benign | Likely Benign | 0.043 | Likely Benign | -1.02 | Neutral | 0.799 | Possibly Damaging | 0.098 | Benign | 4.12 | Benign | 0.03 | Affected | 3.77 | 5 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.3983G>C | R1328P 2D  AIThe SynGAP1 missense variant R1328P (ClinVar ID 1258976.0) is classified as Benign in ClinVar and is observed in gnomAD (6‑33451857‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and Foldetta results are unavailable. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also indicates a likely benign outcome; no Foldetta stability data are reported. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar designation and not contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33451857-G-C | -1.220 | Likely Benign | 0.466 | Ambiguous | Likely Benign | 0.060 | Likely Benign | -2.01 | Neutral | 0.927 | Possibly Damaging | 0.452 | Possibly Damaging | 4.06 | Benign | 0.01 | Affected | 3.77 | 5 | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||
| c.3995C>T | T1332M 2D  AISynGAP1 missense variant T1332M is listed as Benign in ClinVar (ID 794425) and is present in gnomAD (6‑33451869‑C‑T). Functional prediction tools show mixed results: benign calls come from REVEL, ESM1b, and FATHMM, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized returned an uncertain result, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is a tie, and no Foldetta stability data are available. Overall, the majority of evidence points toward a pathogenic effect, which contradicts the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 1 | 6-33451869-C-T | 20 | 1.86e-5 | -4.107 | Likely Benign | 0.948 | Likely Pathogenic | Ambiguous | 0.252 | Likely Benign | -3.63 | Deleterious | 1.000 | Probably Damaging | 0.991 | Probably Damaging | 2.95 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | -1 | 2.6 | 30.09 | ||||||||||||||||||||||||||||
| c.3G>A | M1I 2D  AIThe SynGAP1 missense variant M1I is listed in ClinVar (ID 833646.0) with an uncertain significance annotation and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM all classify the substitution as benign, while SIFT uniquely predicts it to be pathogenic. The consensus score from the SGM framework, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy predictors are incomplete: AlphaMissense‑Optimized and the Foldetta stability assessment are unavailable for this variant. Taking the collective evidence into account, the variant is most likely benign, and this assessment does not conflict with the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | -5.397 | Likely Benign | 0.227 | Likely Benign | -0.17 | Neutral | 0.001 | Benign | 0.000 | Benign | 4.25 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 1 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||
| c.4000A>G | N1334D 2D  AIThe SynGAP1 missense variant N1334D (ClinVar ID 3653769.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33451874‑A‑G). Functional prediction tools show a split: benign calls come from REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic calls come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. High‑accuracy assessments give a benign result from AlphaMissense‑Optimized, an inconclusive SGM Consensus (a 2‑vs‑2 majority vote among AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN), and no available Foldetta stability data. Overall, the majority of predictions (5/10) indicate pathogenicity, and the high‑accuracy tools do not overturn this trend. Therefore, the variant is most likely pathogenic, which does not contradict its ClinVar status of Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33451874-A-G | -4.584 | Likely Benign | 0.674 | Likely Pathogenic | Likely Benign | 0.126 | Likely Benign | -3.06 | Deleterious | 0.886 | Possibly Damaging | 0.522 | Possibly Damaging | 3.55 | Benign | 0.00 | Affected | 3.77 | 5 | 1 | 2 | 0.0 | 0.98 | ||||||||||||||||||||||||||||||
| c.4003G>A | G1335S 2D  AIThe SynGAP1 missense variant G1335S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33451877‑G‑A). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) all predict a pathogenic outcome. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence indicates that G1335S is most likely pathogenic, which is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Conflicting | 2 | 6-33451877-G-A | 3 | 2.37e-6 | -4.495 | Likely Benign | 0.986 | Likely Pathogenic | Likely Pathogenic | 0.362 | Likely Benign | -3.79 | Deleterious | 1.000 | Probably Damaging | 0.997 | Probably Damaging | 2.04 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.4006G>A | E1336K 2D  AISynGAP1 missense variant E1336K is listed in ClinVar as benign (ClinVar ID 984837.0) and is present in gnomAD (6‑33451880‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus remains benign; Foldetta stability analysis is unavailable. Overall, the majority of predictions lean toward a benign impact, aligning with the ClinVar designation, though a single high‑accuracy tool suggests pathogenicity. Thus, the variant is most likely benign, and this conclusion does not contradict its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 2 | 6-33451880-G-A | 6 | 4.20e-6 | -4.697 | Likely Benign | 0.977 | Likely Pathogenic | Likely Pathogenic | 0.272 | Likely Benign | -2.44 | Neutral | 0.748 | Possibly Damaging | 0.079 | Benign | 3.23 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | 1 | -0.4 | -0.94 | |||||||||||||||||||||||||||
| c.4008G>C | E1336D 2D  AIThe SynGAP1 missense variant E1336D is listed in ClinVar (ID 3323942.0) as benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus result is a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yielding a benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, consistent with the ClinVar benign designation. Thus, the variant is most likely benign and does not contradict ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | -3.344 | Likely Benign | 0.596 | Likely Pathogenic | Likely Benign | 0.062 | Likely Benign | -1.92 | Neutral | 0.001 | Benign | 0.003 | Benign | 3.30 | Benign | 0.00 | Affected | 3.77 | 5 | 2 | 3 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||
| c.4013G>A | R1338Q 2D  AIThe SynGAP1 missense variant R1338Q is listed in ClinVar (ID 450879.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451887‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which reports it as “Likely Benign.” In contrast, polyPhen‑2 HumDiv and SIFT predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; a Foldetta stability analysis is not available. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | 6-33451887-G-A | 12 | 8.40e-6 | -3.494 | Likely Benign | 0.317 | Likely Benign | Likely Benign | 0.076 | Likely Benign | -1.87 | Neutral | 0.896 | Possibly Damaging | 0.194 | Benign | 3.81 | Benign | 0.02 | Affected | 3.77 | 5 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.401G>A | S134N 2D  AIThe SynGAP1 missense variant S134N is listed in ClinVar with an “Uncertain” status (ClinVar ID 2819575.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Those that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The remaining tools—AlphaMissense‑Optimized and the SGM‑Consensus—are inconclusive; the consensus score is “Likely Benign” based on a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as likely benign, and Foldetta (combining FoldX‑MD and Rosetta) has no available result. Overall, the balance of evidence points to a benign impact, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -5.534 | Likely Benign | 0.813 | Likely Pathogenic | Ambiguous | 0.075 | Likely Benign | -1.62 | Neutral | 0.001 | Benign | 0.002 | Benign | 3.90 | Benign | 0.00 | Affected | 3.61 | 5 | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||
| c.4021G>A | A1341T 2D  AIThe SynGAP1 missense variant A1341T is listed in ClinVar (ID 837815.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451895‑G‑A). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report benign or likely benign. Only SIFT predicts a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for A1341T, which is consistent with the ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | 6-33451895-G-A | 45 | 3.44e-5 | -3.224 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.099 | Likely Benign | -0.58 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.09 | Benign | 0.03 | Affected | 3.77 | 5 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.4021G>T | A1341S 2D  AIThe SynGAP1 missense variant A1341S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6-33451895-G-T). All available in silico predictors agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is benign; Foldetta results are not available. Based on the collective predictions, the variant is most likely benign, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451895-G-T | -2.867 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.099 | Likely Benign | 0.80 | Neutral | 0.000 | Benign | 0.001 | Benign | 4.40 | Benign | 1.00 | Tolerated | 3.77 | 5 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||
| c.404G>A | R135Q 2D  AIThe SynGAP1 missense variant R135Q is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33432701‑G‑A). Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM; pathogenic predictions come from SIFT, ESM1b, and AlphaMissense‑Default. The remaining high‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is a 2‑to‑2 tie, and Foldetta stability analysis is not available. Overall, the balance of evidence favors a benign effect, and this conclusion does not conflict with the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33432701-G-A | 5 | 3.84e-6 | -8.011 | Likely Pathogenic | 0.853 | Likely Pathogenic | Ambiguous | 0.087 | Likely Benign | -1.94 | Neutral | 0.327 | Benign | 0.100 | Benign | 3.76 | Benign | 0.02 | Affected | 3.61 | 5 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||||
| c.406C>T | R136W 2D  AIThe SynGAP1 missense variant R136W is listed in ClinVar (ID 1479441.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, and FATHMM, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 HumDiv, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts a deleterious effect, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a pathogenic impact, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 2 | -10.453 | Likely Pathogenic | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.237 | Likely Benign | -4.71 | Deleterious | 0.965 | Probably Damaging | 0.416 | Benign | 3.45 | Benign | 0.00 | Affected | 3.61 | 5 | 2 | -3 | 3.6 | 30.03 | ||||||||||||||||||||||||||||||
| c.407G>A | R136Q 2D  AIThe SynGAP1 R136Q variant is listed in ClinVar as benign and is present in gnomAD (6‑33432704‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, ESM1b, and AlphaMissense‑Default. AlphaMissense‑Optimized returns an uncertain result. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive due to a 2‑vs‑2 split, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no reported result for this variant. Based on the available predictions, the variant is most likely benign, which aligns with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Benign | 1 | 6-33432704-G-A | 13 | 9.17e-6 | -11.146 | Likely Pathogenic | 0.950 | Likely Pathogenic | Ambiguous | 0.190 | Likely Benign | -2.26 | Neutral | 0.957 | Probably Damaging | 0.342 | Benign | 3.52 | Benign | 0.01 | Affected | 3.61 | 5 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||||
| c.407G>C | R136P 2D  AIThe SynGAP1 missense variant R136P is listed in ClinVar (ID 579340.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect are REVEL and FATHMM, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus—predict a pathogenic outcome. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates pathogenicity. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 1 | -11.952 | Likely Pathogenic | 0.981 | Likely Pathogenic | Likely Pathogenic | 0.277 | Likely Benign | -3.72 | Deleterious | 0.910 | Possibly Damaging | 0.578 | Possibly Damaging | 3.47 | Benign | 0.00 | Affected | 3.61 | 5 | 0 | -2 | 2.9 | -59.07 | ||||||||||||||||||||||||||||||
| c.416G>A | S139N 2D  AIThe SynGAP1 missense variant S139N is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33432713‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only AlphaMissense‑Default predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33432713-G-A | 3 | 2.22e-6 | -4.584 | Likely Benign | 0.688 | Likely Pathogenic | Likely Benign | 0.109 | Likely Benign | -0.75 | Neutral | 0.149 | Benign | 0.047 | Benign | 4.14 | Benign | 0.24 | Tolerated | 3.61 | 5 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||
| c.431C>T | T144M 2D  AIThe SynGAP1 missense variant T144M is listed in ClinVar with an “Uncertain” status (ClinVar ID 2231966.0) and is present in the gnomAD database (gnomAD ID 6‑33432728‑C‑T). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, and FATHMM. Those that predict a pathogenic effect comprise PROVEAN, polyPhen‑2 HumDiv, SIFT, ESM1b, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Pathogenic.” High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain,” SGM‑Consensus as “Likely Pathogenic,” and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the majority of computational predictions lean toward a pathogenic impact, and this assessment does not contradict the ClinVar designation of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 2 | 6-33432728-C-T | 2 | 1.30e-6 | -11.228 | Likely Pathogenic | 0.922 | Likely Pathogenic | Ambiguous | 0.118 | Likely Benign | -3.16 | Deleterious | 0.913 | Possibly Damaging | 0.333 | Benign | 3.73 | Benign | 0.00 | Affected | 3.61 | 5 | -1 | -1 | 2.6 | 30.09 | |||||||||||||||||||||||||||
| c.43G>A | A15T 2D  AIThe SynGAP1 missense variant A15T is listed in ClinVar (ID 1925632.0) with an “Uncertain” clinical significance and is present in gnomAD (6‑33420307‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as “Likely Benign”; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33420307-G-A | 4 | 2.60e-6 | -3.720 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.086 | Likely Benign | -0.08 | Neutral | 0.602 | Possibly Damaging | 0.017 | Benign | 4.16 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.43G>C | A15P 2D  AIThe SynGAP1 missense variant A15P is listed in ClinVar (ID 3688743.0) with an *Uncertain* clinical significance and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as *Likely Benign*. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -3.436 | Likely Benign | 0.097 | Likely Benign | Likely Benign | 0.146 | Likely Benign | -0.23 | Neutral | 0.880 | Possibly Damaging | 0.123 | Benign | 4.09 | Benign | 0.00 | Affected | 1 | -1 | -3.4 | 26.04 | ||||||||||||||||||||||||||||||||
| c.44C>T | A15V 2D  AIThe SynGAP1 missense variant A15V is listed in ClinVar (ID 1801174.0) with an “Uncertain” status and is present in gnomAD (6‑33420308‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while polyPhen‑2 HumDiv and SIFT predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this does not contradict the ClinVar designation, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33420308-C-T | 1 | 6.49e-7 | -3.560 | Likely Benign | 0.161 | Likely Benign | Likely Benign | 0.105 | Likely Benign | 0.20 | Neutral | 0.602 | Possibly Damaging | 0.015 | Benign | 4.19 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.451G>C | D151H 2D  AIThe SynGAP1 missense variant D151H is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33432748‑G‑C). Prediction tools that agree on a benign effect include REVEL and FATHMM, whereas the remaining tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized) all predict a pathogenic outcome. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as “Likely Pathogenic.” No Foldetta stability result is available for this variant. Overall, the majority of computational evidence indicates that D151H is most likely pathogenic, which does not contradict the current ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 1 | 6-33432748-G-C | 2 | 1.26e-6 | -11.747 | Likely Pathogenic | 0.994 | Likely Pathogenic | Likely Pathogenic | 0.335 | Likely Benign | -3.90 | Deleterious | 0.999 | Probably Damaging | 0.995 | Probably Damaging | 3.86 | Benign | 0.00 | Affected | 3.61 | 5 | -1 | 1 | 0.3 | 22.05 | |||||||||||||||||||||||||||
| c.453C>A | D151E 2D  AIThe SynGAP1 D151E variant is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM. Those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain,” SGM‑Consensus as “Likely Benign,” and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -5.662 | Likely Benign | 0.886 | Likely Pathogenic | Ambiguous | 0.142 | Likely Benign | -2.02 | Neutral | 0.984 | Probably Damaging | 0.967 | Probably Damaging | 3.99 | Benign | 0.11 | Tolerated | 3.61 | 5 | 3 | 2 | 0.0 | 14.03 | ||||||||||||||||||||||||||||||
| c.455G>A | R152Q 2D  AIThe SynGAP1 missense variant R152Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33432752‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM, whereas those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic, two benign). High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic; the SGM Consensus remains unavailable, and Foldetta (combining FoldX‑MD and Rosetta outputs) has no reported result for this variant. Overall, the preponderance of evidence (seven pathogenic versus three benign predictions) indicates that the variant is most likely pathogenic, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33432752-G-A | 5 | 3.14e-6 | -10.336 | Likely Pathogenic | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.181 | Likely Benign | -2.34 | Neutral | 0.997 | Probably Damaging | 0.968 | Probably Damaging | 3.89 | Benign | 0.00 | Affected | 3.61 | 5 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||||
| c.458C>A | T153N 2D  AIThe SynGAP1 missense variant T153N is listed in ClinVar (ID 984906.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) predict pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence supports a benign classification, and this is consistent with the ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | -0.739 | Likely Benign | 0.226 | Likely Benign | Likely Benign | 0.161 | Likely Benign | 0.88 | Neutral | 0.888 | Possibly Damaging | 0.537 | Possibly Damaging | 4.23 | Benign | 0.81 | Tolerated | 3.61 | 5 | 0 | 0 | -2.8 | 13.00 | ||||||||||||||||||||||||||||||
| c.467T>G | F156C 2D  AIThe SynGAP1 missense variant F156C is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect are REVEL and FATHMM, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—all predict a pathogenic or likely pathogenic outcome. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus reports it as “Likely Pathogenic.” No Foldetta stability analysis is available for this variant. Overall, the majority of predictions, including the high‑accuracy tools, indicate that F156C is most likely pathogenic, which is consistent with its ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 1 | -13.658 | Likely Pathogenic | 0.988 | Likely Pathogenic | Likely Pathogenic | 0.297 | Likely Benign | -3.54 | Deleterious | 0.999 | Probably Damaging | 0.990 | Probably Damaging | 3.92 | Benign | 0.00 | Affected | -4 | -2 | -0.3 | -44.04 | ||||||||||||||||||||||||||||||||
| c.470G>A | R157H 2D  AIThe SynGAP1 missense variant R157H (ClinVar ID 2065231.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33432767‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie and is therefore inconclusive. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no reported result for this variant. Overall, the balance of predictions leans toward pathogenic, but the high‑accuracy tools do not provide a definitive verdict. This assessment does not contradict the ClinVar status, which remains Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33432767-G-A | 1 | 6.20e-7 | -10.235 | Likely Pathogenic | 0.604 | Likely Pathogenic | Likely Benign | 0.254 | Likely Benign | -2.23 | Neutral | 0.999 | Probably Damaging | 0.987 | Probably Damaging | 3.80 | Benign | 0.00 | Affected | 3.74 | 4 | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||||||||
| c.484C>G | R162G 2D  AIThe SynGAP1 missense variant R162G is listed in ClinVar (ID 2703066.0) with an uncertain significance status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign (three benign votes versus one pathogenic). High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta stability analysis is unavailable. Overall, the majority of predictions support a benign impact, and this is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -6.985 | Likely Benign | 0.664 | Likely Pathogenic | Likely Benign | 0.190 | Likely Benign | -0.73 | Neutral | 0.487 | Possibly Damaging | 0.272 | Benign | 4.09 | Benign | 0.78 | Tolerated | 3.74 | 4 | -2 | -3 | 4.1 | -99.14 | ||||||||||||||||||||||||||||||
| c.484C>T | R162C 2D  AIThe SynGAP1 missense variant R162C is listed in ClinVar as Pathogenic and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and FATHMM, whereas tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive, and Foldetta stability analysis is unavailable. Overall, the available predictions are split evenly between benign and pathogenic, with no single method providing decisive evidence. Thus, the variant’s pathogenicity remains uncertain based on computational predictions, which contradicts the ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Pathogenic | 2 | -8.157 | Likely Pathogenic | 0.787 | Likely Pathogenic | Ambiguous | 0.150 | Likely Benign | -2.05 | Neutral | 0.988 | Probably Damaging | 0.513 | Possibly Damaging | 4.00 | Benign | 0.11 | Tolerated | 3.74 | 4 | -4 | -3 | 7.0 | -53.05 | |||||||||||||||||||||||||||||||
| c.485G>A | R162H 2D  AIThe SynGAP1 missense variant R162H is listed in ClinVar with an uncertain significance and is present in the gnomAD database (variant ID 6‑33432782‑G‑A). Functional prediction tools cluster into two groups: benign calls are made by REVEL, PROVEAN, SIFT, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic calls come from polyPhen‑2 (HumDiv and HumVar) and ESM1b; AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also yields a benign verdict. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the preponderance of evidence points to a benign effect, which does not contradict the ClinVar uncertain classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33432782-G-A | 2 | 1.24e-6 | -9.730 | Likely Pathogenic | 0.480 | Ambiguous | Likely Benign | 0.167 | Likely Benign | -1.13 | Neutral | 0.957 | Probably Damaging | 0.513 | Possibly Damaging | 4.03 | Benign | 0.12 | Tolerated | 3.74 | 4 | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||||||||
| c.48G>A | M16I 2D  AIThe SynGAP1 missense variant M16I is listed in ClinVar with an “Uncertain” status (ClinVar ID 1424213.0) and is present in gnomAD (6‑33420312‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33420312-G-A | 1 | 6.49e-7 | -2.198 | Likely Benign | 0.722 | Likely Pathogenic | Likely Benign | 0.057 | Likely Benign | -0.15 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.28 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 1 | 2.6 | -18.03 | |||||||||||||||||||||||||||
| c.491G>A | R164Q 2D  AISynGAP1 missense variant R164Q is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33432788‑G‑A). Functional prediction tools show mixed results: benign predictions come from REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic predictions come from polyPhen‑2 HumDiv, SIFT, ESM1b, and AlphaMissense‑Default. High‑accuracy assessments indicate that AlphaMissense‑Optimized predicts a benign effect, while the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta results are not available. Overall, the balance of evidence slightly favors a benign interpretation, and this does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33432788-G-A | 2 | 1.24e-6 | -11.208 | Likely Pathogenic | 0.600 | Likely Pathogenic | Likely Benign | 0.184 | Likely Benign | -1.86 | Neutral | 0.957 | Probably Damaging | 0.342 | Benign | 3.82 | Benign | 0.00 | Affected | 3.74 | 4 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||||
| c.502C>T | H168Y 2D  AIThe SynGAP1 missense variant H168Y is listed in ClinVar (ID 956914.0) as benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are SIFT and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | -8.914 | Likely Pathogenic | 0.264 | Likely Benign | Likely Benign | 0.065 | Likely Benign | -1.53 | Neutral | 0.192 | Benign | 0.062 | Benign | 4.18 | Benign | 0.01 | Affected | 4.32 | 3 | 0 | 2 | 1.9 | 26.03 | ||||||||||||||||||||||||||||||
| c.505G>A | D169N 2D  AIThe SynGAP1 missense variant D169N is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: six methods (REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Optimized) predict a benign effect, while three (SIFT, ESM1b, AlphaMissense‑Default) predict pathogenicity. High‑accuracy assessments are mixed: AlphaMissense‑Optimized indicates benign, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie and is therefore inconclusive. No Foldetta stability data are available. Overall, the balance of evidence leans toward a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | -10.713 | Likely Pathogenic | 0.761 | Likely Pathogenic | Likely Benign | 0.110 | Likely Benign | -2.04 | Neutral | 0.079 | Benign | 0.052 | Benign | 4.07 | Benign | 0.01 | Affected | 3.74 | 4 | 2 | 1 | 0.0 | -0.98 | |||||||||||||||||||||||||||||||
| c.508C>T | R170W 2D  AIThe SynGAP1 missense variant R170W is listed in ClinVar (ID 1310195.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect are REVEL and FATHMM, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—all predict a pathogenic or likely pathogenic outcome. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus indicates it is likely pathogenic; Foldetta results are unavailable. Overall, the preponderance of evidence points to a pathogenic effect, which is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 2 | -11.660 | Likely Pathogenic | 0.978 | Likely Pathogenic | Likely Pathogenic | 0.241 | Likely Benign | -4.28 | Deleterious | 0.999 | Probably Damaging | 0.849 | Possibly Damaging | 3.84 | Benign | 0.00 | Affected | 3.74 | 4 | 2 | -3 | 3.6 | 30.03 | ||||||||||||||||||||||||||||||
| c.509G>A | R170Q 2D  AISynGAP1 missense variant R170Q is listed in ClinVar as Pathogenic and is not reported in gnomAD. Computational predictors show a split: benign calls come from REVEL, PROVEAN, polyPhen‑2 HumVar, and FATHMM, while pathogenic calls come from polyPhen‑2 HumDiv, SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is Uncertain, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive; Foldetta stability analysis is unavailable. Thus, no single method or high‑accuracy consensus strongly supports pathogenicity. The variant is most likely benign according to the current computational evidence, which contradicts the ClinVar pathogenic designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Pathogenic/Likely path. | 6 | -9.021 | Likely Pathogenic | 0.798 | Likely Pathogenic | Ambiguous | 0.221 | Likely Benign | -2.31 | Neutral | 0.947 | Possibly Damaging | 0.342 | Benign | 3.91 | Benign | 0.00 | Affected | 3.74 | 4 | 1 | 1 | 1.0 | -28.06 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||||||
| c.50C>T | S17F 2D  AIThe SynGAP1 missense variant S17F is listed in ClinVar with an “Uncertain” status (ClinVar ID 3451958.0) and is present in gnomAD (ID 6‑33420314‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33420314-C-T | 10 | 6.49e-6 | -3.888 | Likely Benign | 0.637 | Likely Pathogenic | Likely Benign | 0.048 | Likely Benign | -0.99 | Neutral | 0.486 | Possibly Damaging | 0.032 | Benign | 3.99 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -3 | 3.6 | 60.10 | |||||||||||||||||||||||||||
| c.514C>T | R172W 2D  AIThe SynGAP1 missense variant R172W is listed in ClinVar (ID 996892.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33435156‑C‑T). Prediction tools that agree on a benign effect include REVEL and FATHMM. Those that predict a pathogenic effect comprise PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default; the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is “Likely Pathogenic.” AlphaMissense‑Optimized is uncertain, and Foldetta results are unavailable. High‑accuracy assessments therefore indicate a likely pathogenic outcome (SGM‑Consensus) with no definitive stabilizing‑folding evidence. Overall, the majority of computational predictions support a pathogenic classification, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 2 | 6-33435156-C-T | 9 | 5.58e-6 | -10.258 | Likely Pathogenic | 0.878 | Likely Pathogenic | Ambiguous | 0.228 | Likely Benign | -3.61 | Deleterious | 0.997 | Probably Damaging | 0.803 | Possibly Damaging | 3.95 | Benign | 0.00 | Affected | 3.61 | 5 | 2 | -3 | 3.6 | 30.03 | |||||||||||||||||||||||||||
| c.515G>A | R172Q 2D  AISynGAP1 missense variant R172Q is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33435157‑G‑A). Functional prediction tools that agree on benign impact include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Optimized. Those that predict pathogenicity are polyPhen‑2 HumDiv and SIFT, while ESM1b and AlphaMissense‑Default are inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also returns benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of evidence points to a benign effect, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33435157-G-A | 3 | 1.86e-6 | -7.245 | In-Between | 0.465 | Ambiguous | Likely Benign | 0.135 | Likely Benign | -1.72 | Neutral | 0.804 | Possibly Damaging | 0.091 | Benign | 4.04 | Benign | 0.04 | Affected | 3.61 | 5 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||||
| c.526A>C | S176R 2D  AIThe SynGAP1 missense variant S176R is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, and FATHMM, whereas polyPhen‑2 HumDiv, AlphaMissense‑Default, and AlphaMissense‑Optimized predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus remains likely benign; Foldetta results are unavailable. Overall, the balance of evidence favors a benign interpretation, and this assessment does not conflict with the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -6.492 | Likely Benign | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.247 | Likely Benign | 0.94 | Neutral | 0.718 | Possibly Damaging | 0.168 | Benign | 4.16 | Benign | 0.87 | Tolerated | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||
| c.526A>G | S176G 2D  AIThe SynGAP1 missense variant S176G is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33435168‑A‑G). Consensus among most in silico predictors is benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Optimized all report a benign effect. No tool predicts pathogenicity. Two predictors are inconclusive: ESM1b and AlphaMissense‑Default, which are grouped under uncertain. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, while the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) remains uncertain, and Foldetta stability analysis is unavailable. Overall, the computational evidence overwhelmingly favors a benign impact, which does not contradict the ClinVar uncertain classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33435168-A-G | 1 | 6.20e-7 | -7.541 | In-Between | 0.360 | Ambiguous | Likely Benign | 0.066 | Likely Benign | -1.08 | Neutral | 0.131 | Benign | 0.039 | Benign | 4.08 | Benign | 0.22 | Tolerated | 3.54 | 6 | 0 | 1 | 0.4 | -30.03 | ||||||||||||||||||||||||||||
| c.53A>G | Y18C 2D  AIThe SynGAP1 missense variant Y18C is listed in ClinVar with an uncertain significance (ClinVar ID 1967233.0) and is present in gnomAD (ID 6‑33420317‑A‑G). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. In contrast, polyPhen‑2 HumDiv and SIFT predict pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for Y18C, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33420317-A-G | 44 | 2.88e-5 | -2.658 | Likely Benign | 0.251 | Likely Benign | Likely Benign | 0.102 | Likely Benign | -0.56 | Neutral | 0.872 | Possibly Damaging | 0.206 | Benign | 4.04 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -2 | 3.8 | -60.04 | |||||||||||||||||||||||||||
| c.558G>C | L186F 2D  AIThe SynGAP1 missense variant L186F is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), and FATHMM. In contrast, tools that predict a pathogenic effect are PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Pathogenic.” High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (majority vote) also indicates likely pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a pathogenic effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 1 | -11.861 | Likely Pathogenic | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.132 | Likely Benign | -3.03 | Deleterious | 0.009 | Benign | 0.012 | Benign | 3.50 | Benign | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | ||||||||||||||||||||||||||||||||
| c.583G>C | A195P 2D  AIThe SynGAP1 missense variant A195P is listed in ClinVar as Pathogenic (ClinVar ID 375527.0) and is not reported in gnomAD. Prediction tools that indicate a benign effect are REVEL and FATHMM, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus—predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Pathogenic.” High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic and the SGM‑Consensus as likely pathogenic; Foldetta results are unavailable. Overall, the preponderance of evidence supports a pathogenic classification, which aligns with the ClinVar status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Likely Pathogenic | 1 | -9.715 | Likely Pathogenic | 0.978 | Likely Pathogenic | Likely Pathogenic | 0.152 | Likely Benign | -3.03 | Deleterious | 0.997 | Probably Damaging | 0.916 | Probably Damaging | 4.00 | Benign | 0.04 | Affected | 3.54 | 6 | 1 | -1 | -3.4 | 26.04 | ||||||||||||||||||||||||||||||
| c.59C>G | P20R 2D  AIThe SynGAP1 missense variant P20R is listed in ClinVar (ID 566521.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic outcome are polyPhen‑2 (HumDiv and HumVar) and SIFT. AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -3.548 | Likely Benign | 0.434 | Ambiguous | Likely Benign | 0.146 | Likely Benign | -0.15 | Neutral | 0.972 | Probably Damaging | 0.804 | Possibly Damaging | 4.33 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||
| c.59C>T | P20L 2D  AIThe SynGAP1 missense variant P20L (ClinVar ID 1185912.0) is listed as “Uncertain” in ClinVar and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 3 | -3.289 | Likely Benign | 0.464 | Ambiguous | Likely Benign | 0.100 | Likely Benign | -0.44 | Neutral | 0.909 | Possibly Damaging | 0.713 | Possibly Damaging | 4.27 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||
| c.5G>A | S2N 2D  AIThe SynGAP1 missense variant S2N is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33420269‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the preponderance of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33420269-G-A | 3 | 1.96e-6 | -4.104 | Likely Benign | 0.207 | Likely Benign | Likely Benign | 0.092 | Likely Benign | -0.36 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.06 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||
| c.662A>G | E221G 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 E221G missense variant is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include polyPhen‑2 HumVar and FATHMM, while the majority of other in silico predictors (REVEL, PROVEAN, polyPhen‑2 HumDiv, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) indicate a pathogenic impact; FoldX, Rosetta, Foldetta, and premPS are inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta as uncertain. Based on the collective evidence, the variant is most likely pathogenic, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | PH | Uncertain | 1 | -12.221 | Likely Pathogenic | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.863 | Likely Pathogenic | 1.40 | Ambiguous | 0.1 | 1.74 | Ambiguous | 1.57 | Ambiguous | 0.71 | Ambiguous | -5.56 | Deleterious | 0.596 | Possibly Damaging | 0.201 | Benign | 5.79 | Benign | 0.00 | Affected | 0 | -2 | 3.1 | -72.06 | ||||||||||||||||||||||
| c.68A>G | D23G 2D  AIThe SynGAP1 missense variant D23G is listed in ClinVar (ID 3644551.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of computational evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” classification but leans toward a benign interpretation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.622 | Likely Benign | 0.684 | Likely Pathogenic | Likely Benign | 0.100 | Likely Benign | -2.45 | Neutral | 0.805 | Possibly Damaging | 0.539 | Possibly Damaging | 3.50 | Benign | 0.00 | Affected | 1 | -1 | 3.1 | -58.04 | ||||||||||||||||||||||||||||||||
| c.70G>A | V24I 2D  AIThe SynGAP1 missense variant V24I is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6-33423479-G-A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Taken together, the overwhelming majority of computational evidence supports a benign impact for V24I, and this conclusion does not contradict the ClinVar designation, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33423479-G-A | 9 | 5.58e-6 | -3.701 | Likely Benign | 0.137 | Likely Benign | Likely Benign | 0.069 | Likely Benign | -0.25 | Neutral | 0.043 | Benign | 0.031 | Benign | 3.96 | Benign | 0.00 | Affected | 4.32 | 1 | 3 | 4 | 0.3 | 14.03 | |||||||||||||||||||||||||||
| c.718G>A | D240N 2D  AIThe SynGAP1 missense variant D240N is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Benign predictions are provided by FoldX, Rosetta, Foldetta, premPS, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic predictions come from REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and the SGM‑Consensus. High‑accuracy methods give a split: AlphaMissense‑Optimized predicts benign, SGM‑Consensus predicts pathogenic, and Foldetta predicts benign. Overall, the majority of tools favor a benign effect, and this consensus does not contradict the ClinVar uncertain status. Thus, the variant is most likely benign based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | PH | Uncertain | 1 | -12.942 | Likely Pathogenic | 0.755 | Likely Pathogenic | Likely Benign | 0.701 | Likely Pathogenic | 0.22 | Likely Benign | 0.9 | 0.47 | Likely Benign | 0.35 | Likely Benign | 0.37 | Likely Benign | -4.37 | Deleterious | 0.993 | Probably Damaging | 0.984 | Probably Damaging | 5.88 | Benign | 0.01 | Affected | 2 | 1 | 0.0 | -0.98 | ||||||||||||||||||||||
| c.719A>G | D240G 2D  AIThe SynGAP1 missense variant D240G is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Benign predictions are provided by premPS and FATHMM, whereas pathogenic predictions are made by REVEL, Rosetta, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), and Foldetta. FoldX‑MD is inconclusive, and AlphaMissense‑Optimized is uncertain. High‑accuracy methods show that AlphaMissense‑Optimized is inconclusive, SGM Consensus predicts pathogenic, and Foldetta predicts pathogenic. Overall, the majority of evidence points to a pathogenic effect, which is consistent with the ClinVar uncertain status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | PH | Uncertain | 1 | -12.825 | Likely Pathogenic | 0.951 | Likely Pathogenic | Ambiguous | 0.912 | Likely Pathogenic | 1.85 | Ambiguous | 0.1 | 2.72 | Destabilizing | 2.29 | Destabilizing | 0.24 | Likely Benign | -6.19 | Deleterious | 0.993 | Probably Damaging | 0.984 | Probably Damaging | 5.79 | Benign | 0.01 | Affected | 1 | -1 | 3.1 | -58.04 | ||||||||||||||||||||||
| c.73C>T | R25W 2D  AIThe SynGAP1 missense variant R25W is listed in ClinVar with an “Uncertain” status (ClinVar ID 2993054.0) and is present in gnomAD (ID 6‑33423482‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is not in conflict with the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33423482-C-T | 6 | 3.72e-6 | -5.133 | Likely Benign | 0.549 | Ambiguous | Likely Benign | 0.158 | Likely Benign | -1.60 | Neutral | 0.994 | Probably Damaging | 0.919 | Probably Damaging | 3.92 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | 2 | 3.6 | 30.03 | |||||||||||||||||||||||||||
| c.74G>A | R25Q 2D  AIThe SynGAP1 missense variant R25Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33423483‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33423483-G-A | 15 | 9.29e-6 | -4.126 | Likely Benign | 0.212 | Likely Benign | Likely Benign | 0.038 | Likely Benign | -0.70 | Neutral | 0.829 | Possibly Damaging | 0.614 | Possibly Damaging | 4.01 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.767A>G | N256S 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant N256S is listed in ClinVar as Pathogenic (ClinVar ID 2584352.0) and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from FoldX, Rosetta, Foldetta, premPS, and FATHMM, while pathogenic calls come from SGM‑Consensus, REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy subset gives AlphaMissense‑Optimized as Uncertain, SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) as Benign. Overall, the majority of predictions support a pathogenic effect, aligning with the ClinVar classification. Therefore, the variant is most likely pathogenic, and this assessment does not contradict the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Likely Pathogenic | 1 | -10.640 | Likely Pathogenic | 0.950 | Likely Pathogenic | Ambiguous | 0.707 | Likely Pathogenic | 0.31 | Likely Benign | 0.2 | 0.36 | Likely Benign | 0.34 | Likely Benign | 0.48 | Likely Benign | -4.33 | Deleterious | 0.997 | Probably Damaging | 0.970 | Probably Damaging | 5.87 | Benign | 0.02 | Affected | 3.39 | 15 | 1 | 1 | 2.7 | -27.03 | ||||||||||||||||||||
| c.76G>A | G26R 2D  AIThe SynGAP1 missense variant G26R is listed in ClinVar as a benign alteration (ClinVar ID 1521495.0) and is present in the gnomAD database (gnomAD ID 6‑33423485‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of computational evidence supports a benign impact, aligning with the ClinVar designation and indicating no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33423485-G-A | 3 | 1.86e-6 | -2.946 | Likely Benign | 0.678 | Likely Pathogenic | Likely Benign | 0.189 | Likely Benign | -2.22 | Neutral | 0.994 | Probably Damaging | 0.990 | Probably Damaging | 3.87 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||
| c.772C>T | R258C 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 R258C missense variant is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33437677‑C‑T). Prediction tools that agree on a benign effect include only FATHMM. All other evaluated predictors—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—indicate a pathogenic or likely pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, the SGM‑Consensus as likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) as uncertain. Based on the preponderance of pathogenic predictions, the variant is most likely pathogenic, which does not contradict its current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | 6-33437677-C-T | 1 | 6.20e-7 | -10.285 | Likely Pathogenic | 0.790 | Likely Pathogenic | Ambiguous | 0.771 | Likely Pathogenic | 1.17 | Ambiguous | 0.4 | 1.76 | Ambiguous | 1.47 | Ambiguous | 0.87 | Ambiguous | -6.79 | Deleterious | 1.000 | Probably Damaging | 0.993 | Probably Damaging | 5.77 | Benign | 0.00 | Affected | 3.39 | 15 | -3 | -4 | 7.0 | -53.05 | |||||||||||||||||
| c.791T>C | L264P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant L264P is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that assess pathogenicity all converge on a deleterious effect: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict pathogenicity. No tool in the dataset reports a benign outcome. High‑accuracy methods reinforce this view: AlphaMissense‑Optimized is pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is “Likely Pathogenic”; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, is pathogenic. Based on the unanimous computational evidence, the variant is most likely pathogenic, a conclusion that contradicts the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | -12.285 | Likely Pathogenic | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.767 | Likely Pathogenic | 5.73 | Destabilizing | 0.3 | 6.57 | Destabilizing | 6.15 | Destabilizing | 2.65 | Destabilizing | -6.43 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.49 | Pathogenic | 0.00 | Affected | -3 | -3 | -5.4 | -16.04 | ||||||||||||||||||||||
| c.82T>C | S28P 2D  AIThe SynGAP1 missense variant S28P is listed in ClinVar (ID 1500161.0) with an uncertain significance designation and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while only SIFT indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta stability analysis is unavailable. Overall, the collective evidence points to a benign classification for S28P, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -3.309 | Likely Benign | 0.051 | Likely Benign | Likely Benign | 0.047 | Likely Benign | 1.37 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.53 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | -1 | -0.8 | 10.04 | ||||||||||||||||||||||||||||||
| c.851T>C | L284P 2D  AIThe SynGAP1 missense variant L284P is listed in ClinVar as Pathogenic (ClinVar ID 3344808.0) and is not reported in gnomAD. Prediction tools that assess functional impact uniformly classify the variant as pathogenic: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts a benign effect. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates Likely Pathogenic; and Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, also predicts pathogenic. Based on the unanimous computational evidence, the variant is most likely pathogenic, and this conclusion aligns with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Likely Pathogenic | 1 | -15.588 | Likely Pathogenic | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.794 | Likely Pathogenic | 5.83 | Destabilizing | 0.2 | 5.81 | Destabilizing | 5.82 | Destabilizing | 1.89 | Destabilizing | -6.17 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.64 | Pathogenic | 0.00 | Affected | -3 | -3 | -5.4 | -16.04 | ||||||||||||||||||||||
| c.860A>C | D287A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant D287A is listed in ClinVar with an Uncertain significance status and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, FoldX, Rosetta, Foldetta, and premPS, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Pathogenic, and Foldetta (integrating FoldX‑MD and Rosetta) as benign. The overall tally favors pathogenicity (8 tools vs 5 benign), but the conflicting high‑accuracy results leave uncertainty. Thus, the variant is most likely pathogenic according to the majority of predictions, which does not contradict its ClinVar Uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | -14.686 | Likely Pathogenic | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.484 | Likely Benign | 0.30 | Likely Benign | 0.1 | -0.04 | Likely Benign | 0.13 | Likely Benign | 0.40 | Likely Benign | -7.35 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.58 | Pathogenic | 0.01 | Affected | 3.38 | 23 | -2 | 0 | 5.3 | -44.01 | ||||||||||||||||||||
| c.862G>A | D288N 2D  3DClick to see structure in 3D Viewer AISynGAP1 D288N is listed in ClinVar with an uncertain significance (ClinVar ID 2572204.0) and is present in gnomAD (6‑33437767‑G‑A). Computational predictors are divided: benign calls come from REVEL, FoldX, Rosetta, Foldetta, premPS, and AlphaMissense‑Optimized, while pathogenic calls come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, Foldetta as benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as likely pathogenic. Because the majority of high‑accuracy tools predict benign and the overall split of predictions is even, the variant is most likely benign, which does not contradict the ClinVar status of uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | 6-33437767-G-A | 2 | 1.24e-6 | -10.535 | Likely Pathogenic | 0.521 | Ambiguous | Likely Benign | 0.321 | Likely Benign | -0.39 | Likely Benign | 0.1 | 0.01 | Likely Benign | -0.19 | Likely Benign | -0.03 | Likely Benign | -3.73 | Deleterious | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 1.78 | Pathogenic | 0.05 | Affected | 3.38 | 23 | 1 | 2 | 0.0 | -0.98 | |||||||||||||||||
| c.866T>C | M289T 2D  AIThe SynGAP1 missense variant M289T is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools that classify the variant as benign include REVEL, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and FATHMM. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized scores the variant as benign; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is labeled likely benign; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, also predicts a benign effect. Taken together, the majority of evidence indicates that M289T is most likely benign, and this conclusion does not contradict the current ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 1 | -4.668 | Likely Benign | 0.238 | Likely Benign | Likely Benign | 0.222 | Likely Benign | 0.73 | Ambiguous | 0.1 | 0.17 | Likely Benign | 0.45 | Likely Benign | -0.01 | Likely Benign | -0.47 | Neutral | 0.801 | Possibly Damaging | 0.315 | Benign | 1.83 | Pathogenic | 0.57 | Tolerated | -1 | -1 | -2.6 | -30.09 | ||||||||||||||||||||||
| c.86T>C | M29T 2D  AIThe SynGAP1 missense variant M29T is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that M29T is most likely benign, and this conclusion does not contradict the current ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.167 | Likely Benign | 0.122 | Likely Benign | Likely Benign | 0.199 | Likely Benign | -0.37 | Neutral | 0.018 | Benign | 0.184 | Benign | 4.33 | Benign | 0.00 | Affected | 4.32 | 1 | -1 | -1 | -2.6 | -30.09 | ||||||||||||||||||||||||||||||
| c.878G>A | R293H 2D  AISynGAP1 missense variant R293H is listed in ClinVar with an uncertain significance (ClinVar ID 3901513.0) and is not reported in gnomAD. Prediction tools that indicate a benign effect include REVEL and premPS, whereas the remaining 13 tools—FoldX, Rosetta, Foldetta, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus—predict a pathogenic outcome. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized scores the variant as pathogenic; the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports pathogenic; and Foldetta, which integrates FoldX‑MD and Rosetta stability predictions, classifies the variant as pathogenic. Overall, the preponderance of evidence indicates that R293H is most likely pathogenic, a conclusion that does not contradict the current ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | -13.009 | Likely Pathogenic | 0.973 | Likely Pathogenic | Likely Pathogenic | 0.438 | Likely Benign | 4.45 | Destabilizing | 2.3 | 2.12 | Destabilizing | 3.29 | Destabilizing | 0.32 | Likely Benign | -4.60 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.45 | Pathogenic | 0.04 | Affected | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||
| c.88C>T | H30Y 2D  AIThe SynGAP1 H30Y missense variant (ClinVar ID 972248.0) is listed as “Uncertain” and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic outcome are polyPhen‑2 HumVar and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as “Likely Benign”; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -3.047 | Likely Benign | 0.115 | Likely Benign | Likely Benign | 0.082 | Likely Benign | -1.84 | Neutral | 0.273 | Benign | 0.478 | Possibly Damaging | 3.99 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 2 | 1.9 | 26.03 | ||||||||||||||||||||||||||||||
| c.892C>T | P298S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant P298S is listed in ClinVar as Benign (ClinVar ID 2965590.0) and is present in gnomAD (ID 6‑33437797‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, the SGM‑Consensus as Likely Benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) as Uncertain. No evidence from FoldX, Rosetta, or premPS is available to support either outcome. Overall, the majority of predictions support a benign impact, aligning with the ClinVar designation. Thus, the variant is most likely benign and does not contradict the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Benign | 1 | 6-33437797-C-T | 5 | 3.10e-6 | -6.342 | Likely Benign | 0.144 | Likely Benign | Likely Benign | 0.189 | Likely Benign | 1.38 | Ambiguous | 0.2 | 1.41 | Ambiguous | 1.40 | Ambiguous | 0.58 | Ambiguous | -1.20 | Neutral | 0.991 | Probably Damaging | 0.898 | Possibly Damaging | 2.03 | Pathogenic | 0.85 | Tolerated | 3.39 | 20 | -1 | 1 | 0.8 | -10.04 | |||||||||||||||||
| c.910G>A | D304N 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant D304N is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, FoldX, Rosetta, Foldetta, premPS, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as pathogenic, and Foldetta as benign. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | C2 | Uncertain | 1 | -6.194 | Likely Benign | 0.391 | Ambiguous | Likely Benign | 0.345 | Likely Benign | 0.30 | Likely Benign | 0.1 | -0.08 | Likely Benign | 0.11 | Likely Benign | 0.21 | Likely Benign | -4.18 | Deleterious | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 1.81 | Pathogenic | 0.03 | Affected | 3.38 | 23 | 1 | 2 | 0.0 | -0.98 | |||||||||||||||||||||
| c.929A>G | E310G 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant E310G is listed in ClinVar as Pathogenic (ClinVar ID 2732842.0) and is not reported in gnomAD. Prediction tools that assess the variant’s effect largely agree on a deleterious outcome: REVEL, FoldX, Rosetta, Foldetta, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict pathogenicity, while only premPS predicts a benign effect. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is pathogenic; and Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, also predicts pathogenicity. Consequently, the variant is most likely pathogenic, and this prediction is consistent with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Pathogenic | 1 | -14.132 | Likely Pathogenic | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.848 | Likely Pathogenic | 2.38 | Destabilizing | 0.7 | 3.56 | Destabilizing | 2.97 | Destabilizing | 0.36 | Likely Benign | -6.43 | Deleterious | 1.000 | Probably Damaging | 0.996 | Probably Damaging | 1.12 | Pathogenic | 0.00 | Affected | 3.38 | 19 | -2 | 0 | 3.1 | -72.06 | ||||||||||||||||||||
| c.92G>A | R31Q 2D  AIThe SynGAP1 missense variant R31Q is listed in ClinVar (ID 1977609.0) with an “Uncertain” status and is present in gnomAD (6‑33423501‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” classification and suggests the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33423501-G-A | 7 | 4.34e-6 | -4.434 | Likely Benign | 0.136 | Likely Benign | Likely Benign | 0.051 | Likely Benign | -0.92 | Neutral | 0.829 | Possibly Damaging | 0.614 | Possibly Damaging | 4.01 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.937G>A | E313K 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant E313K is listed in ClinVar as Benign (ClinVar ID 3695040.0) and is not reported in gnomAD. Prediction tools that report a benign effect are absent; all available predictors that provide a definitive call classify the variant as pathogenic. These include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized predicts Pathogenic, the SGM‑Consensus indicates Likely Pathogenic, while Foldetta (combining FoldX‑MD and Rosetta outputs) is Uncertain. Based on the overwhelming pathogenic predictions, the variant is most likely pathogenic, which contradicts its ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Likely Benign | 1 | -12.902 | Likely Pathogenic | 0.959 | Likely Pathogenic | Likely Pathogenic | 0.575 | Likely Pathogenic | 0.64 | Ambiguous | 0.6 | 1.40 | Ambiguous | 1.02 | Ambiguous | 0.75 | Ambiguous | -3.31 | Deleterious | 1.000 | Probably Damaging | 0.995 | Probably Damaging | 1.90 | Pathogenic | 0.02 | Affected | 0 | 1 | -0.4 | -0.94 | ||||||||||||||||||||||
| c.958G>A | V320I 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant V320I is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that uniformly indicate a benign effect include REVEL, FoldX, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only FATHMM predicts a pathogenic outcome, while Rosetta remains inconclusive. High‑accuracy assessments corroborate the benign trend: AlphaMissense‑Optimized is benign; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is “Likely Benign”; and Foldetta, which integrates FoldX‑MD and Rosetta outputs, also predicts benign. Overall, the preponderance of evidence points to a benign effect for V320I, and this conclusion does not conflict with the current ClinVar designation of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 1 | -5.220 | Likely Benign | 0.111 | Likely Benign | Likely Benign | 0.027 | Likely Benign | -0.27 | Likely Benign | 0.2 | 0.66 | Ambiguous | 0.20 | Likely Benign | 0.01 | Likely Benign | -0.21 | Neutral | 0.198 | Benign | 0.114 | Benign | 1.77 | Pathogenic | 0.45 | Tolerated | 3.38 | 23 | 3 | 4 | 0.3 | 14.03 | ||||||||||||||||||||
| c.961C>T | R321C 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant R321C is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33437866‑C‑T). Prediction tools that agree on a benign effect include REVEL, premPS, and AlphaMissense‑Optimized. Those that agree on a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM. Five tools (SGM‑Consensus, FoldX, Rosetta, AlphaMissense‑Default, and Foldetta) report uncertain or inconclusive results. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as pathogenic, and Foldetta as uncertain. Overall, the majority of predictions (six out of eleven) support a pathogenic impact, while three support benign and five are inconclusive. Thus, the variant is most likely pathogenic based on current computational evidence, and this does not contradict its ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Conflicting | 2 | 6-33437866-C-T | 9 | 5.58e-6 | -10.025 | Likely Pathogenic | 0.387 | Ambiguous | Likely Benign | 0.495 | Likely Benign | 0.57 | Ambiguous | 0.1 | 0.56 | Ambiguous | 0.57 | Ambiguous | 0.18 | Likely Benign | -4.59 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.89 | Pathogenic | 0.01 | Affected | 3.38 | 23 | -3 | -4 | 7.0 | -53.05 | |||||||||||||||||
| c.971G>A | R324Q 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R324Q is listed in ClinVar with an uncertain significance (ClinVar ID 2572558.0) and is present in gnomAD (ID 6‑33437876‑G‑A). Prediction tools that classify the variant as benign include REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict pathogenicity are premPS, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. The high‑accuracy AlphaMissense‑Optimized score is benign, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also indicates a likely benign outcome. Protein‑stability predictions from FoldX, Rosetta, and the combined Foldetta method are all uncertain. Overall, the consensus of available computational evidence points to a benign effect for R324Q, which is consistent with its ClinVar status of uncertain significance rather than a pathogenic designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 3 | 6-33437876-G-A | 3 | 1.86e-6 | -5.001 | Likely Benign | 0.173 | Likely Benign | Likely Benign | 0.307 | Likely Benign | 0.56 | Ambiguous | 0.1 | 0.63 | Ambiguous | 0.60 | Ambiguous | 1.02 | Destabilizing | -1.17 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 1.92 | Pathogenic | 0.41 | Tolerated | 3.39 | 22 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||
| c.1730C>G | A577G 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant A577G is listed in ClinVar as Benign (ClinVar ID 1010280.0) and is present in gnomAD (ID 6‑33440782‑C‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. Predictions that are inconclusive (FoldX, Rosetta, Foldetta, premPS) are treated as unavailable. High‑accuracy methods give a benign verdict: AlphaMissense‑Optimized is benign, the SGM‑Consensus is Likely Benign, and Foldetta is uncertain. Overall, the majority of reliable predictions support a benign impact, which is consistent with the ClinVar status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign/Likely benign | 2 | 6-33440782-C-G | 1 | 6.20e-7 | -5.717 | Likely Benign | 0.268 | Likely Benign | Likely Benign | 0.443 | Likely Benign | 0.83 | Ambiguous | 0.0 | 1.02 | Ambiguous | 0.93 | Ambiguous | 0.86 | Ambiguous | -1.84 | Neutral | 0.997 | Probably Damaging | 0.990 | Probably Damaging | -1.31 | Pathogenic | 0.31 | Tolerated | 3.37 | 34 | 1 | 0 | -2.2 | -14.03 | 158.7 | 23.6 | 0.0 | 0.0 | 0.0 | 0.0 | X | Potentially Benign | Ala577 is located near the end and outer surface of an α-helix (res. Arg563-Glu578), where its methyl group does not form any particular interactions in the WT simulations. The introduced residue, glycine, is known as an “α-helix breaker.” However, the residue swap caused only minor helix shortening in one of the replica simulations for the variant system. Regardless, the residue swap seems to be well tolerated based on the variant simulations. | ||||||||
| c.1556A>C | E519A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 E519A missense variant is listed in ClinVar as Pathogenic (ClinVar ID 1029087.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, FoldX, Foldetta, premPS, SIFT, and FATHMM. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, ESM1b, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Uncertain predictions from Rosetta and AlphaMissense‑Optimized are treated as unavailable. High‑accuracy results are: AlphaMissense‑Optimized – unavailable; SGM‑Consensus – Pathogenic; Foldetta – Benign. Overall, the predictions are balanced, but the high‑accuracy Foldetta result leans toward benign while the consensus leans toward pathogenic, leaving the assessment inconclusive. Based on the available predictions, the variant is most likely benign, contradicting the ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Pathogenic | 1 | -8.557 | Likely Pathogenic | 0.904 | Likely Pathogenic | Ambiguous | 0.384 | Likely Benign | -0.05 | Likely Benign | 0.0 | 0.55 | Ambiguous | 0.25 | Likely Benign | 0.00 | Likely Benign | -5.23 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 3.33 | Benign | 0.10 | Tolerated | 3.37 | 35 | 0 | -1 | 5.3 | -58.04 | 162.4 | 83.5 | -0.1 | 0.1 | -0.2 | 0.0 | X | Potentially Benign | Glu519 is located at the beginning of an α-α loop between the two α-helices (res. Gly502-Tyr518 and Ala533-Val560). In the WT simulations, the carboxylate side chain of Glu519 does not make any specific interactions. Accordingly, the Ala residue swap does not show any negative structural effects in the variant simulations. However, it should be noted that Glu519 faces the missing part of the N-terminal in the model, and thus its potential role in maintaining the tertiary structure might be de-emphasized in the current model. | |||||||||||
| c.1256A>G | E419G 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant E419G is listed in ClinVar with an uncertain significance (ClinVar ID 2004834.0) and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and FATHMM, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as likely pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenic, SGM‑Consensus confirms likely pathogenic, and the Foldetta stability analysis is inconclusive. No evidence from FoldX, Rosetta, or premPS is available. Overall, the preponderance of predictions indicates that E419G is most likely pathogenic, which contrasts with the current ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -10.589 | Likely Pathogenic | 0.956 | Likely Pathogenic | Likely Pathogenic | 0.469 | Likely Benign | 1.41 | Ambiguous | 0.0 | 1.94 | Ambiguous | 1.68 | Ambiguous | 0.83 | Ambiguous | -6.42 | Deleterious | 1.000 | Probably Damaging | 0.997 | Probably Damaging | 3.31 | Benign | 0.02 | Affected | 3.37 | 29 | 0 | -2 | 3.1 | -72.06 | 165.3 | 110.8 | 0.0 | 0.0 | -0.1 | 0.0 | X | Potentially Pathogenic | The carboxylate group of Glu419, located on an α helix (res. Met414-Glu436), forms a salt bridge with the side chain of either Arg716 or Lys418 from an opposing helix (res. Pro713-Arg726). The backbone amide group of Glu419 does not form H-bonds, resulting in a slight bend in the α helix. Thus, although glycine is known as an “α helix breaker,” the residue swap does not disrupt the continuity or integrity of the α helix. However, because Gly419 cannot form a salt bridge with the guanidinium group of the Arg716 side chain, the C2-GAP domain tertiary structure could be compromised during folding. | |||||||||||
| c.670A>G | T224A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T224A is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33435521‑A‑G). Prediction tools that agree on a benign effect include REVEL, FoldX, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN and AlphaMissense‑Default. The remaining tools (Rosetta, Foldetta, premPS, ESM1b) return uncertain or inconclusive results. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta as uncertain. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Uncertain | 3 | 6-33435521-A-G | 2 | 1.24e-6 | -7.379 | In-Between | 0.651 | Likely Pathogenic | Likely Benign | 0.464 | Likely Benign | 0.33 | Likely Benign | 0.1 | 1.05 | Ambiguous | 0.69 | Ambiguous | 0.91 | Ambiguous | -2.96 | Deleterious | 0.243 | Benign | 0.079 | Benign | 5.57 | Benign | 0.57 | Tolerated | 3.41 | 13 | 1 | 0 | 2.5 | -30.03 | 169.0 | 41.4 | -0.5 | 1.1 | -0.4 | 0.0 | X | X | Uncertain | The introduced residue Ala224 is located on the outer surface of an anti-parallel β sheet strand (res. Cys219-Thr224). Unlike the hydroxyl group of the Thr224 side chain in the WT model, the methyl side chain of Ala224 cannot form hydrogen bonds with nearby residues Ser204, Ser226, and Gly227. Without these hydrogen-bonding interactions at the β sheet surface, the secondary structure element becomes unstable and unfolds during the variant simulations. However, since the model ends abruptly at the N-terminus, no definite conclusions can be drawn from the simulations. | ||||||||
| c.1622C>G | A541G 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant A541G is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33438865‑C‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. The remaining tools (FoldX, Rosetta, Foldetta, premPS, AlphaMissense‑Default, and ESM1b) return uncertain or inconclusive results. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive, and Foldetta is also inconclusive. Overall, the balance of evidence leans toward a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 1 | 6-33438865-C-G | 2 | 1.24e-6 | -7.233 | In-Between | 0.341 | Ambiguous | Likely Benign | 0.421 | Likely Benign | 0.67 | Ambiguous | 0.0 | 0.94 | Ambiguous | 0.81 | Ambiguous | 0.76 | Ambiguous | -1.48 | Neutral | 0.999 | Probably Damaging | 0.995 | Probably Damaging | -1.31 | Pathogenic | 0.57 | Tolerated | 3.37 | 35 | 1 | 0 | -2.2 | -14.03 | 170.1 | 23.6 | 0.0 | 0.0 | 0.0 | 0.0 | X | Potentially Pathogenic | Ala541 is located on the outer surface of an α-helix (res. Ala533-Val560). The methyl group of Ala541 is on the surface and does not form any interactions. Glycine, known as an “α-helix breaker,” weakens the integrity of the helix. Indeed, in the variant simulations, the hydrogen bond formation between Gly541 and the backbone carbonyl of Ala537 is disrupted. | |||||||||
| c.1621G>C | A541P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant A541P is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that classify the variant as benign include only SIFT, whereas the remaining tools—REVEL, FoldX, Rosetta, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict it to be pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates likely pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is pathogenic, the SGM Consensus is likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) predicts pathogenicity. No predictions are inconclusive or missing. Overall, the collective evidence points to a pathogenic effect for A541P, which is in contrast to the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -14.733 | Likely Pathogenic | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.594 | Likely Pathogenic | 2.47 | Destabilizing | 0.3 | 7.26 | Destabilizing | 4.87 | Destabilizing | 0.86 | Ambiguous | -3.16 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | -1.34 | Pathogenic | 0.07 | Tolerated | 3.37 | 35 | 1 | -1 | -3.4 | 26.04 | 170.4 | -11.2 | 0.1 | 0.0 | 0.1 | 0.0 | X | Potentially Pathogenic | Ala541 is located on the outer surface of an α-helix (res. Ala533-Val560). The methyl group of Ala541 is on the surface and does not form any interactions. Proline lacks a free backbone amide group, and thus, Pro541 is unable to form a hydrogen bond with the carbonyl group of Ala537 in the variant simulations. Consequently, Pro541 disrupts the continuity of the secondary structure element, causing the α-helix to bend slightly in the variant simulations. | |||||||||||
| c.1487A>G | E496G 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 E496G missense variant is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that assess the variant’s effect fall into two groups: no tool predicts a benign outcome, while eight tools (REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default) all predict a pathogenic effect. The SGM‑Consensus, which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely pathogenic outcome. High‑accuracy assessments are mixed: AlphaMissense‑Optimized is uncertain, the SGM‑Consensus remains likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is uncertain. Overall, the preponderance of evidence points to a pathogenic effect, contradicting the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -13.529 | Likely Pathogenic | 0.850 | Likely Pathogenic | Ambiguous | 0.825 | Likely Pathogenic | 1.83 | Ambiguous | 0.1 | 1.76 | Ambiguous | 1.80 | Ambiguous | 0.92 | Ambiguous | -6.16 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | -1.45 | Pathogenic | 0.02 | Affected | 3.37 | 35 | 0 | -2 | 3.1 | -72.06 | 173.9 | 103.1 | 0.0 | 0.0 | -0.7 | 0.0 | X | X | Potentially Pathogenic | Glu496 is located in the α-helix (res. Leu489-Glu519), and its carboxylate group forms salt bridges with the neighbouring residues Lys492 and Arg499 in the WT simulations. Glu496 also forms a hydrogen bond with Ser449 on an opposing helix (res. Val441-Ser457). In the variant simulations, Gly496 cannot form these salt bridges, which could weaken the secondary structure. Additionally, the loss of the hydrogen bond with Ser449 on the opposite helix can weaken the tertiary structure assembly. Moreover, glycine is an α-helix breaker, and it is seen to weaken the integrity of the helix as the hydrogen bonding between the backbone atoms of Gly496 and Ala493 breaks down. Also, due to its location at the GAP-Ras interface, the interaction of Glu496 with Arg499 and Lys492 might play a role in complex association and stability, which cannot be fully addressed using the SynGAP solvent-only simulations. | ||||||||||
| c.1997A>G | E666G 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant E666G is listed in ClinVar as Benign (ClinVar ID 1115026.0) and is present in gnomAD (ID 6‑33441256‑A‑G). Functional prediction tools that agree on pathogenicity include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and the SGM‑Consensus. Only FATHMM predicts a benign effect. Predictions marked Uncertain (FoldX, Rosetta, Foldetta, premPS, AlphaMissense‑Optimized) are treated as unavailable. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN), and Foldetta as Uncertain. Overall, the majority of evidence points to a pathogenic impact, which contradicts the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Benign | 1 | 6-33441256-A-G | 10 | 6.20e-6 | -12.261 | Likely Pathogenic | 0.911 | Likely Pathogenic | Ambiguous | 0.522 | Likely Pathogenic | 1.57 | Ambiguous | 0.1 | 1.46 | Ambiguous | 1.52 | Ambiguous | 0.93 | Ambiguous | -6.25 | Deleterious | 1.000 | Probably Damaging | 0.970 | Probably Damaging | 3.37 | Benign | 0.02 | Affected | 3.38 | 28 | 0 | -2 | 3.1 | -72.06 | 173.9 | 98.5 | 0.0 | 0.0 | -0.7 | 0.0 | X | Potentially Pathogenic | In the WT simulations, the carboxylate group of Glu666, located on the α-helix (res. Ser641-Glu666), is involved in a highly coordinated hydrogen-bonding network between residues from two α-helices (res. Ser641-Glu666 and res. Arg563-Glu578) and from the α-α loop connecting the two α-helices (res. Ser641-Glu666 and res. Leu685-Val699), such as Lys566, Thr672, and Asn669. In the variant simulations, the carbonyl group of Gly666 occasionally forms hydrogen bonds with Lys566 and Asn669. However, Gly666 lacks a side chain and thus cannot maintain as well-coordinated a hydrogen-bond network as Glu666 in the WT, which may affect the tertiary structure assembly. | ||||||||
| c.1594A>C | T532P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T532P is listed in ClinVar as Benign (ClinVar ID 1598909.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM Consensus (majority vote). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments—AlphaMissense‑Optimized, the SGM Consensus, and Foldetta (combining FoldX‑MD and Rosetta outputs)—all indicate a benign impact. No prediction or folding‑stability result is missing or inconclusive. Based on the collective evidence, the variant is most likely benign, and this conclusion is consistent with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign | 1 | -2.143 | Likely Benign | 0.061 | Likely Benign | Likely Benign | 0.201 | Likely Benign | -0.30 | Likely Benign | 0.2 | 0.06 | Likely Benign | -0.12 | Likely Benign | 0.08 | Likely Benign | -0.90 | Neutral | 0.005 | Benign | 0.008 | Benign | -1.28 | Pathogenic | 0.18 | Tolerated | 3.37 | 35 | 0 | -1 | -0.9 | -3.99 | 174.2 | 35.1 | 0.4 | 0.0 | 0.1 | 0.0 | X | Potentially Benign | Thr532 is located on an α-α loop between the two α-helices (res. Gly502-Tyr518 and Ala533-Val560) facing the membrane. In the WT simulations, the hydroxyl group of Thr532 occasionally forms hydrogen bonds with the backbone atoms of other loop residues without any specific interaction. In the variant simulations, the Pro532 residue swap does not cause structural changes. Although hydrophilic residues seem more favorable in the loop, the pyrrolidine side chain of proline is well suited for unstructured protein regions such as loops. However, due to its location at the SynGAP-membrane interface, the effect of the residue swap cannot be fully addressed using the SynGAP solvent-only simulations. | |||||||||||
| c.913A>G | T305A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 T305A variant is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33437818‑A‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus as benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) as uncertain. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Conflicting | 2 | 6-33437818-A-G | 13 | 8.05e-6 | -4.307 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.144 | Likely Benign | 1.30 | Ambiguous | 0.6 | 1.55 | Ambiguous | 1.43 | Ambiguous | 0.77 | Ambiguous | -2.10 | Neutral | 0.939 | Possibly Damaging | 0.645 | Possibly Damaging | 1.76 | Pathogenic | 0.12 | Tolerated | 3.40 | 20 | 1 | 0 | 2.5 | -30.03 | 177.9 | 43.5 | -0.2 | 0.1 | 0.4 | 0.0 | Uncertain | The hydroxyl group of Thr305, located at the beginning of an anti-parallel β strand (res. Thr305-Asn315), hydrogen bonds with the carboxylate groups of Glu270 and Asp304 in the anti-parallel β strand and the adjacent β hairpin loop, respectively. In the variant simulations, the methyl group of the Ala305 side chain cannot hydrogen bond with either of the acidic residues, which could weaken the integrity of the tertiary structure and the β hairpin loop. Indeed, the guanidinium group of Arg299 does not acquire its central hairpin loop position due to the residue swap.β hairpins are potential nucleation sites during the initial stages of protein folding, so even minor changes in them could be significant. Due to its location near the membrane surface, the residue swap could also affect the C2 loop dynamics and SynGAP-membrane association. However, this is beyond the scope of the solvent-only simulations to unravel. | |||||||||
| c.2087T>C | L696P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant L696P is listed in ClinVar as Pathogenic (ClinVar ID 1699350.0) and is not reported in gnomAD. Prediction tools that classify the variant as benign include only FATHMM; all other evaluated algorithms—REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized—report it as pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a pathogenic effect. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts pathogenic, the SGM Consensus (majority vote) is pathogenic, and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, predicts a destabilizing, pathogenic outcome. Taken together, the overwhelming majority of predictions and the high‑accuracy tools classify the variant as pathogenic, fully consistent with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Pathogenic | 1 | -16.926 | Likely Pathogenic | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.678 | Likely Pathogenic | 6.66 | Destabilizing | 0.2 | 10.84 | Destabilizing | 8.75 | Destabilizing | 2.13 | Destabilizing | -6.58 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 3.00 | Benign | 0.00 | Affected | 3.46 | 13 | -3 | -3 | -5.4 | -16.04 | 180.6 | 65.9 | 0.1 | 0.0 | -0.6 | 0.1 | X | Potentially Pathogenic | The isobutyl side chain of Leu696, located in the middle of an α-helix (res. Leu685-Gln702), engages in hydrophobic packing with nearby residues (e.g., Leu441, Leu431, Leu692, Leu714) in the inter-helix space. Prolines lack a free amide group necessary for hydrogen bonding with the carbonyl group of Leu692 in the same manner as Leu696 in the WT. Consequently, the residue swap with proline disrupts the continuity of the secondary structure element in the variant simulations. Additionally, the side chain of Pro696 is not as optimal as Leu696 for hydrophobic packing in the inter-helix space. | |||||||||||
| c.886T>G | S296A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S296A (ClinVar ID 469166.0) is listed as ClinVar status Uncertain and is not present in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, Rosetta, Foldetta, premPS, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign or neutral. In contrast, polyPhen‑2 (HumDiv and HumVar) and FATHMM predict pathogenic. Grouping by consensus, the benign‑predicting tools outnumber the pathogenic ones. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is also benign; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, reports a benign effect. No evidence from the available tools suggests a deleterious impact. Therefore, the variant is most likely benign, and this conclusion is consistent with its ClinVar status of Uncertain rather than pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 1 | -6.847 | Likely Benign | 0.247 | Likely Benign | Likely Benign | 0.209 | Likely Benign | 0.50 | Ambiguous | 0.3 | -0.26 | Likely Benign | 0.12 | Likely Benign | 0.35 | Likely Benign | -1.79 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.97 | Pathogenic | 0.65 | Tolerated | 3.40 | 16 | 1 | 1 | 2.6 | -16.00 | 182.5 | 26.6 | -0.2 | 0.1 | -0.5 | 0.0 | X | Potentially Pathogenic | The hydroxyl group of the Ser296 side chain, located in an anti-parallel β sheet strand (res. Met289-Pro298), stably hydrogen bonds with the carboxylate group of Asp330 in a neighboring β strand (res. Ala322-Asp332). The backbone carbonyl group of Ser296 also hydrogen bonds with the guanidinium group of Arg279 in another nearby β strand (res. Arg279-Cys285). In the variant simulations, the methyl group of the Ala296 side chain cannot hydrogen bond with Asp330, causing the carboxylate group positioning to fluctuate more than in the WT simulations.Although the residue swap does not seem to affect the anti-parallel β sheet assembly during the simulations, it is possible that the Ser296-Asp330 hydrogen bond plays a crucial role in maintaining the C2 domain fold. Notably, because Ser296 is located near the membrane interface, the potential effect of the residue swap on the SynGAP-membrane association cannot be addressed by solvent-only simulations. | |||||||||||
| c.1517T>C | L506P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant L506P is listed in ClinVar (ID 975474.0) as Pathogenic and is not reported in gnomAD. All available in‑silico predictors classify the variant as pathogenic: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts a benign effect. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized is Pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is Likely Pathogenic; and Foldetta (combining FoldX‑MD and Rosetta outputs) is Pathogenic. Based on the unanimous computational evidence, the variant is most likely pathogenic, which aligns with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Pathogenic | 1 | -12.088 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.737 | Likely Pathogenic | 5.48 | Destabilizing | 0.7 | 10.19 | Destabilizing | 7.84 | Destabilizing | 2.50 | Destabilizing | -6.96 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 1.55 | Pathogenic | 0.00 | Affected | 3.37 | 35 | -3 | -3 | -5.4 | -16.04 | 182.6 | 64.9 | 0.1 | 0.0 | 0.2 | 0.1 | X | Potentially Pathogenic | Leu506 is located in the middle of an α-helix (res. Gly502-Tyr518) within the inter-helix space of two helices (res. Gly502-Tyr518 and res. Glu582-Met603). In the WT simulations, the iso-butyl side chain of Leu506 hydrophobically packs with residues in the inter-helix space (e.g., Ile510, Phe597, Leu598, Ala601). In the variant simulations, the cyclic five-membered pyrrolidine ring of Pro506 is not as optimal as Leu506 for hydrophobic packing with nearby residues. Additionally, Pro506 cannot maintain the hydrogen bond with the backbone oxygen of Gly502 as Leu506 does in the WT, which disrupts the secondary structure element. | |||||||||||
| c.2075T>C | L692P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant L692P is listed in ClinVar with an “Uncertain” status (ClinVar ID 847082.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect are limited to FATHMM, while the remaining tools (REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized) uniformly predict a pathogenic impact. High‑accuracy assessments further support this: AlphaMissense‑Optimized predicts pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates “Likely Pathogenic”; and Foldetta, which integrates FoldX‑MD and Rosetta outputs, also predicts pathogenic. Based on the overwhelming consensus of pathogenic predictions, the variant is most likely pathogenic, which does not contradict its current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -16.447 | Likely Pathogenic | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.668 | Likely Pathogenic | 9.19 | Destabilizing | 0.1 | 13.20 | Destabilizing | 11.20 | Destabilizing | 1.69 | Destabilizing | -6.98 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 3.06 | Benign | 0.00 | Affected | 3.42 | 17 | -3 | -3 | -5.4 | -16.04 | 186.2 | 62.8 | -0.2 | 0.1 | -0.7 | 0.3 | X | Potentially Pathogenic | The isobutyl side chain of Leu692, located in the middle of an α-helix (res. Leu685-Gln702), engages in hydrophobic packing with nearby residues (e.g., Leu441, Leu431, Leu696) in the inter-helix space. Prolines lack a free amide group necessary for hydrogen bonding with the carbonyl group of Glu688 in the same manner as Leu692 in the WT. Consequently, the residue swap with proline disrupts the continuity of the secondary structure element in the variant simulations. Additionally, the side chain of Pro692 is not as optimal as Leu692 for hydrophobic packing in the inter-helix space. | |||||||||||
| c.667A>G | T223A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T223A is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (ID 6‑33435518‑A‑G). Functional prediction tools that agree on a benign effect include FoldX, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are REVEL and PROVEAN. Predictions that are inconclusive are Rosetta, Foldetta, premPS, and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also resolves to benign; and Foldetta, which integrates FoldX‑MD and Rosetta outputs, remains uncertain. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Uncertain | 1 | 6-33435518-A-G | 3 | 1.86e-6 | -7.076 | In-Between | 0.316 | Likely Benign | Likely Benign | 0.574 | Likely Pathogenic | 0.30 | Likely Benign | 0.1 | 0.77 | Ambiguous | 0.54 | Ambiguous | 0.74 | Ambiguous | -3.36 | Deleterious | 0.231 | Benign | 0.058 | Benign | 5.74 | Benign | 0.09 | Tolerated | 3.41 | 13 | 1 | 0 | 2.5 | -30.03 | 186.4 | 44.0 | 0.0 | 0.0 | 0.0 | 0.0 | X | X | Uncertain | The introduced residue Ala223 is located on the outer surface of an anti-parallel β sheet strand (res. Cys219-Thr224). Unlike the hydroxyl group of the Thr223 side chain in the WT protein, the methyl side chain of Ala223 cannot form hydrogen bonds with nearby residues Thr228 and Lys207. Without these hydrogen-bonding interactions at the β sheet surface, the secondary structure element becomes unstable and partially unfolds in the variant simulations. However, since the model ends abruptly at the N-terminus, no definite conclusions can be drawn from the simulations. | ||||||||
| c.1768A>G | S590G 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant S590G is listed in ClinVar (ID 1721675.0) with an uncertain significance status and is present in gnomAD (6‑33440820‑A‑G). Functional prediction tools that report a benign effect include REVEL, SIFT, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and ESM1b. The high‑accuracy consensus (SGM Consensus) derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN yields a pathogenic majority. Foldetta, which integrates FoldX‑MD and Rosetta outputs, is inconclusive, as are FoldX, Rosetta, and premPS. Overall, the majority of evidence points toward a pathogenic impact, which does not contradict the ClinVar uncertain status but suggests a higher likelihood of pathogenicity. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Conflicting | 2 | 6-33440820-A-G | 14 | 8.67e-6 | -14.277 | Likely Pathogenic | 0.574 | Likely Pathogenic | Likely Benign | 0.379 | Likely Benign | 0.67 | Ambiguous | 0.1 | 1.28 | Ambiguous | 0.98 | Ambiguous | 0.71 | Ambiguous | -3.92 | Deleterious | 1.000 | Probably Damaging | 0.922 | Probably Damaging | 3.42 | Benign | 0.06 | Tolerated | 3.37 | 35 | 1 | 0 | 0.4 | -30.03 | 186.7 | 49.4 | 0.0 | 0.0 | 0.1 | 0.0 | X | Potentially Pathogenic | In the WT simulations, the hydroxyl group of Ser590, located on an α helix (res. Glu582-Met603), forms hydrogen bonds with the backbone carbonyl of Ala634 and/or the carboxamide group of the Asn635 side chain at the end of the opposing α helix (res. Thr619-Ala634).The residue swap could weaken the integrity of the α helix, as glycine is known as an “α helix breaker.” However, no discernible difference was observed between the WT and variant simulations in this regard. Importantly, Gly590 cannot form hydrogen bonds with the opposing helix in the same way that serine can, which could weaken the tertiary structure assembly between the two helices. | ||||||||
| c.1306G>A | E436K 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant E436K is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include only FATHMM, whereas the remaining evaluated algorithms (REVEL, Rosetta, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) uniformly predict a pathogenic impact; FoldX, Foldetta, and premPS are inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as likely pathogenic, and Foldetta as uncertain. Overall, the preponderance of evidence points to a pathogenic effect for E436K, which does not conflict with the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -13.869 | Likely Pathogenic | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.829 | Likely Pathogenic | 0.56 | Ambiguous | 0.1 | 2.86 | Destabilizing | 1.71 | Ambiguous | 0.82 | Ambiguous | -3.77 | Deleterious | 0.994 | Probably Damaging | 0.951 | Probably Damaging | 4.71 | Benign | 0.02 | Affected | 3.37 | 29 | 0 | 1 | -0.4 | -0.94 | 186.8 | 39.8 | 0.0 | 0.0 | -0.2 | 0.0 | X | X | X | Potentially Pathogenic | The carboxylate group of Glu436, located on the α helix (res. Met414-Glu436), forms a salt bridge with the amino group of the Lys444 side chain on an opposing α helix (res. Val441-Ser457). The backbone carbonyl of Glu436 also H-bonds with the Lys444 side chain, which helps keep the ends of the two α helices tightly connected. In contrast, in the variant simulations, the salt bridge formation with Lys444 is not possible. Instead, the repelled Lys436 side chain rotates outward, causing a change in the α helix backbone H-bonding: the amide group of Lys444 H-bonds with the carbonyl of Ala433 instead of the carbonyl of Cys432. | |||||||||
| c.2116G>A | E706K 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant E706K is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM all classify the change as benign. In contrast, ESM1b and AlphaMissense‑Default predict a pathogenic impact. Tools that return uncertain results—FoldX, Rosetta, Foldetta, and AlphaMissense‑Optimized—do not provide decisive evidence. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic versus two benign calls). High‑accuracy assessments are likewise ambiguous: AlphaMissense‑Optimized is uncertain, Foldetta is uncertain, and the SGM Consensus remains inconclusive. Overall, the preponderance of evidence points to a benign effect, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 1 | -10.519 | Likely Pathogenic | 0.833 | Likely Pathogenic | Ambiguous | 0.080 | Likely Benign | 1.17 | Ambiguous | 0.1 | 0.51 | Ambiguous | 0.84 | Ambiguous | 0.08 | Likely Benign | -1.51 | Neutral | 0.345 | Benign | 0.028 | Benign | 4.15 | Benign | 0.52 | Tolerated | 3.47 | 10 | 0 | 1 | -0.4 | -0.94 | 187.1 | 49.2 | 0.0 | 0.0 | 0.4 | 0.1 | X | Uncertain | The carboxylate side chain of Glu706, located at the end and outer surface of an α-helix (res. Thr704-Gly712), forms a salt bridge with Lys710 and a hydrogen bond with its own backbone amino group at the helix end in the WT simulations. Although Lys706 is unable to make these transient interactions in the variant simulations, there is no apparent negative effect on the protein structure due to the residue swap. However, because the model ends abruptly at the C-terminus, no definite conclusions can be drawn based on the simulations. | ||||||||||||
| c.2014A>G | T672A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T672A is listed in ClinVar as Benign (ClinVar ID 2154412.0) and is present in gnomAD (variant ID 6‑33441273‑A‑G). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only PROVEAN predicts a pathogenic outcome. Uncertain results are reported for FoldX, Rosetta, Foldetta, and premPS. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as Likely Benign, and Foldetta as Uncertain. Overall, the preponderance of evidence points to a benign effect, and this conclusion is consistent with the ClinVar designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign | 1 | 6-33441273-A-G | 3 | 1.86e-6 | -6.524 | Likely Benign | 0.109 | Likely Benign | Likely Benign | 0.046 | Likely Benign | 0.51 | Ambiguous | 0.3 | 1.15 | Ambiguous | 0.83 | Ambiguous | 0.65 | Ambiguous | -3.20 | Deleterious | 0.006 | Benign | 0.002 | Benign | 3.44 | Benign | 0.12 | Tolerated | 3.40 | 25 | 1 | 0 | 2.5 | -30.03 | 188.5 | 42.5 | -0.1 | 0.3 | 0.2 | 0.0 | X | Potentially Pathogenic | The hydroxyl group of Thr672, located in an entangled α-α loop connecting the two α-helices (res. Ser641-Glu666 and res. Leu685-Val699), is involved in a highly coordinated hydrogen-bonding network between residues from two α-helices (res. Ser641-Glu666 and res. Arg563-Glu578) and from the α-α loop itself, such as Lys566, Glu666, and Asn669. In the variant simulations, Ala672 can only form a hydrogen bond with Lys566 via its backbone carbonyl group. Consequently, it cannot maintain the Lys566-Glu666 salt bridge through hydrogen bonding, leading to a significant disruption of the intricate and stable hydrogen-bond network between the loop and the helices. | ||||||||
| c.1403T>A | M468K 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant M468K is listed in ClinVar (ID 642691.0) as Pathogenic and is not reported in gnomAD. All available in silico predictors classify the variant as pathogenic: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts a benign effect. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields Likely Pathogenic; and Foldetta (combining FoldX‑MD and Rosetta outputs) predicts pathogenic. Thus, the variant is most likely pathogenic, and this prediction aligns with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Pathogenic | 1 | -16.982 | Likely Pathogenic | 0.978 | Likely Pathogenic | Likely Pathogenic | 0.828 | Likely Pathogenic | 3.21 | Destabilizing | 0.1 | 3.30 | Destabilizing | 3.26 | Destabilizing | 2.57 | Destabilizing | -4.61 | Deleterious | 0.878 | Possibly Damaging | 0.922 | Probably Damaging | -1.34 | Pathogenic | 0.04 | Affected | 3.37 | 31 | 0 | -1 | -5.8 | -3.02 | 188.7 | 69.3 | 0.0 | 0.0 | -0.1 | 0.2 | X | X | Potentially Pathogenic | The thioether group of Met468, located in the middle of an α helix (res. Ala461–Phe476), interacts with hydrophobic residues (e.g., Phe464, Leu465, Leu489) in an inter-helix space formed by two other α helices (res. Ala461–Phe476, res. Thr488–Gly502). In the variant simulations, the positively charged side chain of Lys468 rotates outward to escape the hydrophobic niche, forming an H-bond with the hydroxyl group of the Ser471 side chain and a salt bridge with the carboxylate group of the Glu472 side chain. This residue swap also disrupts the methionine-aromatic stacking with the phenyl ring of the Phe464 side chain. Although no large-scale structural changes are observed during the variant simulations, the importance of hydrophobic packing suggests that the effects could be more pronounced during protein folding. | ||||||||||
| c.2071A>C | T691P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T691P is listed in ClinVar (ID 648126.0) as Pathogenic and is not reported in gnomAD. Across the broad panel of in‑silico predictors, three tools (REVEL, SIFT, FATHMM) classify the change as benign, whereas the remaining 11 predictors (FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and the SGM‑Consensus score) report it as pathogenic. High‑accuracy assessments further support a deleterious effect: the AlphaMissense‑Optimized model is inconclusive, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is pathogenic, and the Foldetta stability analysis (combining FoldX‑MD and Rosetta outputs) is pathogenic. Taken together, the preponderance of evidence indicates that T691P is most likely pathogenic, which is consistent with its ClinVar classification and does not contradict the database status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Pathogenic | 1 | -13.801 | Likely Pathogenic | 0.905 | Likely Pathogenic | Ambiguous | 0.214 | Likely Benign | 5.04 | Destabilizing | 0.4 | 6.09 | Destabilizing | 5.57 | Destabilizing | 1.27 | Destabilizing | -3.43 | Deleterious | 1.000 | Probably Damaging | 0.952 | Probably Damaging | 3.43 | Benign | 0.06 | Tolerated | 3.43 | 14 | 0 | -1 | -0.9 | -3.99 | 188.9 | 33.0 | 0.1 | 0.0 | -0.6 | 0.0 | X | X | Potentially Pathogenic | The hydroxyl side chain of Thr691, located in an α-helix (res. Leu696-Leu685), can form hydrogen bonds with the backbone carbonyl and the side chain guanidinium group of Arg687. This interaction facilitates the simultaneous formation of salt bridges between Arg687 and Glu688 on the same α-helix. Additionally, Thr691 occasionally interacts with the thioether side chain of Met409 in an anti-parallel β-sheet of the C2 domain (res. Ile411-Ala399), although this interaction is not consistently maintained throughout the WT simulations. In the variant simulations, the pyrrolidine side chain of Pro691 lacks hydrogen bond donors, making a similar setup impossible. Moreover, proline lacks a free amide group necessary for hydrogen bonding with the carbonyl group of Arg687, introducing a slight bend in the α-helix and compromising its integrity. | ||||||||||
| c.1771G>C | A591P 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant A591P is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools largely converge on a pathogenic effect: pathogenic predictions come from FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and the SGM‑Consensus (Likely Pathogenic). Benign predictions are limited to REVEL and FATHMM. High‑accuracy assessments reinforce the pathogenic view: AlphaMissense‑Optimized predicts pathogenic, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is Likely Pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) also indicates pathogenic. Consequently, the variant is most likely pathogenic, a conclusion that contrasts with its ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -14.479 | Likely Pathogenic | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.404 | Likely Benign | 3.78 | Destabilizing | 0.3 | 7.29 | Destabilizing | 5.54 | Destabilizing | 1.45 | Destabilizing | -4.41 | Deleterious | 0.995 | Probably Damaging | 0.853 | Possibly Damaging | 3.35 | Benign | 0.01 | Affected | 3.37 | 35 | 1 | -1 | -3.4 | 26.04 | 191.5 | -10.1 | 0.2 | 0.1 | 0.4 | 0.1 | X | Potentially Pathogenic | The methyl group of the Ala591 side chain, located in the middle of an α helix (res. Glu582-Met603), packs against hydrophobic residues (e.g., Ile483, Phe484) of an opposing partially helical loop (res. Phe476-Asn487).In the variant simulations, Pro591 lacks a free backbone amide group and, therefore, cannot form a hydrogen bond with the backbone carbonyl of Arg587 as Ala591 does in the WT. This notably weakens the α helix integrity and compromises the continuity of the helix. In reality, the effect on the structure during protein folding could be more severe. | |||||||||||
| c.1729G>A | A577T 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant A577T is listed in ClinVar as benign (ClinVar ID 2195056.0) and is present in gnomAD (ID 6‑33440781‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) as uncertain. No other high‑confidence stability predictions are available. Overall, the consensus of the available predictions indicates that the variant is most likely benign, which aligns with its ClinVar classification and does not contradict the reported status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign | 1 | 6-33440781-G-A | 6 | 3.72e-6 | -5.311 | Likely Benign | 0.322 | Likely Benign | Likely Benign | 0.427 | Likely Benign | 0.86 | Ambiguous | 0.1 | 0.54 | Ambiguous | 0.70 | Ambiguous | 0.54 | Ambiguous | -1.47 | Neutral | 0.999 | Probably Damaging | 0.987 | Probably Damaging | -1.31 | Pathogenic | 0.47 | Tolerated | 3.37 | 34 | 1 | 0 | -2.5 | 30.03 | 191.9 | -43.4 | 0.0 | 0.0 | 0.7 | 0.1 | X | Potentially Benign | Ala577 is located near the end and outer surface of an α-helix (res. Arg563-Glu578), where its methyl group does not form any particular interactions in the WT simulations. In the variant simulations, the hydroxyl group of the Thr577 side chain hydrogen bonds with the backbone atoms of Arg573 and Lys574 within the same helix, which has the potential to weaken the stability of the secondary structure element. Regardless, the residue swap seems to be well tolerated based on the variant simulations. | ||||||||
| c.1631G>C | R544P 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R544P is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Across the available in‑silico predictors, none indicate a benign effect; all 13 tools (REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) predict a pathogenic outcome. High‑accuracy methods reinforce this view: AlphaMissense‑Optimized is pathogenic, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta) is pathogenic. Consequently, the variant is most likely pathogenic based on current predictions, which contradicts the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 2 | -16.905 | Likely Pathogenic | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.762 | Likely Pathogenic | 4.70 | Destabilizing | 0.1 | 4.19 | Destabilizing | 4.45 | Destabilizing | 1.14 | Destabilizing | -4.88 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | -1.48 | Pathogenic | 0.05 | Affected | 3.37 | 35 | 0 | -2 | 2.9 | -59.07 | 192.0 | 123.8 | 0.1 | 0.0 | -0.3 | 0.0 | X | X | Potentially Pathogenic | Arg544 is located in the middle of an α-helix (res. Ala533-Val560). In the WT simulations, the guanidinium side chain of Arg544 forms a salt bridge with the carboxylate groups of Glu548 on the same α-helix, and with Glu651 and Glu656 on an opposing α-helix (res. Glu666-Asp644). In the variant simulations, the pyrrolidine side chain of Pro544 cannot form any of the salt bridges that Arg544 does in the WT, potentially weakening the tertiary structure assembly. Additionally, Pro544 lacks the amide group, and thus, unlike Arg544 in the WT, is unable to form a hydrogen bond with the carbonyl of Gln540. This disruption breaks the continuity of the secondary structure element, causing the α-helix to bend slightly in the variant simulations. These negative structural effects could be more pronounced during protein folding and are likely to be undermined in the MD simulations. | ||||||||||
| c.1898T>C | L633P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant L633P (ClinVar ID 858973.0) is listed as Pathogenic and is not reported in gnomAD. Prediction tools that classify the variant as benign include only FATHMM. All other evaluated tools—SGM‑Consensus, REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict it to be pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized scores it as Pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates Likely Pathogenic; and Foldetta, which integrates FoldX‑MD and Rosetta outputs, predicts Pathogenic. Based on the overwhelming consensus of pathogenic predictions and the ClinVar designation, the variant is most likely pathogenic, with no contradiction to its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Pathogenic/Likely path. | 2 | -15.669 | Likely Pathogenic | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.693 | Likely Pathogenic | 6.60 | Destabilizing | 0.2 | 10.15 | Destabilizing | 8.38 | Destabilizing | 2.42 | Destabilizing | -6.97 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.70 | Benign | 0.00 | Affected | 3.37 | 34 | -3 | -3 | -5.4 | -16.04 | 193.2 | 65.1 | 0.0 | 0.0 | 0.1 | 0.0 | X | Potentially Pathogenic | The iso-butyl side chain of Leu633, located in the middle of an α helix (res. Glu617-Asn635), packs hydrophobically with nearby residues (e.g., Leu653, Val629, Leu551) in the WT simulations.In the variant simulations, the pyrrolidine side chain of Pro633 is not as optimal for hydrophobic packing as Leu633 in the WT. Additionally, proline lacks a free backbone amide group, so Pro633 cannot form a hydrogen bond with the backbone carbonyl group of Val629, which disrupts the continuity of the secondary structure element. | |||||||||||
| c.1045C>T | P349S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 P349S missense variant is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. Those that predict a pathogenic impact are Rosetta, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. Predictions that are inconclusive or uncertain are FoldX, ESM1b, and premPS. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) as pathogenic. Overall, the majority of tools, including the high‑accuracy methods, predict a pathogenic effect. Thus, the variant is most likely pathogenic, which does not contradict its current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | C2 | Uncertain | 1 | -7.654 | In-Between | 0.217 | Likely Benign | Likely Benign | 0.277 | Likely Benign | 1.92 | Ambiguous | 0.1 | 2.28 | Destabilizing | 2.10 | Destabilizing | 0.87 | Ambiguous | -6.13 | Deleterious | 1.000 | Probably Damaging | 0.996 | Probably Damaging | 1.66 | Pathogenic | 0.06 | Tolerated | 3.37 | 25 | 1 | -1 | 0.8 | -10.04 | 194.9 | -18.1 | -0.1 | 0.0 | 0.2 | 0.1 | X | X | Potentially Pathogenic | The cyclic pyrrolidine side chain of Pro349, located at the end of an anti-parallel β sheet strand (res. Gly341-Pro349), allows the strand to end and make a tight turn before a short α helical section within a loop connecting to another β strand (res. Thr359-Pro364). In the variant simulations, the hydroxyl group of Ser349 forms a hydrogen bond with the backbone amide group of Ala351 in the short helical section. Conversely, the backbone amide group of Ser349 (absent in proline) does not form any intra-protein hydrogen bonds. However, the β strand end connects to the α helical section in a more stable and consistent manner compared to the WT. Although the residue swap does not cause major adverse effects on the protein structure in the simulations, it is possible that the tight turn at the β strand end could not be created during folding without the presence of proline. | |||||||||||
| c.1322T>C | V441A 2D  3DClick to see structure in 3D Viewer AISynGAP1 variant V441A is listed in ClinVar as uncertain and is present in gnomAD (ID 6‑33438227‑T‑C). Consensus from most in silico predictors favors a benign effect: REVEL, FoldX, Rosetta, Foldetta, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Optimized all report benign. Pathogenic predictions come from PROVEAN, polyPhen‑2 HumDiv, and ESM1b, while premPS and AlphaMissense‑Default remain uncertain. High‑accuracy assessments give a mixed picture: AlphaMissense‑Optimized predicts benign; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields pathogenic; Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, reports benign. Overall, the preponderance of evidence points to a benign impact, aligning with the ClinVar uncertain designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Conflicting | 2 | 6-33438227-T-C | 3 | 1.86e-6 | -9.439 | Likely Pathogenic | 0.359 | Ambiguous | Likely Benign | 0.053 | Likely Benign | -0.14 | Likely Benign | 0.0 | 0.33 | Likely Benign | 0.10 | Likely Benign | 0.95 | Ambiguous | -2.92 | Deleterious | 0.513 | Possibly Damaging | 0.214 | Benign | 3.44 | Benign | 0.93 | Tolerated | 3.37 | 29 | 0 | 0 | -2.4 | -28.05 | 195.0 | 44.6 | 0.0 | 0.1 | 0.5 | 0.0 | X | X | Uncertain | The iso-propyl side chain of Val441, located on the outer surface of an α helix (res. Asn440-Thr458), does not interact with other residues in the WT simulations. In the variant simulations, the methyl side chain of Ala441 is similarly hydrophobic and does not form any interactions on the outer helix surface. Although the residue swap does not negatively affect the protein structure based on the simulations, it is noteworthy that the residue faces the RasGTPase interface. Thus, the effect of the residue swap on the SynGAP-Ras complex formation or GTPase activation cannot be fully addressed using the SynGAP solvent-only simulations. | ||||||||
| c.2015C>A | T672K 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant T672K is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, FoldX, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are SGM‑Consensus, PROVEAN, polyPhen‑2 HumDiv, ESM1b, and AlphaMissense‑Default. Uncertain predictions come from Foldetta, premPS, and Rosetta. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta as inconclusive. Overall, the majority of tools lean toward a benign interpretation, but the high‑accuracy consensus is split, leaving the variant’s impact uncertain. Thus, the variant is most likely benign based on the bulk of predictions, and this does not contradict its ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -12.192 | Likely Pathogenic | 0.698 | Likely Pathogenic | Likely Benign | 0.065 | Likely Benign | 0.20 | Likely Benign | 0.5 | 1.21 | Ambiguous | 0.71 | Ambiguous | 0.72 | Ambiguous | -4.31 | Deleterious | 0.745 | Possibly Damaging | 0.051 | Benign | 3.40 | Benign | 0.07 | Tolerated | 3.40 | 25 | 0 | -1 | -3.2 | 27.07 | 195.1 | 7.0 | 0.4 | 0.7 | 0.4 | 0.1 | X | X | Potentially Pathogenic | The hydroxyl group of Thr672, located in an entangled α-α loop connecting the two α-helices (res. Ser641-Glu666 and res. Leu685-Val699), is involved in a highly coordinated hydrogen-bonding network between residues from two α-helices (res. Ser641-Glu666 and res. Arg563-Glu578) and from the α-α loop itself, such as Lys566, Glu666, and Asn669. In the variant simulations, Lys672 can only form a hydrogen bond with the amino group of the Lys566 side chain via its backbone carbonyl group. Consequently, it cannot maintain the Lys566-Glu666 salt bridge through hydrogen bonding. However, the amino group of Lys periodically forms a salt bridge with the carboxylate group of Glu666, which prevents a drastic disruption of the hydrogen-bond network that keeps the loop close to the helices. | ||||||||||
| c.1714T>G | W572G 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant W572G is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that assess pathogenicity all converge on a deleterious effect: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report a pathogenic outcome. No tool in the dataset predicts a benign effect. High‑accuracy assessments reinforce this consensus: AlphaMissense‑Optimized is pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is “Likely Pathogenic”; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, is pathogenic. Based on the uniform pathogenic predictions from both general and high‑accuracy tools, the variant is most likely pathogenic, a conclusion that contradicts its current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -17.692 | Likely Pathogenic | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.900 | Likely Pathogenic | 6.57 | Destabilizing | 0.2 | 7.57 | Destabilizing | 7.07 | Destabilizing | 1.83 | Destabilizing | -11.98 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | -1.24 | Pathogenic | 0.00 | Affected | 3.37 | 35 | -7 | -2 | 0.5 | -129.16 | 195.2 | 127.9 | 0.0 | 0.0 | -1.0 | 0.0 | X | Potentially Pathogenic | The introduced residue Gly572, located in an α-helix (res. Arg563-Glu578), is considerably smaller than the tryptophan it replaced. The indole ring of the Trp572 side chain lies in a hydrophobic inter-helix space, where it makes extensive hydrophobic interactions with nearby residues such as Met470, Phe569, Leu588, and Ile589. In the variant simulations, all these favorable packing interactions are completely removed, as the introduced residue Gly572 essentially lacks a side chain altogether. Although not observed in the simulations, the residue swap could also weaken the integrity of the helix (res. Arg563-Glu578), as glycine is known as an “α-helix breaker.” Overall, the residue swap is highly likely to cause critical protein folding problems that are underestimated based on the effects seen in the variant simulations. | |||||||||||
| c.1904A>G | N635S 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant N635S is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6-33440956-A-G). Functional prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, SIFT, and ESM1b. Predictions that are inconclusive or unavailable are FoldX, Rosetta, Foldetta, and premPS. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive, and Foldetta is also inconclusive. Overall, the majority of available predictions lean toward a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Conflicting | 4 | 6-33440956-A-G | 10 | 6.20e-6 | -9.002 | Likely Pathogenic | 0.101 | Likely Benign | Likely Benign | 0.104 | Likely Benign | 0.80 | Ambiguous | 0.1 | 0.67 | Ambiguous | 0.74 | Ambiguous | 0.95 | Ambiguous | -4.45 | Deleterious | 0.261 | Benign | 0.044 | Benign | 3.06 | Benign | 0.05 | Affected | 3.37 | 34 | 1 | 1 | 2.7 | -27.03 | 196.0 | 30.9 | 0.1 | 0.0 | -0.3 | 0.2 | X | Uncertain | In the WT simulations, the carboxamide side chain of Asn635, located on the outer surface of an α helix (res. Glu617-Asn635), forms hydrogen bonds with Gln631 on the same α helix and with the hydroxyl side chain of Ser590 on an opposing α helix (res. Glu582-Met603).In the variant simulations, the side chain of Ser635 is shorter than asparagine and thus prefers to hydrogen bond with the carbonyl group of Gln631 on the same helix and, to a lesser extent, with Ser590 compared to Asn635 in the WT. Ser635 forms hydrogen bonds with the backbone atoms of the same helix, which may destabilize the helix, although this is not clearly evident in the simulations. The weakening of the hydrogen bond between Ser635 and Ser590 in the variant may also weaken the tertiary structure assembly between the helices.Additionally, Asn635 is at the GTPase interface. However, the implication of the residue swap on the complex formation with the GTPase cannot be investigated using solvent-only simulations. | |||||||||
| c.1108G>A | G370S 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant G370S is listed in ClinVar with an uncertain significance and is present in gnomAD (variant ID 6‑33438013‑G‑A). Consensus predictions from standard in silico tools cluster into two groups: benign (REVEL, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized) and pathogenic (FoldX, FATHMM). Two tools report uncertainty: Rosetta and Foldetta. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is Likely Benign; Foldetta remains uncertain. Overall, the preponderance of evidence points to a benign effect, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 1 | 6-33438013-G-A | 15 | 9.31e-6 | -3.533 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.282 | Likely Benign | 2.83 | Destabilizing | 2.0 | 1.05 | Ambiguous | 1.94 | Ambiguous | -0.02 | Likely Benign | 0.47 | Neutral | 0.000 | Benign | 0.000 | Benign | 1.33 | Pathogenic | 0.77 | Tolerated | 3.42 | 19 | 1 | 0 | -0.4 | 30.03 | 196.6 | -49.6 | 0.9 | 2.2 | -0.1 | 0.4 | Uncertain | Gly370 is located in the Gly-rich Ω loop (res. Pro364- Pro398) between two anti-parallel β sheet strands (res. Thr359-Pro364, res. Ala399-Ile411). Because, the Ω loop is assumed to be directly interacting with the membrane, it is only seen to move arbitrarily throughout the WT solvent simulations. The Ω loop is potentially playing a crucial loop in the SynGAP-membrane complex association, stability and dynamics, regardless, this aspect cannot be addressed through the solvent simulations only. The Ω-loops are known to have a major role in protein functions that requires flexibility and thus, they are rich in glycines, prolines and to a lesser extent, hydrophilic residues to ensure maximum flexibility. Thus, Ser370 in the variant is potentially tolerated in the Ω loop. However, since the effect on the Gly-rich Ω loop dynamics can only be well-studied through the SynGAP-membrane complex, no definite conclusions can be withdrawn. | |||||||||
| c.1667A>G | N556S 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant N556S (ClinVar ID 941099.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33438910‑A‑G). Functional prediction tools that agree on a benign effect include REVEL, Rosetta, Foldetta, premPS, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. The high‑accuracy AlphaMissense‑Optimized score is benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑vs‑2 split, and Foldetta predicts a benign effect. No other high‑accuracy or folding‑stability methods provide additional evidence. Overall, the majority of predictions support a benign impact, which does not contradict the ClinVar Uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 1 | 6-33438910-A-G | 3 | 1.86e-6 | -6.576 | Likely Benign | 0.197 | Likely Benign | Likely Benign | 0.449 | Likely Benign | 0.52 | Ambiguous | 0.1 | 0.14 | Likely Benign | 0.33 | Likely Benign | 0.16 | Likely Benign | -3.60 | Deleterious | 1.000 | Probably Damaging | 0.989 | Probably Damaging | -1.22 | Pathogenic | 0.14 | Tolerated | 3.37 | 35 | 1 | 1 | 2.7 | -27.03 | 198.8 | 31.0 | 0.0 | 0.0 | -0.5 | 0.2 | X | Potentially Benign | Asn556 is located on the outer surface of an α-helix (res. Ala533-Val560). The carboxamide group of Asn556 forms hydrogen bonds with nearby residues such as Lys553 and Cys552. It also forms a hydrogen bond with the backbone carbonyl group of Cys552, which weakens the α-helix integrity. In the variant simulations, the hydroxyl group of Ser556 forms a more stable hydrogen bond with the backbone carbonyl oxygen of the same helix residue, Cys552, compared to Asn556 in the WT. Serine has a slightly lower propensity to reside in an α-helix than asparagine, which may exacerbate the negative effect on the α-helix integrity. However, the residue swap does not cause negative structural effects during the simulations. | |||||||||
| c.1025A>C | Y342S 2D  3DClick to see structure in 3D Viewer AISynGAP1 variant Y342S is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include only REVEL, whereas the majority of algorithms predict a pathogenic impact: FoldX, Rosetta, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), and the Foldetta stability assessment (combining FoldX‑MD and Rosetta). Uncertain results come from premPS, ESM1b, and AlphaMissense‑Optimized. High‑accuracy methods specifically give AlphaMissense‑Optimized as uncertain, SGM‑Consensus as pathogenic, and Foldetta as pathogenic. Overall, the preponderance of evidence points to a pathogenic effect, which contradicts the ClinVar uncertain classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 2 | -7.996 | In-Between | 0.925 | Likely Pathogenic | Ambiguous | 0.407 | Likely Benign | 3.03 | Destabilizing | 0.1 | 2.87 | Destabilizing | 2.95 | Destabilizing | 0.93 | Ambiguous | -6.60 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.75 | Pathogenic | 0.04 | Affected | 3.37 | 25 | -3 | -2 | 0.5 | -76.10 | 200.1 | 77.8 | 0.0 | 0.0 | -0.2 | 0.1 | Potentially Pathogenic | The phenol ring of Tyr342, located at the end of an anti-parallel β sheet strand (res. Gly341-Pro349), faces outward in the C2 domain. In the WT simulations, the phenol ring of Tyr342 contributes to a triple tyrosine stack (Tyr342, Tyr328, and Tyr281) that links together three anti-parallel β sheet strands. Additionally, it shields Gly344 from the solvent, reducing its exposure and providing stability for the β-sandwich. This motif also contributes to a twist formation in the β sheet.In the variant simulations, the Ser342 side chain cannot participate in the stack formation. Instead, the hydroxyl group of the Ser342 side chain forms a hydrogen bond with the imidazole ring of His326 in a neighboring β strand (res. Ala322-Asp330). This disrupts the formation of a hydrogen bond between His326 and the carboxylate group of the Glu283 side chain from another β strand (res. Arg279-Cys285). Although these changes in surface interactions could weaken the characteristic twist that strengthens the β sheet fold, no major structural effects are observed in the variant simulations. The residue swap could also affect the SynGAP-membrane association, as the hydroxyl group of Ser342 could form hydrogen bonds with membrane-facing loop residues. However, this phenomenon cannot be addressed using solvent-only simulations. | ||||||||||||
| c.667A>T | T223S 2D  3DClick to see structure in 3D Viewer AISynGAP1 T223S is listed in ClinVar as a variant of uncertain significance and is present in the gnomAD database (ID 6‑33435518‑A‑T). Functional prediction tools that reach consensus classify the variant as benign: FoldX, Foldetta, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Optimized. Tools that predict pathogenicity include REVEL, PROVEAN, and SIFT. Predictions that are inconclusive or uncertain are Rosetta, premPS, AlphaMissense‑Default, and ESM1b. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, Foldetta is benign, while the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 1‑to‑1 split between benign and pathogenic calls. Overall, the majority of evidence points to a benign effect, which does not contradict the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Conflicting | 2 | 6-33435518-A-T | 3 | 1.86e-6 | -7.714 | In-Between | 0.410 | Ambiguous | Likely Benign | 0.535 | Likely Pathogenic | 0.26 | Likely Benign | 0.1 | 0.50 | Ambiguous | 0.38 | Likely Benign | 0.62 | Ambiguous | -2.86 | Deleterious | 0.421 | Benign | 0.058 | Benign | 5.80 | Benign | 0.02 | Affected | 3.41 | 13 | 1 | 1 | -0.1 | -14.03 | 200.7 | 17.3 | -0.2 | 0.2 | 0.0 | 0.0 | X | Uncertain | The introduced residue Ser223 is located on the outer surface of an anti-parallel β sheet strand (res. Cys219-Thr224). Its hydroxyl group forms hydrogen bonds with nearby residues Thr228 and Lys207 in the variant simulations, similar to the hydroxyl group of Thr223 in the WT simulations. These hydrogen-bonding interactions at the β sheet surface contribute to the stability of the secondary structure element and may prevent it from unfolding. However, since the model ends abruptly at the N-terminus, no definite conclusions can be drawn from the simulations. | |||||||||
| c.1604G>C | S535T 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S535T is catalogued in ClinVar as benign (ClinVar ID 537005.0) and is observed in gnomAD (variant ID 6‑33438847‑G‑C). In silico prediction tools largely agree on a benign effect: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores. Only FATHMM predicts a pathogenic outcome. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as likely benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign; the SGM Consensus is likely benign; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, also reports a benign effect. Overall, the consensus of predictive tools and high‑accuracy methods indicates that the variant is most likely benign, consistent with its ClinVar classification and presence in gnomAD. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign | 1 | 6-33438847-G-C | 14 | 8.67e-6 | -3.886 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.177 | Likely Benign | 0.45 | Likely Benign | 0.1 | -0.27 | Likely Benign | 0.09 | Likely Benign | 0.17 | Likely Benign | -0.81 | Neutral | 0.000 | Benign | 0.001 | Benign | -1.25 | Pathogenic | 0.25 | Tolerated | 3.37 | 35 | 1 | 1 | 0.1 | 14.03 | 201.3 | -17.3 | -0.1 | 0.7 | -0.2 | 0.1 | X | Potentially Benign | Ser535 is located near the terminal end of an α-helix (res. Ala533-Val560) close to the membrane interface. In the WT simulations, the hydroxyl side chain of Ser535 forms hydrogen bonds with nearby residues (e.g., His539, Glu538) without any specific interactions. These hydrogen bonds disrupt the structure of the terminal end of the α-helix (Ala533-Ser535), causing it to weaken or unfold during the WT simulations. In the variant simulations, Thr535, a hydrophilic residue with a hydroxyl group of almost the same size as Ser, interacts more frequently with the preceding loop residues (e.g., Thr532, Cys531) due to its longer side chain. Regardless, the residue swap is tolerated in the simulations with no negative effects. However, due to its location near the SynGAP-membrane interface, the effect of the residue swap cannot be fully addressed using the SynGAP solvent-only simulations. | 10.1016/j.ajhg.2020.11.011 | |||||||
| c.1529T>G | I510S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant I510S is listed in ClinVar as Pathogenic (ClinVar ID 449946.0) and is not reported in gnomAD. Prediction tools that assess the variant’s effect all converge on a deleterious outcome: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all indicate pathogenicity. No tool predicts a benign effect. High‑accuracy assessments further support this: AlphaMissense‑Optimized is uncertain, SGM‑Consensus is pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is pathogenic. Based on the collective evidence, the variant is most likely pathogenic, and this prediction aligns with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Pathogenic | 1 | -11.661 | Likely Pathogenic | 0.955 | Likely Pathogenic | Ambiguous | 0.926 | Likely Pathogenic | 4.00 | Destabilizing | 0.1 | 3.78 | Destabilizing | 3.89 | Destabilizing | 2.34 | Destabilizing | -4.63 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | -1.44 | Pathogenic | 0.00 | Affected | 3.37 | 35 | -1 | -2 | -5.3 | -26.08 | 201.4 | 45.9 | -0.4 | 0.2 | 0.0 | 0.3 | X | Potentially Pathogenic | Ile510 is located in the middle of an α-helix (res. Gly502-Tyr518) within the inter-helix space of three helices (res. Gly502-Tyr518, Ala533-Val560, and res. Glu582-Met603). In the WT simulations, the sec-butyl side chain of Ile510 hydrophobically packs with other residues in the inter-helix space (e.g., Leu506, Leu610, Ile514, Ile602, Leu598). In the variant simulations, the hydroxyl group of Ser510 forms a hydrogen bond with the backbone atoms of Leu506 and Gly511 in the same α-helix, which could further weaken the α-helix integrity. This α-helix already shows weakness in the WT simulations due to Gly511. Although the simulations do not show large-scale effects, the residue swap could have a substantial impact due to the fundamental role of hydrophobic packing during protein folding. | |||||||||||
| c.703T>C | S235P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S235P is listed in ClinVar as Pathogenic (ClinVar ID 1067856.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect are polyPhen‑2 HumVar and FATHMM; all other evaluated algorithms—including REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumDiv, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized—classify the variant as pathogenic. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized predicts pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields a pathogenic verdict; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, also reports pathogenic. No predictions or stability results are missing or inconclusive. **Based on the collective predictions, the variant is most likely pathogenic, and this conclusion aligns with its ClinVar status.** Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | PH | Likely Pathogenic | 1 | -14.857 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.870 | Likely Pathogenic | 4.02 | Destabilizing | 0.1 | 6.91 | Destabilizing | 5.47 | Destabilizing | 1.23 | Destabilizing | -4.24 | Deleterious | 0.917 | Possibly Damaging | 0.446 | Benign | 5.47 | Benign | 0.01 | Affected | 3.40 | 14 | 1 | -1 | -0.8 | 10.04 | 201.5 | 17.0 | 0.1 | 0.0 | -0.6 | 0.0 | X | Potentially Pathogenic | In the WT, the hydroxyl group of Ser235, located in a β-α loop between an anti-parallel β sheet strand (res. Gly227-Phe231) and an α helix (residues Ala236-Val250), forms hydrogen bonds with the GAP domain loop residue Glu680 and with the backbone amide groups of Ala237 and Glu238 from the α helix. In the variant simulations, the pyrrolidine ring of Pro235 cannot stabilize the α helix end or maintain tertiary bonding interactions between the PH and GAP domains via hydrogen bonding as effectively as serine. | |||||||||||
| c.2111G>C | S704T 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S704T is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Consensus from multiple in‑silico predictors shows a predominance of benign calls: REVEL, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus. Only polyPhen‑2 HumDiv predicts a pathogenic effect, while FoldX remains inconclusive. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign; the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is benign; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, also predicts benign. Overall, the aggregate evidence indicates that S704T is most likely benign, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | -4.930 | Likely Benign | 0.265 | Likely Benign | Likely Benign | 0.071 | Likely Benign | 0.80 | Ambiguous | 0.0 | 0.15 | Likely Benign | 0.48 | Likely Benign | 0.29 | Likely Benign | -1.72 | Neutral | 0.525 | Possibly Damaging | 0.107 | Benign | 3.45 | Benign | 0.07 | Tolerated | 3.47 | 10 | 1 | 1 | 0.1 | 14.03 | 201.7 | -18.0 | 0.0 | 0.0 | -0.2 | 0.7 | X | Potentially Benign | Ser704 is located at the end and outer surface of an α-helix (res. Thr704-Gly712), which is connected via a tight turn or loop to another α-helix (res. Asp684-Gln702). The hydroxyl side chain of Ser704 occasionally forms a hydrogen bond with the amide group of Ala707. Similarly, in the variant simulations, the hydroxyl side chain of Thr704 forms hydrogen bonds with the amide groups of Ala707 and Leu708. Thus, the residue swap does not cause any apparent structural change. | |||||||||||
| c.968T>C | L323P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant L323P is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that assess pathogenicity uniformly classify the variant as pathogenic: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool in the dataset predicts a benign effect. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates “Likely Pathogenic”; and Foldetta, which integrates FoldX‑MD and Rosetta outputs, also predicts pathogenic. Based on the unanimous computational evidence, the variant is most likely pathogenic, which does not contradict its current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | -12.507 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.762 | Likely Pathogenic | 3.39 | Destabilizing | 0.6 | 8.46 | Destabilizing | 5.93 | Destabilizing | 2.20 | Destabilizing | -4.80 | Deleterious | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 0.59 | Pathogenic | 0.01 | Affected | 4.29 | 398 | -3 | -3 | -5.4 | -16.04 | 201.9 | 68.2 | 0.0 | 0.1 | 0.6 | 0.3 | X | Potentially Pathogenic | The iso-butyl side chain of Leu323, located at the beginning of an anti-parallel β sheet strand (res. Ala322-Asp330), packs against multiple hydrophobic leucine residues (e.g., Leu264, Leu266, Leu284, Leu286). In contrast, in the variant simulations, the less bulky cyclic five-membered pyrrolidine ring of Pro323 cannot fill the hydrophobic space as effectively as the branched hydrocarbon side chain of leucine. Notably, the backbone amide group of Leu323 forms a hydrogen bond with the backbone carbonyl group of Cys285. Pro323 cannot form this bond due to the absence of the backbone amide group, resulting in partial unfolding of the anti-parallel β sheet end in the variant simulations. | |||||||||||
| c.878G>C | R293P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant R293P is listed in ClinVar as Pathogenic (ClinVar ID 571092.0) and is not reported in gnomAD. Prediction tools that indicate a benign effect include REVEL and premPS, whereas the remaining tools—FoldX, Rosetta, Foldetta, SGM‑Consensus, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic outcome. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized scores the variant as Pathogenic; the SGM Consensus, derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports Likely Pathogenic; and Foldetta, which integrates FoldX‑MD and Rosetta stability predictions, classifies it as Pathogenic. Consequently, the variant is most likely pathogenic, and this prediction is concordant with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Likely Pathogenic | 1 | -16.275 | Likely Pathogenic | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.497 | Likely Benign | 3.62 | Destabilizing | 0.4 | 9.06 | Destabilizing | 6.34 | Destabilizing | 0.47 | Likely Benign | -6.43 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.45 | Pathogenic | 0.01 | Affected | 3.38 | 23 | 0 | -2 | 2.9 | -59.07 | 202.3 | 132.0 | 0.1 | 0.0 | 0.1 | 0.1 | X | X | X | Potentially Pathogenic | The guanidinium group of the Arg293 side chain, located in an anti-parallel β sheet strand (res. Met289-Pro298), packs against the phenol ring of the Tyr281 side chain or forms a salt bridge with the carboxylate group of Glu283 on the outer side of the C2 domain. In the WT simulations, the positively charged side chain of arginine remains outside the hydrophobic C2 domain, resulting in a twist in the β strand. The backbone amide bond of Arg293 potentially maintains this twist by forming a hydrogen bond with the carbonyl group of His210 or the hydroxyl group of Ser211 in the anti-parallel β sheet.Although this twist is also maintained in the variant simulations, replacing the positively charged residue with proline, which lacks the backbone amide group altogether, causes the β strand to unfold. Because Arg293 is positioned at the C2 and PH domain interface, the residue swap could significantly impact the tertiary structure assembly. Notably, Arg293 is located at the SynGAP-Ras interface, and its role in complex formation cannot be fully understood through solvent-only simulations. | |||||||||
| c.1150G>A | G384S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant G384S (gnomAD ID 6-33438055‑G‑A) is listed in ClinVar with an uncertain significance. Functional prediction tools cluster into two groups: benign predictions from REVEL, premPS, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized; pathogenic predictions from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments further support benignity: AlphaMissense‑Optimized predicts benign, the SGM‑Consensus (majority vote) is likely benign, and Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, is inconclusive. No evidence from FoldX or Rosetta alone is available. Overall, the preponderance of evidence points to a benign effect, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 1 | 6-33438055-G-A | 1 | 6.22e-7 | -5.243 | Likely Benign | 0.090 | Likely Benign | Likely Benign | 0.315 | Likely Benign | 1.92 | Ambiguous | 0.2 | 1.66 | Ambiguous | 1.79 | Ambiguous | 0.19 | Likely Benign | -0.67 | Neutral | 0.980 | Probably Damaging | 0.968 | Probably Damaging | 1.33 | Pathogenic | 0.04 | Affected | 4.32 | 2 | 1 | 0 | -0.4 | 30.03 | 202.4 | -49.8 | 0.5 | 1.0 | -0.2 | 0.0 | Uncertain | Gly384 is located in the Gly-rich Ω loop (res. Pro364-Pro398) between two anti-parallel β sheet strands (res. Thr359-Pro364, res. Ala399-Ile411). Because the Ω loop is assumed to directly interact with the membrane, it moves arbitrarily throughout the WT solvent simulations. The Ω loop potentially plays a crucial role in the SynGAP-membrane complex association, stability, and dynamics. However, this aspect cannot be fully addressed through solvent simulations alone.Ω loops are known to play major roles in protein functions that require flexibility, and so they are rich in glycines, prolines, and, to a lesser extent, small hydrophilic residues to ensure maximum flexibility. Thus, the variant’s Ser384 is potentially tolerated in the Ω loop, although the hydroxyl group of Ser384 forms various hydrogen bonds with several other loop residues in the variant simulations. However, since the effects on Gly-rich Ω loop dynamics can only be studied through the SynGAP-membrane complex, no definite conclusions can be drawn. | |||||||||
| c.1771G>A | A591T 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant A591T is listed in ClinVar with an uncertain significance designation and is observed in gnomAD (variant ID 6‑33440823‑G‑A). Functional prediction tools cluster into two groups: benign predictions come from REVEL, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from premPS, PROVEAN, polyPhen‑2 HumDiv, SIFT, ESM1b, and AlphaMissense‑Default. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely pathogenic outcome. High‑accuracy assessments further show AlphaMissense‑Optimized as benign, SGM Consensus as pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) as uncertain. No other folding‑stability metrics are available. Overall, the balance of evidence favors a pathogenic interpretation, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Conflicting | 3 | 6-33440823-G-A | 18 | 1.12e-5 | -9.572 | Likely Pathogenic | 0.704 | Likely Pathogenic | Likely Benign | 0.270 | Likely Benign | 1.61 | Ambiguous | 0.2 | 1.00 | Ambiguous | 1.31 | Ambiguous | 1.19 | Destabilizing | -3.40 | Deleterious | 0.955 | Possibly Damaging | 0.209 | Benign | 3.48 | Benign | 0.01 | Affected | 3.37 | 35 | 1 | 0 | -2.5 | 30.03 | 202.9 | -43.4 | 0.2 | 0.0 | 0.7 | 0.1 | X | Potentially Benign | The methyl group of the Ala591 side chain, located in the middle of an α helix (res. Glu582-Met603), packs against hydrophobic residues (e.g., Ile483, Phe484) of an opposing partially helical loop (res. Phe476-Asn487).In the variant simulations, the hydroxyl group of Thr591 can form hydrogen bonds with the backbone carbonyl of Ile843 in the opposing loop or the backbone carbonyl group of Arg587. These interactions could either reinforce the tertiary assembly or weaken the α helix unity. Additionally, the Thr591 side chain can hydrogen bond with the guanidinium group of the Arg587 side chain, potentially strengthening the α helix unity.Overall, the residue swap does not seem to cause any major negative effects on the protein structure. | ||||||||
| c.2162T>G | I721S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant I721S is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that assess the variant’s effect fall into two groups: the single benign prediction comes from REVEL, while all other evaluated algorithms (FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) predict a pathogenic outcome. High‑accuracy methods reinforce this view: AlphaMissense‑Optimized reports pathogenic, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is “Likely Pathogenic,” and Foldetta (combining FoldX‑MD and Rosetta outputs) also predicts pathogenic. No predictions are missing or inconclusive. Based on the overwhelming consensus of pathogenic predictions, the variant is most likely pathogenic, which does not contradict its current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -14.032 | Likely Pathogenic | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.466 | Likely Benign | 3.91 | Destabilizing | 0.1 | 3.96 | Destabilizing | 3.94 | Destabilizing | 2.28 | Destabilizing | -5.26 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.21 | Pathogenic | 0.00 | Affected | 3.50 | 9 | -1 | -2 | -5.3 | -26.08 | 203.3 | 49.3 | -0.1 | 0.0 | -1.1 | 0.0 | X | Uncertain | The sec-butyl side chain of Ile721, located on an α-helix (res. Leu714-Arg726), engages in hydrophobic packing with other residues in the hydrophobic inter-helix space, such as Phe420, Tyr417, His693, and Leu717. In the variant simulations, the hydroxyl side chain of Ser721 forms hydrogen bonds with nearby residues, such as Leu717 and His693. Although no major structural changes are observed during the variant simulations, the hydrophilic residue Ser721 could disrupt the hydrophobic packing during folding. However, because the model ends abruptly at the C-terminus, no definite conclusions can be drawn based on the simulations. | |||||||||||
| c.865A>G | M289V 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant M289V is reported in ClinVar as Benign (ClinVar ID 2122760.0) and is not present in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus all predict benign, while only FATHMM predicts pathogenic. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized scores benign; the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is benign; and Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, also indicates benign. No prediction or stability result is inconclusive. Overall, the computational evidence strongly supports a benign classification, consistent with the ClinVar status and showing no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Benign | 1 | -4.239 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.150 | Likely Benign | 1.09 | Ambiguous | 0.1 | -0.27 | Likely Benign | 0.41 | Likely Benign | 0.24 | Likely Benign | -0.36 | Neutral | 0.136 | Benign | 0.054 | Benign | 1.80 | Pathogenic | 1.00 | Tolerated | 3.38 | 23 | 2 | 1 | 2.3 | -32.06 | 204.2 | 51.0 | 0.0 | 0.0 | 0.2 | 0.0 | X | Potentially Benign | The hydrophobic residue Met289, located in a β hairpin linking two anti-parallel β sheet strands (res. Met289-Arg299, res. Arg272-Leu286), is swapped for another hydrophobic residue, valine. In the variant simulations, the branched hydrocarbon side chain of Val289 packs against the phenol group of the Tyr291 side chain but is unable to form methionine-aromatic interactions. β hairpins are potential nucleation sites during the initial stages of protein folding, so even minor changes in them could be significant. However, based on the simulations, the residue swap does not cause adverse effects on the structure. | |||||||||||
| c.1393C>G | L465V 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant L465V is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL and SIFT, while the remaining tools—FoldX, Rosetta, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Default—indicate pathogenicity. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is uncertain; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports likely pathogenic; and Foldetta, which combines FoldX‑MD and Rosetta stability outputs, predicts pathogenic. Overall, the majority of evidence points to a pathogenic impact, which is consistent with the ClinVar uncertain status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -9.893 | Likely Pathogenic | 0.838 | Likely Pathogenic | Ambiguous | 0.276 | Likely Benign | 2.46 | Destabilizing | 0.1 | 2.66 | Destabilizing | 2.56 | Destabilizing | 1.21 | Destabilizing | -2.98 | Deleterious | 0.996 | Probably Damaging | 0.992 | Probably Damaging | 2.44 | Pathogenic | 0.10 | Tolerated | 3.37 | 34 | 2 | 1 | 0.4 | -14.03 | 204.3 | 30.9 | 0.0 | 0.0 | -0.4 | 0.6 | X | Potentially Benign | The iso-butyl side chain of Leu465, located in the middle of an α helix (res. Ala461–Phe476), packs with hydrophobic residues (e.g., Phe464, Met468, Tyr497, Ile494) in an inter-helix space formed with two other α helices (res. Ala461–Phe476 and res. Thr488-Gly502). In the variant simulations, the iso-propyl side chain of Val465 is equally sized and similarly hydrophobic as the original side chain of Leu465. Hence, the mutation does not exert any negative effects on the protein structure based on the variant simulations. | |||||||||||
| c.1606T>G | L536V 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant L536V is listed in ClinVar (ID 1690714.0) with an uncertain significance designation and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized; pathogenic predictions arise from REVEL, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely pathogenic verdict. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) remains uncertain. No evidence from FoldX or Rosetta alone is available. Overall, the majority of evidence points toward a pathogenic effect, which does not contradict the ClinVar uncertain status but suggests a higher likelihood of pathogenicity. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -9.014 | Likely Pathogenic | 0.269 | Likely Benign | Likely Benign | 0.586 | Likely Pathogenic | 1.25 | Ambiguous | 0.3 | 1.22 | Ambiguous | 1.24 | Ambiguous | 1.20 | Destabilizing | -2.81 | Deleterious | 0.998 | Probably Damaging | 0.992 | Probably Damaging | -1.34 | Pathogenic | 0.09 | Tolerated | 3.37 | 34 | 2 | 1 | 0.4 | -14.03 | 204.7 | 26.4 | 0.2 | 0.0 | -0.2 | 0.2 | X | Potentially Benign | Leu536 is located on an α-helix (res. Ala533-Val560) at the membrane interface. The iso-butyl group of Leu536 interacts with nearby hydrophobic residues in the preceding loop (e.g., Val526, Pro528, Cys531). In the variant simulations, the iso-propyl side chain of Val536 forms similar hydrophobic interactions as Leu536 in the WT, causing no negative structural effects. | |||||||||||
| c.872A>G | Y291C 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant Y291C is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that assess pathogenicity all converge on a deleterious effect: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict pathogenicity. No tool in the dataset predicts a benign outcome. High‑accuracy methods reinforce this view: AlphaMissense‑Optimized is pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is “Likely Pathogenic”; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, is pathogenic. Based on the unanimous computational evidence, the variant is most likely pathogenic, a conclusion that contradicts the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | -8.997 | Likely Pathogenic | 0.967 | Likely Pathogenic | Likely Pathogenic | 0.505 | Likely Pathogenic | 2.90 | Destabilizing | 0.4 | 3.51 | Destabilizing | 3.21 | Destabilizing | 1.35 | Destabilizing | -7.37 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.76 | Pathogenic | 0.01 | Affected | 3.38 | 23 | 0 | -2 | 3.8 | -60.04 | 205.2 | 66.1 | 0.1 | 0.0 | -0.4 | 0.4 | X | X | Potentially Pathogenic | The phenol group of the Tyr291 side chain, located in an anti-parallel β sheet strand (res. Met289-Pro298), packs against hydrophobic residues of the C2 and PH domains (e.g., Leu317, Leu286, Leu284, Pro208, Val209). The phenol ring of Tyr291 also forms favorable Met-aromatic stacking with the methyl group of Met289. In the variant simulation, the thiol group of the Cys291 side chain is not as suitable for the hydrophobic inter-domain space as the phenol ring of Tyr291. Consequently, the structural unity of the PH domain is weakened and ultimately unfolds in the second simulation. Moreover, the residue swap might result in severe detrimental effects on the C2 domain structure and the C2-PH domain tertiary structure assembly during folding. | ||||||||||
| c.1456G>A | E486K 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant E486K is listed in ClinVar with an uncertain significance status and is not reported in gnomAD. Functional prediction tools show mixed results: benign predictions come from REVEL, FoldX, Rosetta, Foldetta, premPS, SIFT, and FATHMM, whereas pathogenic predictions are reported by PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Default. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenic, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates likely pathogenic, and the Foldetta stability analysis (combining FoldX‑MD and Rosetta) predicts benign. Overall, the majority of high‑confidence tools and the consensus analysis favor a pathogenic interpretation, which contrasts with the ClinVar uncertain designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -14.545 | Likely Pathogenic | 0.988 | Likely Pathogenic | Likely Pathogenic | 0.435 | Likely Benign | 0.06 | Likely Benign | 0.1 | 0.37 | Likely Benign | 0.22 | Likely Benign | 0.41 | Likely Benign | -3.58 | Deleterious | 1.000 | Probably Damaging | 0.988 | Probably Damaging | 3.40 | Benign | 0.12 | Tolerated | 3.37 | 35 | 0 | 1 | -0.4 | -0.94 | 206.8 | 52.1 | -0.3 | 0.1 | 0.2 | 0.0 | X | X | Uncertain | Glu486 is located in an α-α loop connecting the two α-helices (res. Ala461-Phe476 and Leu489-Glu519) at the GAP-Ras interface. It is adjacent to the arginine finger (Arg485) and is expected to closely interact with Ras. The residue swap could affect complex formation with the GTPase and its activation. In the WT simulations, the carboxylate group of Glu486 forms salt bridges with Arg485 and Arg475 on the preceding α-helix (res. Ala461-Phe476). In the variant simulations, Lys486 does not form any specific interactions. Although the amino group of the Lys486 side chain cannot form these salt bridges, no negative effects on the protein structure are observed. Nevertheless, the potential role of Glu486 in SynGAP-Ras complex formation or GTPase activation cannot be fully addressed using the SynGAP solvent-only simulations, and no definite conclusions can be drawn. | ||||||||||
| c.1586T>C | I529T 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant I529T is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus “Likely Benign” call. Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also yields a benign prediction; and Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, predicts benign. Overall, the majority of evidence points to a benign effect, and this is consistent with the ClinVar “Uncertain” classification—there is no contradiction between the predictions and the current ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | -0.539 | Likely Benign | 0.336 | Likely Benign | Likely Benign | 0.343 | Likely Benign | 0.22 | Likely Benign | 0.2 | 0.16 | Likely Benign | 0.19 | Likely Benign | 0.17 | Likely Benign | 0.24 | Neutral | 0.872 | Possibly Damaging | 0.820 | Possibly Damaging | -1.23 | Pathogenic | 0.55 | Tolerated | 3.37 | 35 | 0 | -1 | -5.2 | -12.05 | 207.2 | 29.8 | 0.2 | 0.0 | 0.2 | 0.1 | X | Potentially Benign | Ile529 is located on an α-α loop between the two α-helices (res. Gly502-Tyr518 and Ala533-Val560). In the WT simulations, the sec-butyl side chain of Ile529 faces the membrane interface and shows no specific interactions. In the variant simulations, the hydroxyl group of Thr529 forms a hydrogen bond with the carboxylate side chain of Asp527, but no negative structural changes are observed. However, due to its location near the SynGAP-membrane interface, the effect of the residue swap cannot be fully addressed using the SynGAP solvent-only simulations. | |||||||||||
| c.2068T>C | S690P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S690P is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that indicate a benign effect are REVEL and FATHMM, whereas the remaining tools (FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) all predict a pathogenic outcome. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized scores the variant as pathogenic; the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Pathogenic”; and Foldetta, which integrates FoldX‑MD and Rosetta stability predictions, also classifies the variant as pathogenic. Overall, the preponderance of evidence from multiple independent predictors indicates that the variant is most likely pathogenic, a conclusion that does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -14.568 | Likely Pathogenic | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.431 | Likely Benign | 4.84 | Destabilizing | 0.3 | 4.40 | Destabilizing | 4.62 | Destabilizing | 1.42 | Destabilizing | -4.77 | Deleterious | 0.998 | Probably Damaging | 0.790 | Possibly Damaging | 3.44 | Benign | 0.01 | Affected | 3.42 | 17 | 1 | -1 | -0.8 | 10.04 | 207.5 | 15.1 | 0.1 | 0.0 | -0.1 | 0.2 | X | X | Potentially Pathogenic | The hydroxyl side chain of Ser690, located in an α-helix (res. Leu696-Leu685), forms a hydrogen bond with the backbone carbonyl group of Ser410 in an anti-parallel β-sheet of the C2 domain (res. Ile411-Ala399). In the variant simulations, the pyrrolidine side chain of Pro690 cannot form hydrogen bonds with the C2 domain residue, resulting in the loss of this inter-domain connection. Additionally, prolines lack a free amide group necessary for hydrogen bonding with the carbonyl group of Gly686, introducing a slight bend in the α-helix and compromising its integrity. | ||||||||||
| c.1118G>T | G373V 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant G373V is listed in ClinVar with an uncertain significance and is present in gnomAD (variant ID 6‑33438023‑G‑T). Functional prediction tools that agree on a benign effect include REVEL, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic outcome are FoldX, Foldetta, and SIFT, while Rosetta is inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus as Likely Benign, and Foldetta as pathogenic. Overall, the majority of predictions support a benign impact, and this consensus does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 1 | 6-33438023-G-T | 6 | 5.03e-6 | -6.062 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.428 | Likely Benign | 5.32 | Destabilizing | 3.2 | 0.82 | Ambiguous | 3.07 | Destabilizing | 0.09 | Likely Benign | -0.98 | Neutral | 0.007 | Benign | 0.001 | Benign | 3.90 | Benign | 0.00 | Affected | 3.53 | 16 | -1 | -3 | 4.6 | 42.08 | 207.6 | -68.1 | 1.9 | 1.1 | -0.6 | 0.1 | Uncertain | Gly373 is located in the Gly-rich Ω loop (res. Pro364-Pro398) between two anti-parallel β sheet strands (res. Thr359-Pro364, res. Ala399-Ile411). Because the Ω loop is assumed to directly interact with the membrane, it moves arbitrarily throughout the WT solvent simulations. The Ω loop potentially plays a crucial role in the SynGAP-membrane complex association, stability, and dynamics. However, this aspect cannot be fully addressed through solvent simulations alone.Ω loops are known to play major roles in protein functions that require flexibility, and thus hydrophobic residues like valine are rarely tolerated. Although no negative structural effects are observed in the variant simulations, Val373 may exert drastic effects on the SynGAP-membrane complex dynamics and stability. However, since the effect on the Gly-rich Ω loop dynamics can only be studied through the SynGAP-membrane complex, no definite conclusions can be drawn. | |||||||||
| c.1160G>T | G387V 2D  3DClick to see structure in 3D Viewer AISynGAP1 G387V is listed in ClinVar with an uncertain significance and is present in gnomAD (variant ID 6-33438065-G-T). Functional prediction tools that report a benign effect include REVEL, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are FoldX, Rosetta, SIFT, and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as likely benign, while the Foldetta stability assessment (combining FoldX‑MD and Rosetta) indicates a pathogenic change. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely benign, and Foldetta as pathogenic. Overall, the majority of predictions favor a benign impact, and this consensus does not contradict the ClinVar uncertain status; thus the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 1 | 6-33438065-G-T | 22 | 1.37e-5 | -6.199 | Likely Benign | 0.153 | Likely Benign | Likely Benign | 0.390 | Likely Benign | 5.13 | Destabilizing | 1.8 | 6.44 | Destabilizing | 5.79 | Destabilizing | -0.33 | Likely Benign | -0.54 | Neutral | 0.069 | Benign | 0.077 | Benign | 1.32 | Pathogenic | 0.01 | Affected | 4.32 | 3 | -1 | -3 | 4.6 | 42.08 | 207.7 | -68.4 | -0.7 | 0.8 | -0.5 | 0.1 | Uncertain | Gly387 is located in the Gly-rich Ω loop (res. Pro364-Pro398) between two anti-parallel β sheet strands (res. Thr359-Pro364 and res. Ala399-Ile411). The Ω loop is assumed to directly interact with the membrane, and it is observed to move arbitrarily throughout the WT solvent simulations. This loop potentially plays a crucial role in the SynGAP-membrane complex association, stability, and dynamics. However, this aspect cannot be fully addressed through solvent simulations alone.Ω loops are known to play significant roles in protein functions that require flexibility, and thus hydrophobic residues like valine are rarely tolerated. Although no negative structural effects are visualized in the variant’s simulations, Val387 may exert drastic effects on the SynGAP-membrane complex dynamics and stability. Since the effects on the Gly-rich Ω loop dynamics can only be well studied through the SynGAP-membrane complex, no definite conclusions can be drawn. | |||||||||
Found 757 rows. Show 200 rows per page. Page 3/4 « Previous | Next »