

Table of SynGAP1 Isoform α2 (UniProt Q96PV0-1) Missense Variants.
| c.dna | Variant | SGM Consensus | Domain and Structure information: based on WT protein | Annotated databases | Deep learning-based pathogenicity predictions | Folding stability-based pathogenicity predictions | Sequence/structure-based pathogenicity predictions | Phase Separation | Evolutionary/physical properties | Molecular Dynamics-based analysis | DOI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | IUPred2 | ANCHOR2 | AlphaFold | MobiDB | PhosphoSitePlus | ClinVar | gnomAD | ESM1b | AlphaMissense | FoldX | Rosetta | Foldetta | PremPS | REVEL | PROVEAN | PolyPhen-2 HumDiv | PolyPhen-2 HumVar | FATHMM | SIFT | PSMutPred | PAM | Physical | SASA | Normalized B-factor backbone | Normalized B-factor sidechain | SynGAP Structural Annotation | |||||||||||||||||||||||||||||||||||||||||||||
| Score | Prediction | Score | Prediction | pLDDT | disorder | disorder | LTP | HTP | KL | PTM | Clinical Status | Review | Subm. | ID | Allele count | Allele freq. | LLR score | Prediction | Pathogenicity | Class | Optimized | Average ΔΔG | Prediction | StdDev | ΔΔG | Prediction | ΔΔG | Prediction | ΔΔG | Prediction | Score | Prediction | Score | Prediction | pph2_prob | Prediction | pph2_prob | Prediction | Nervous System Score | Prediction | Prediction | Status | Conservation | Sequences | IP RF | SP RF | Prediction | PAM250 | PAM120 | Hydropathy Δ | MW Δ | Average | Δ | Δ | StdDev | Δ | StdDev | Secondary | Tertiary bonds | Inside out | GAP-Ras interface | At membrane | No effect | MD Alert | Verdict | Description | |||||
| c.3464T>G | V1155G 2D  AIThe SynGAP1 missense variant V1155G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy methods give mixed results: AlphaMissense‑Optimized classifies the variant as pathogenic; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default (pathogenic), ESM1b (benign), FATHMM (benign), and PROVEAN (benign), reports a benign outcome; Foldetta’s stability assessment is unavailable. Overall, the majority of conventional tools lean toward pathogenicity, whereas the high‑accuracy consensus leans benign, leaving the functional impact uncertain. The variant is most likely pathogenic based on the prevailing predictions, and this assessment does not contradict any ClinVar annotation because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.750527 | Disordered | 0.855718 | Binding | 0.335 | 0.857 | 0.500 | -3.018 | Likely Benign | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.209 | Likely Benign | -1.91 | Neutral | 0.997 | Probably Damaging | 0.999 | Probably Damaging | 2.59 | Benign | 0.05 | Affected | 0.2211 | 0.2374 | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.3466G>A | A1156T 2D  AIThe SynGAP1 missense variant A1156T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic outcome: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence from multiple in silico tools points to a pathogenic effect for A1156T, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.720929 | Disordered | 0.871395 | Binding | 0.294 | 0.861 | 0.500 | -3.732 | Likely Benign | 0.988 | Likely Pathogenic | Likely Pathogenic | 0.285 | Likely Benign | -2.99 | Deleterious | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 1.63 | Pathogenic | 0.00 | Affected | 0.1366 | 0.7442 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3472G>T | V1158F 2D  AIThe SynGAP1 missense variant V1158F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, while the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is classified as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as Likely Pathogenic. Foldetta results are unavailable. Overall, the majority of evidence indicates that V1158F is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar annotation exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.599170 | Disordered | 0.877504 | Binding | 0.369 | 0.847 | 0.250 | -3.888 | Likely Benign | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.264 | Likely Benign | -2.66 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.33 | Pathogenic | 0.02 | Affected | 0.0792 | 0.3577 | -1 | -1 | -1.4 | 48.04 | |||||||||||||||||||||||||||||||||||||||
| c.3443T>G | M1148R 2D  AIThe SynGAP1 missense variant M1148R is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus, REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. High‑accuracy assessments reinforce the benign prediction: AlphaMissense‑Optimized scores the variant as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. No Foldetta stability analysis is available for this variant. Overall, the majority of computational evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.774279 | Binding | 0.343 | 0.835 | 0.875 | -2.303 | Likely Benign | 0.669 | Likely Pathogenic | Likely Benign | 0.129 | Likely Benign | -1.77 | Neutral | 0.255 | Benign | 0.113 | Benign | 2.54 | Benign | 0.00 | Affected | 0.1624 | 0.0888 | 0 | -1 | -6.4 | 24.99 | |||||||||||||||||||||||||||||||||||||||
| c.3415C>A | Q1139K 2D  AIThe SynGAP1 missense variant Q1139K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.908098 | Disordered | 0.721191 | Binding | 0.313 | 0.866 | 1.000 | -4.768 | Likely Benign | 0.222 | Likely Benign | Likely Benign | 0.253 | Likely Benign | -1.70 | Neutral | 0.004 | Benign | 0.006 | Benign | 5.44 | Benign | 0.00 | Affected | 0.1667 | 0.4139 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.3421C>G | P1141A 2D  AIThe SynGAP1 missense variant P1141A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive because it yields a 2‑to‑2 split. Foldetta, a protein‑folding‑stability method that combines FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy tools therefore provide mixed evidence: AlphaMissense‑Optimized indicates benign, while the consensus and stability analyses are unavailable. Overall, the majority of individual predictors (five versus four) favor a pathogenic interpretation, and this does not contradict any ClinVar annotation because none exists. Thus, the variant is most likely pathogenic based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.930790 | Disordered | 0.716087 | Binding | 0.364 | 0.852 | 1.000 | -3.885 | Likely Benign | 0.120 | Likely Benign | Likely Benign | 0.076 | Likely Benign | -3.67 | Deleterious | 0.856 | Possibly Damaging | 0.492 | Possibly Damaging | 1.00 | Pathogenic | 0.00 | Affected | 0.3525 | 0.5135 | 1 | -1 | 3.4 | -26.04 | ||||||||||||||||||||||||||||||||||||||||
| c.3421C>T | P1141S 2D  AIThe SynGAP1 missense variant P1141S is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs 2 pathogenic). Foldetta results are unavailable. Overall, five of the nine evaluated tools predict pathogenicity while four predict benignity, giving a slight majority toward a deleterious effect. Thus, the variant is most likely pathogenic based on current computational predictions, and this assessment does not contradict any ClinVar status because the variant has not yet been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.930790 | Disordered | 0.716087 | Binding | 0.364 | 0.852 | 1.000 | -2.574 | Likely Benign | 0.187 | Likely Benign | Likely Benign | 0.094 | Likely Benign | -3.58 | Deleterious | 0.856 | Possibly Damaging | 0.492 | Possibly Damaging | 0.99 | Pathogenic | 0.00 | Affected | 0.3379 | 0.5566 | 1 | -1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||||||||||||
| c.3422C>A | P1141Q 2D  AIThe SynGAP1 missense variant P1141Q is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 benign vs 2 pathogenic), and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.930790 | Disordered | 0.716087 | Binding | 0.364 | 0.852 | 1.000 | -4.331 | Likely Benign | 0.274 | Likely Benign | Likely Benign | 0.136 | Likely Benign | -2.72 | Deleterious | 0.074 | Benign | 0.047 | Benign | 0.97 | Pathogenic | 0.00 | Affected | 0.1449 | 0.5186 | 0 | -1 | -1.9 | 31.01 | ||||||||||||||||||||||||||||||||||||||||
| c.3422C>G | P1141R 2D  AIThe SynGAP1 missense variant P1141R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect comprise PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus indicates a likely pathogenic outcome; a Foldetta stability analysis is unavailable. Overall, the majority of computational predictions (seven pathogenic vs. three benign) support a pathogenic classification. This consensus does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.930790 | Disordered | 0.716087 | Binding | 0.364 | 0.852 | 1.000 | -4.768 | Likely Benign | 0.626 | Likely Pathogenic | Likely Benign | 0.120 | Likely Benign | -3.90 | Deleterious | 0.913 | Possibly Damaging | 0.690 | Possibly Damaging | 0.97 | Pathogenic | 0.00 | Affected | 0.1329 | 0.3614 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.3422C>T | P1141L 2D  AIThe SynGAP1 missense variant P1141L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicting benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—leans pathogenic (two pathogenic versus one benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from both general and high‑accuracy tools indicates that P1141L is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.930790 | Disordered | 0.716087 | Binding | 0.364 | 0.852 | 1.000 | -3.817 | Likely Benign | 0.352 | Ambiguous | Likely Benign | 0.110 | Likely Benign | -4.64 | Deleterious | 0.954 | Possibly Damaging | 0.759 | Possibly Damaging | 0.98 | Pathogenic | 0.00 | Affected | 0.2147 | 0.6099 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||||||
| c.3424T>A | S1142T 2D  AIThe SynGAP1 missense variant S1142T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.719935 | Binding | 0.276 | 0.844 | 1.000 | -3.712 | Likely Benign | 0.100 | Likely Benign | Likely Benign | 0.106 | Likely Benign | -1.63 | Neutral | 0.611 | Possibly Damaging | 0.324 | Benign | 2.69 | Benign | 0.00 | Affected | 0.1548 | 0.6427 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3424T>G | S1142A 2D  AIThe SynGAP1 missense variant S1142A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) confirms a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that S1142A is most likely benign, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.719935 | Binding | 0.276 | 0.844 | 1.000 | -3.694 | Likely Benign | 0.085 | Likely Benign | Likely Benign | 0.074 | Likely Benign | -1.31 | Neutral | 0.002 | Benign | 0.015 | Benign | 2.73 | Benign | 0.00 | Affected | 0.4689 | 0.5319 | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.3425C>A | S1142Y 2D  AIThe SynGAP1 missense variant S1142Y is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized predicts a benign outcome, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta results are unavailable. Overall, the majority of conventional predictors lean toward pathogenicity, but the single high‑accuracy benign prediction and the inconclusive consensus temper this view. Thus, the variant is most likely pathogenic based on the current predictions, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.719935 | Binding | 0.276 | 0.844 | 1.000 | -6.146 | Likely Benign | 0.569 | Likely Pathogenic | Likely Benign | 0.200 | Likely Benign | -3.39 | Deleterious | 0.971 | Probably Damaging | 0.876 | Possibly Damaging | 2.64 | Benign | 0.00 | Affected | 0.0849 | 0.5429 | -3 | -2 | -0.5 | 76.10 | ||||||||||||||||||||||||||||||||||||||||
| c.3425C>G | S1142C 2D  AIThe SynGAP1 missense variant S1142C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic predictions come from PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; ESM1b remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields benign, and Foldetta data are unavailable. Consequently, the variant is most likely benign based on the collective evidence, and this conclusion does not conflict with any ClinVar annotation because no ClinVar entry exists for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.719935 | Binding | 0.276 | 0.844 | 1.000 | -7.355 | In-Between | 0.182 | Likely Benign | Likely Benign | 0.146 | Likely Benign | -2.89 | Deleterious | 0.992 | Probably Damaging | 0.866 | Possibly Damaging | 2.70 | Benign | 0.00 | Affected | 0.1170 | 0.6016 | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||||||
| c.3425C>T | S1142F 2D  AIThe SynGAP1 missense variant S1142F has no ClinVar record and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized, while those that predict a pathogenic outcome are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a benign majority, and Foldetta data are unavailable. Overall, the balance of evidence—especially from the high‑accuracy tools—suggests that the variant is most likely benign. This conclusion does not contradict ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.719935 | Binding | 0.276 | 0.844 | 1.000 | -5.074 | Likely Benign | 0.508 | Ambiguous | Likely Benign | 0.206 | Likely Benign | -3.70 | Deleterious | 0.918 | Possibly Damaging | 0.827 | Possibly Damaging | 2.65 | Benign | 0.00 | Affected | 0.0861 | 0.5603 | -3 | -2 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||||||||||||
| c.3427A>C | T1143P 2D  AIThe SynGAP1 missense variant T1143P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic effect. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.722918 | Binding | 0.275 | 0.837 | 1.000 | -2.307 | Likely Benign | 0.155 | Likely Benign | Likely Benign | 0.183 | Likely Benign | 0.80 | Neutral | 0.999 | Probably Damaging | 0.966 | Probably Damaging | 2.69 | Benign | 0.24 | Tolerated | 0.1818 | 0.4108 | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||||||
| c.3427A>G | T1143A 2D  AIThe SynGAP1 missense variant T1143A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Based on the preponderance of benign predictions and the consensus from high‑accuracy methods, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.722918 | Binding | 0.275 | 0.837 | 1.000 | -3.340 | Likely Benign | 0.159 | Likely Benign | Likely Benign | 0.061 | Likely Benign | -1.19 | Neutral | 0.877 | Possibly Damaging | 0.675 | Possibly Damaging | 2.80 | Benign | 0.43 | Tolerated | 0.3252 | 0.3546 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3427A>T | T1143S 2D  AIThe SynGAP1 missense variant T1143S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods indicates that T1143S is most likely benign, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.722918 | Binding | 0.275 | 0.837 | 1.000 | -2.427 | Likely Benign | 0.150 | Likely Benign | Likely Benign | 0.086 | Likely Benign | -1.34 | Neutral | 0.573 | Possibly Damaging | 0.230 | Benign | 2.76 | Benign | 0.74 | Tolerated | 0.2810 | 0.3640 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3421C>A | P1141T 2D  AIThe SynGAP1 missense variant P1141T is not reported in ClinVar (ClinVar status: not reported) and is absent from gnomAD (gnomAD: not present). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign vs. two pathogenic). High‑accuracy methods give AlphaMissense‑Optimized a benign prediction; the SGM Consensus remains inconclusive, and Foldetta (combining FoldX‑MD and Rosetta) has no available result. Overall, the balance of evidence, particularly the benign call from the high‑accuracy AlphaMissense‑Optimized model, suggests that the variant is most likely benign, and this assessment does not contradict the current ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.930790 | Disordered | 0.716087 | Binding | 0.364 | 0.852 | 1.000 | -4.090 | Likely Benign | 0.176 | Likely Benign | Likely Benign | 0.106 | Likely Benign | -3.85 | Deleterious | 0.856 | Possibly Damaging | 0.723 | Possibly Damaging | 0.98 | Pathogenic | 0.00 | Affected | 0.1594 | 0.6145 | 0 | -1 | 0.9 | 3.99 | ||||||||||||||||||||||||||||||||||||||||
| c.341A>T | K114M 2D  AIThe SynGAP1 missense variant K114M is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) is unavailable for this variant. Overall, the majority of evidence points toward a benign effect, and there is no ClinVar entry to contradict this conclusion. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.649749 | Binding | 0.381 | 0.879 | 0.750 | -3.953 | Likely Benign | 0.877 | Likely Pathogenic | Ambiguous | 0.120 | Likely Benign | -1.89 | Neutral | 0.992 | Probably Damaging | 0.615 | Possibly Damaging | 3.90 | Benign | 0.00 | Affected | 0.1809 | 0.4075 | 0 | -1 | 5.8 | 3.02 | |||||||||||||||||||||||||||||||||||||||
| c.3415C>G | Q1139E 2D  AIThe SynGAP1 missense variant Q1139E is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign or likely benign outcome, whereas only SIFT classifies it as pathogenic. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates likely benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the consensus of the available predictions points to a benign effect, and this is consistent with the lack of ClinVar evidence for pathogenicity. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.908098 | Disordered | 0.721191 | Binding | 0.313 | 0.866 | 1.000 | -3.065 | Likely Benign | 0.204 | Likely Benign | Likely Benign | 0.285 | Likely Benign | -1.34 | Neutral | 0.112 | Benign | 0.089 | Benign | 5.36 | Benign | 0.00 | Affected | 0.1360 | 0.2269 | 2 | 2 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.3416A>C | Q1139P 2D  AIThe SynGAP1 missense variant Q1139P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. In contrast, polyPhen‑2 HumDiv and SIFT predict pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence indicates the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.908098 | Disordered | 0.721191 | Binding | 0.313 | 0.866 | 1.000 | -3.753 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.437 | Likely Benign | -1.74 | Neutral | 0.812 | Possibly Damaging | 0.396 | Benign | 5.28 | Benign | 0.00 | Affected | 0.2199 | 0.5349 | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||||||||||||
| c.3416A>G | Q1139R 2D  AIThe SynGAP1 missense variant Q1139R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of ClinVar annotation—there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.908098 | Disordered | 0.721191 | Binding | 0.313 | 0.866 | 1.000 | -4.249 | Likely Benign | 0.267 | Likely Benign | Likely Benign | 0.315 | Likely Benign | -1.91 | Neutral | 0.126 | Benign | 0.138 | Benign | 5.47 | Benign | 0.00 | Affected | 0.1342 | 0.2405 | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||||||||||
| c.3416A>T | Q1139L 2D  AIThe SynGAP1 missense variant Q1139L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. In contrast, PROVEAN and SIFT predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.908098 | Disordered | 0.721191 | Binding | 0.313 | 0.866 | 1.000 | -1.689 | Likely Benign | 0.153 | Likely Benign | Likely Benign | 0.472 | Likely Benign | -3.75 | Deleterious | 0.224 | Benign | 0.237 | Benign | 5.30 | Benign | 0.00 | Affected | 0.0745 | 0.5599 | -2 | -2 | 7.3 | -14.97 | |||||||||||||||||||||||||||||||||||||||
| c.3417G>C | Q1139H 2D  AIThe SynGAP1 missense variant Q1139H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.908098 | Disordered | 0.721191 | Binding | 0.313 | 0.866 | 1.000 | -3.962 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.265 | Likely Benign | -2.40 | Neutral | 0.002 | Benign | 0.007 | Benign | 5.41 | Benign | 0.00 | Affected | 0.1313 | 0.3801 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.3417G>T | Q1139H 2D  AIThe SynGAP1 missense variant Q1139H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the preponderance of benign predictions and the lack of contradictory evidence, the variant is most likely benign. This assessment does not conflict with ClinVar status, as no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.908098 | Disordered | 0.721191 | Binding | 0.313 | 0.866 | 1.000 | -3.962 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.265 | Likely Benign | -2.40 | Neutral | 0.002 | Benign | 0.007 | Benign | 5.41 | Benign | 0.00 | Affected | 0.1313 | 0.3801 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.3418A>C | T1140P 2D  AIThe SynGAP1 missense variant T1140P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools, polyPhen‑2 HumDiv and HumVar, predict a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for T1140P, and this conclusion is consistent with the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.926919 | Disordered | 0.708094 | Binding | 0.293 | 0.854 | 1.000 | -1.921 | Likely Benign | 0.126 | Likely Benign | Likely Benign | 0.058 | Likely Benign | -1.49 | Neutral | 0.761 | Possibly Damaging | 0.478 | Possibly Damaging | 2.61 | Benign | 0.32 | Tolerated | 0.2044 | 0.3837 | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||||||
| c.3418A>G | T1140A 2D  AIThe SynGAP1 missense variant T1140A is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.926919 | Disordered | 0.708094 | Binding | 0.293 | 0.854 | 1.000 | -3.838 | Likely Benign | 0.107 | Likely Benign | Likely Benign | 0.061 | Likely Benign | -0.36 | Neutral | 0.002 | Benign | 0.003 | Benign | 2.71 | Benign | 1.00 | Tolerated | 0.3949 | 0.3463 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3418A>T | T1140S 2D  AIThe SynGAP1 missense variant T1140S is not reported in ClinVar and is absent from gnomAD, indicating no documented clinical or population evidence. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification. Foldetta results are not available, so they do not influence the assessment. Overall, the variant is most likely benign, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.926919 | Disordered | 0.708094 | Binding | 0.293 | 0.854 | 1.000 | -2.805 | Likely Benign | 0.105 | Likely Benign | Likely Benign | 0.052 | Likely Benign | -0.27 | Neutral | 0.025 | Benign | 0.010 | Benign | 3.25 | Benign | 0.91 | Tolerated | 0.3401 | 0.3504 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3419C>A | T1140K 2D  AIThe SynGAP1 missense variant T1140K is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The high‑accuracy consensus, SGM‑Consensus, is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN and therefore reports a likely benign outcome. AlphaMissense‑Optimized also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence—including the high‑accuracy consensus—points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.926919 | Disordered | 0.708094 | Binding | 0.293 | 0.854 | 1.000 | -4.053 | Likely Benign | 0.657 | Likely Pathogenic | Likely Benign | 0.068 | Likely Benign | -1.65 | Neutral | 0.611 | Possibly Damaging | 0.257 | Benign | 2.63 | Benign | 0.22 | Tolerated | 0.1196 | 0.2950 | 0 | -1 | -3.2 | 27.07 | |||||||||||||||||||||||||||||||||||||||
| c.3419C>T | T1140I 2D  AIThe SynGAP1 missense variant T1140I is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all classify the change as benign, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports it as likely benign. Only polyPhen‑2 HumDiv predicts a pathogenic outcome, and AlphaMissense‑Default remains uncertain. High‑accuracy assessments reinforce the benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates a likely benign effect. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods indicates that the variant is most likely benign, with no conflict with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.926919 | Disordered | 0.708094 | Binding | 0.293 | 0.854 | 1.000 | -5.205 | Likely Benign | 0.368 | Ambiguous | Likely Benign | 0.058 | Likely Benign | -1.85 | Neutral | 0.611 | Possibly Damaging | 0.398 | Benign | 2.61 | Benign | 0.08 | Tolerated | 0.0948 | 0.4648 | 0 | -1 | 5.2 | 12.05 | |||||||||||||||||||||||||||||||||||||||
| c.341A>C | K114T 2D 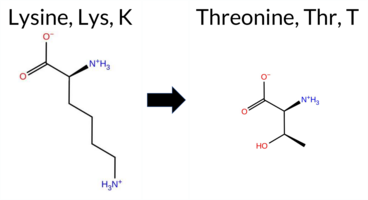 AIThe SynGAP1 missense variant K114T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which collectively indicate a likely benign outcome. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, the SGM‑Consensus score is likely benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Based on the overall distribution of predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.649749 | Binding | 0.381 | 0.879 | 0.750 | -3.366 | Likely Benign | 0.804 | Likely Pathogenic | Ambiguous | 0.091 | Likely Benign | -1.48 | Neutral | 0.759 | Possibly Damaging | 0.190 | Benign | 3.95 | Benign | 0.00 | Affected | 0.2796 | 0.3391 | 0 | -1 | 3.2 | -27.07 | |||||||||||||||||||||||||||||||||||||||
| c.341A>G | K114R 2D 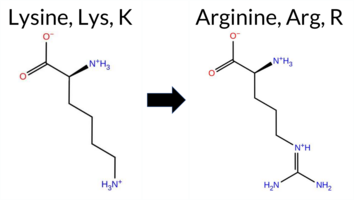 AIThe SynGAP1 missense variant K114R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect; there is no conflict with ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.649749 | Binding | 0.381 | 0.879 | 0.750 | -1.861 | Likely Benign | 0.108 | Likely Benign | Likely Benign | 0.041 | Likely Benign | -1.02 | Neutral | 0.005 | Benign | 0.003 | Benign | 4.09 | Benign | 0.00 | Affected | 0.5668 | 0.1498 | Weaken | 3 | 2 | -0.6 | 28.01 | ||||||||||||||||||||||||||||||||||||||
| c.3428C>A | T1143K 2D  AIThe SynGAP1 missense variant T1143K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, whereas the SGM‑Consensus (majority vote) supports a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact for T1143K, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.722918 | Binding | 0.275 | 0.837 | 1.000 | -3.498 | Likely Benign | 0.811 | Likely Pathogenic | Ambiguous | 0.163 | Likely Benign | -2.03 | Neutral | 0.986 | Probably Damaging | 0.895 | Possibly Damaging | 2.78 | Benign | 0.14 | Tolerated | 0.1266 | 0.2936 | 0 | -1 | -3.2 | 27.07 | |||||||||||||||||||||||||||||||||||||||
| c.3428C>G | T1143R 2D  AIThe SynGAP1 missense variant T1143R is not reported in ClinVar and is absent from gnomAD, indicating no known population frequency data. Functional prediction tools cluster into two groups: benign predictions include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from AlphaMissense‑Default, polyPhen‑2 HumDiv, and polyPhen‑2 HumVar. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also favors benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.722918 | Binding | 0.275 | 0.837 | 1.000 | -2.882 | Likely Benign | 0.782 | Likely Pathogenic | Likely Benign | 0.175 | Likely Benign | -2.31 | Neutral | 0.996 | Probably Damaging | 0.951 | Probably Damaging | 2.73 | Benign | 0.09 | Tolerated | 0.1087 | 0.3060 | -1 | -1 | -3.8 | 55.08 | |||||||||||||||||||||||||||||||||||||||
| c.3428C>T | T1143I 2D  AIThe SynGAP1 missense variant T1143I is not reported in ClinVar and has no entries in gnomAD, indicating it has not been catalogued in these databases. Functional prediction tools largely agree that the substitution is benign: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all classify it as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports it as likely benign. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic effect. The AlphaMissense‑Default score is uncertain, and no Foldetta stability assessment is available. High‑accuracy predictions from AlphaMissense‑Optimized and the SGM‑Consensus both support a benign outcome, while the absence of a Foldetta result leaves the structural impact unresolved. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the lack of ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.722918 | Binding | 0.275 | 0.837 | 1.000 | -3.554 | Likely Benign | 0.560 | Ambiguous | Likely Benign | 0.163 | Likely Benign | -1.87 | Neutral | 0.999 | Probably Damaging | 0.966 | Probably Damaging | 2.68 | Benign | 0.10 | Tolerated | 0.1013 | 0.4274 | 0 | -1 | 5.2 | 12.05 | |||||||||||||||||||||||||||||||||||||||
| c.3437C>A | P1146H 2D  AIThe SynGAP1 missense variant P1146H is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include ESM1b, FATHMM, and AlphaMissense‑Optimized, whereas a majority of tools (REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT) predict a pathogenic impact; AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a benign outcome (2 benign vs. 1 pathogenic, 1 uncertain). Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion does not contradict the ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.732173 | Binding | 0.415 | 0.837 | 1.000 | -4.428 | Likely Benign | 0.518 | Ambiguous | Likely Benign | 0.529 | Likely Pathogenic | -4.93 | Deleterious | 1.000 | Probably Damaging | 0.983 | Probably Damaging | 5.46 | Benign | 0.00 | Affected | 0.1625 | 0.4512 | 0 | -2 | -1.6 | 40.02 | ||||||||||||||||||||||||||||||||||||||||
| c.3437C>G | P1146R 2D  AIThe SynGAP1 missense variant P1146R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a pathogenic effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. Tools that agree on a benign effect are ESM1b, FATHMM, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts a benign outcome, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑vs‑2 split, and Foldetta results are unavailable. Overall, the majority of available predictions (seven pathogenic versus three benign) indicate that the variant is most likely pathogenic. This conclusion is not contradicted by ClinVar status, as the variant has no existing ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.732173 | Binding | 0.415 | 0.837 | 1.000 | -3.826 | Likely Benign | 0.729 | Likely Pathogenic | Likely Benign | 0.603 | Likely Pathogenic | -4.81 | Deleterious | 0.996 | Probably Damaging | 0.967 | Probably Damaging | 5.50 | Benign | 0.00 | Affected | 0.1315 | 0.3193 | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||||||||
| c.3437C>T | P1146L 2D  AISynGAP1 missense variant P1146L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include ESM1b, FATHMM, and AlphaMissense‑Optimized, whereas a separate group predicts pathogenicity: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), and SIFT. AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also favors a benign outcome, while Foldetta results are unavailable. Overall, the majority of conventional predictors indicate pathogenicity, but the most accurate tools lean benign. Thus, the variant is most likely pathogenic based on the collective predictions, and this assessment does not contradict the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.732173 | Binding | 0.415 | 0.837 | 1.000 | -2.182 | Likely Benign | 0.483 | Ambiguous | Likely Benign | 0.564 | Likely Pathogenic | -5.25 | Deleterious | 0.992 | Probably Damaging | 0.912 | Probably Damaging | 5.51 | Benign | 0.00 | Affected | 0.2151 | 0.6446 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||||||
| c.3439A>C | T1147P 2D  AIThe SynGAP1 missense variant T1147P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.831250 | Disordered | 0.746520 | Binding | 0.345 | 0.839 | 0.875 | -2.849 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.425 | Likely Benign | -2.17 | Neutral | 0.000 | Benign | 0.002 | Benign | 5.41 | Benign | 0.02 | Affected | 0.1925 | 0.4490 | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||||||
| c.3439A>G | T1147A 2D  AIThe SynGAP1 missense variant T1147A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also indicates likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign effect, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.831250 | Disordered | 0.746520 | Binding | 0.345 | 0.839 | 0.875 | -3.115 | Likely Benign | 0.061 | Likely Benign | Likely Benign | 0.188 | Likely Benign | -1.60 | Neutral | 0.001 | Benign | 0.004 | Benign | 5.54 | Benign | 0.03 | Affected | 0.3816 | 0.3705 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3439A>T | T1147S 2D  AIThe SynGAP1 missense variant T1147S is not reported in ClinVar and is absent from gnomAD, indicating no documented clinical or population evidence. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification. Foldetta results are not available, so they do not influence the assessment. Overall, the variant is most likely benign, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.831250 | Disordered | 0.746520 | Binding | 0.345 | 0.839 | 0.875 | -2.593 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.173 | Likely Benign | -0.51 | Neutral | 0.126 | Benign | 0.096 | Benign | 5.48 | Benign | 0.08 | Tolerated | 0.3129 | 0.3757 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.343C>A | Q115K 2D  AIThe SynGAP1 missense variant Q115K is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign interpretation: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all predict benign, while the majority of other predictors (polyPhen‑2 HumDiv and HumVar) indicate pathogenic. When predictions are grouped by agreement, the benign‑oriented tools outnumber the pathogenic ones, and the single uncertain call from AlphaMissense‑Default does not alter the overall trend. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized classifies the variant as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports it as Likely Benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Based on the preponderance of benign predictions and the high‑accuracy consensus, the variant is most likely benign; this conclusion is consistent with the absence of a ClinVar pathogenic claim. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.657256 | Binding | 0.327 | 0.878 | 0.750 | -3.365 | Likely Benign | 0.507 | Ambiguous | Likely Benign | 0.101 | Likely Benign | -0.49 | Neutral | 0.924 | Possibly Damaging | 0.857 | Possibly Damaging | 4.18 | Benign | 0.40 | Tolerated | 0.1704 | 0.3678 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.3440C>A | T1147K 2D  AIThe SynGAP1 missense variant T1147K is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Based on the preponderance of benign predictions and the lack of pathogenic evidence, T1147K is most likely benign, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.831250 | Disordered | 0.746520 | Binding | 0.345 | 0.839 | 0.875 | -3.921 | Likely Benign | 0.564 | Ambiguous | Likely Benign | 0.386 | Likely Benign | -2.29 | Neutral | 0.126 | Benign | 0.096 | Benign | 5.50 | Benign | 0.02 | Affected | 0.1148 | 0.3200 | 0 | -1 | -3.2 | 27.07 | |||||||||||||||||||||||||||||||||||||||
| c.3440C>G | T1147R 2D  AIThe SynGAP1 missense variant T1147R is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in‑silico tools cluster around a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support this view: AlphaMissense‑Optimized reports a benign effect, and the SGM‑Consensus likewise indicates Likely Benign; Foldetta results are unavailable. Taken together, the preponderance of evidence points to a benign impact for T1147R, and this conclusion does not contradict any existing ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.831250 | Disordered | 0.746520 | Binding | 0.345 | 0.839 | 0.875 | -3.234 | Likely Benign | 0.534 | Ambiguous | Likely Benign | 0.442 | Likely Benign | -2.44 | Neutral | 0.411 | Benign | 0.139 | Benign | 5.46 | Benign | 0.01 | Affected | 0.1014 | 0.2742 | -1 | -1 | -3.8 | 55.08 | |||||||||||||||||||||||||||||||||||||||
| c.3440C>T | T1147I 2D  AIThe SynGAP1 missense variant T1147I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.831250 | Disordered | 0.746520 | Binding | 0.345 | 0.839 | 0.875 | -3.552 | Likely Benign | 0.151 | Likely Benign | Likely Benign | 0.423 | Likely Benign | -2.03 | Neutral | 0.259 | Benign | 0.059 | Benign | 5.42 | Benign | 0.01 | Affected | 0.1029 | 0.4968 | 0 | -1 | 5.2 | 12.05 | |||||||||||||||||||||||||||||||||||||||
| c.3442A>C | M1148L 2D  AIThe SynGAP1 missense variant M1148L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and there is no conflict with ClinVar status because no ClinVar classification exists. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.774279 | Binding | 0.343 | 0.835 | 0.875 | -1.777 | Likely Benign | 0.093 | Likely Benign | Likely Benign | 0.068 | Likely Benign | -1.13 | Neutral | 0.016 | Benign | 0.016 | Benign | 2.62 | Benign | 0.00 | Affected | 4.32 | 2 | 0.1641 | 0.4135 | 4 | 2 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||||
| c.3442A>T | M1148L 2D  AIThe SynGAP1 missense variant M1148L is listed in ClinVar (ID 1010061.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this is consistent with the ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.774279 | Binding | 0.343 | 0.835 | 0.875 | Uncertain | 1 | -1.777 | Likely Benign | 0.093 | Likely Benign | Likely Benign | 0.068 | Likely Benign | -1.13 | Neutral | 0.016 | Benign | 0.016 | Benign | 2.62 | Benign | 0.00 | Affected | 4.32 | 2 | 0.1641 | 0.4135 | 4 | 2 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||
| c.3443T>A | M1148K 2D 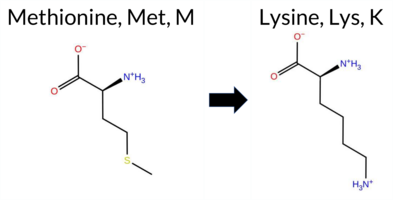 AIThe SynGAP1 missense variant M1148K is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus, REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.774279 | Binding | 0.343 | 0.835 | 0.875 | -2.527 | Likely Benign | 0.624 | Likely Pathogenic | Likely Benign | 0.105 | Likely Benign | -1.58 | Neutral | 0.144 | Benign | 0.085 | Benign | 2.55 | Benign | 0.00 | Affected | 0.1539 | 0.1056 | 0 | -1 | -5.8 | -3.02 | |||||||||||||||||||||||||||||||||||||||
| c.3436C>G | P1146A 2D  AIThe SynGAP1 missense variant P1146A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, tools that predict a pathogenic outcome are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for P1146A, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.732173 | Binding | 0.415 | 0.837 | 1.000 | -3.896 | Likely Benign | 0.203 | Likely Benign | Likely Benign | 0.300 | Likely Benign | -3.56 | Deleterious | 0.972 | Probably Damaging | 0.760 | Possibly Damaging | 5.53 | Benign | 0.00 | Affected | 0.3516 | 0.4791 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.3436C>A | P1146T 2D  AIThe SynGAP1 missense variant P1146T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and polyPhen‑2 HumVar, as well as the SGM‑Consensus call of “Likely Benign.” In contrast, PROVEAN, polyPhen‑2 HumDiv, and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. Foldetta results are not available. Overall, the majority of evidence points to a benign effect for P1146T, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.732173 | Binding | 0.415 | 0.837 | 1.000 | -3.494 | Likely Benign | 0.272 | Likely Benign | Likely Benign | 0.454 | Likely Benign | -4.11 | Deleterious | 0.573 | Possibly Damaging | 0.334 | Benign | 5.51 | Benign | 0.00 | Affected | 0.1478 | 0.5789 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.342G>C | K114N 2D  AIThe SynGAP1 missense variant K114N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, while the SGM‑Consensus remains likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.649749 | Binding | 0.381 | 0.879 | 0.750 | -3.851 | Likely Benign | 0.937 | Likely Pathogenic | Ambiguous | 0.045 | Likely Benign | -1.41 | Neutral | 0.608 | Possibly Damaging | 0.190 | Benign | 3.95 | Benign | 0.00 | Affected | 0.4590 | 0.1877 | 1 | 0 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||||||||
| c.342G>T | K114N 2D  AIThe SynGAP1 missense variant K114N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.649749 | Binding | 0.381 | 0.879 | 0.750 | -3.851 | Likely Benign | 0.937 | Likely Pathogenic | Ambiguous | 0.045 | Likely Benign | -1.41 | Neutral | 0.608 | Possibly Damaging | 0.190 | Benign | 3.95 | Benign | 0.00 | Affected | 0.4590 | 0.1877 | 1 | 0 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||||||||
| c.3430T>A | L1144M 2D 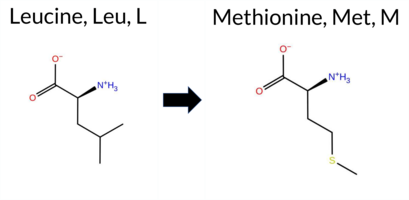 AIThe SynGAP1 missense variant L1144M is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: **benign** – REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized; **pathogenic** – polyPhen‑2 (HumDiv and HumVar) and SIFT. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports Likely Benign. Foldetta predictions are unavailable. Overall, the majority of evidence points to a benign effect for L1144M, and this conclusion does not conflict with ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.726803 | Binding | 0.277 | 0.840 | 1.000 | -4.787 | Likely Benign | 0.199 | Likely Benign | Likely Benign | 0.277 | Likely Benign | -0.25 | Neutral | 0.999 | Probably Damaging | 0.979 | Probably Damaging | 5.38 | Benign | 0.05 | Affected | 0.0846 | 0.3863 | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||||||
| c.3430T>G | L1144V 2D  AIThe SynGAP1 missense variant L1144V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools, polyPhen‑2 HumDiv and HumVar, predict pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy methods reinforce this view: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.726803 | Binding | 0.277 | 0.840 | 1.000 | -4.025 | Likely Benign | 0.151 | Likely Benign | Likely Benign | 0.227 | Likely Benign | -0.04 | Neutral | 0.952 | Possibly Damaging | 0.770 | Possibly Damaging | 5.43 | Benign | 0.70 | Tolerated | 0.1553 | 0.3297 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3431T>C | L1144S 2D  AIThe SynGAP1 missense variant L1144S has no ClinVar entry and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic calls come from REVEL, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. Grouping by consensus, the majority of tools (four benign vs. five pathogenic) lean slightly toward pathogenicity, but the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—labels the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM Consensus also indicates Likely Benign. Foldetta, a protein‑folding stability predictor, did not return a result for this variant. Overall, the computational evidence most strongly suggests the variant is benign, and this conclusion does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.726803 | Binding | 0.277 | 0.840 | 1.000 | -2.248 | Likely Benign | 0.683 | Likely Pathogenic | Likely Benign | 0.580 | Likely Pathogenic | -1.69 | Neutral | 1.000 | Probably Damaging | 0.979 | Probably Damaging | 5.41 | Benign | 0.01 | Affected | 0.3045 | 0.0863 | -3 | -2 | -4.6 | -26.08 | |||||||||||||||||||||||||||||||||||||||
| c.3432G>C | L1144F 2D  AIThe SynGAP1 missense variant L1144F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for the variant, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.726803 | Binding | 0.277 | 0.840 | 1.000 | -4.375 | Likely Benign | 0.406 | Ambiguous | Likely Benign | 0.415 | Likely Benign | -1.71 | Neutral | 0.999 | Probably Damaging | 0.969 | Probably Damaging | 5.69 | Benign | 0.04 | Affected | 0.0685 | 0.3283 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.3432G>T | L1144F 2D  AIThe SynGAP1 missense variant L1144F is not reported in ClinVar and has no entry in gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic predictions come from polyPhen‑2 (HumDiv and HumVar) and SIFT. AlphaMissense‑Default remains uncertain. The high‑accuracy consensus, SGM‑Consensus, classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Based on the aggregate evidence, the variant is most likely benign. This assessment does not contradict ClinVar status, as no ClinVar claim exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.726803 | Binding | 0.277 | 0.840 | 1.000 | -4.375 | Likely Benign | 0.406 | Ambiguous | Likely Benign | 0.415 | Likely Benign | -1.71 | Neutral | 0.999 | Probably Damaging | 0.969 | Probably Damaging | 5.69 | Benign | 0.04 | Affected | 0.0685 | 0.3283 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.3433A>C | N1145H 2D  AIThe SynGAP1 missense variant N1145H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while polyPhen‑2 (HumDiv and HumVar) and SIFT predict pathogenic. Grouping by consensus, the benign‑predicting tools outnumber the pathogenic ones. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized scores benign, and the SGM Consensus—derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also indicates a likely benign outcome. Foldetta results are unavailable. Overall, the computational evidence supports a benign classification for the variant, and this is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.922952 | Disordered | 0.722723 | Binding | 0.284 | 0.850 | 1.000 | -2.974 | Likely Benign | 0.187 | Likely Benign | Likely Benign | 0.391 | Likely Benign | -2.42 | Neutral | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 5.41 | Benign | 0.02 | Affected | 0.1520 | 0.7233 | 2 | 1 | 0.3 | 23.04 | |||||||||||||||||||||||||||||||||||||||
| c.3433A>G | N1145D 2D  AIThe SynGAP1 missense variant N1145D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (HumDiv and HumVar) and AlphaMissense‑Default predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar status, as none is assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.922952 | Disordered | 0.722723 | Binding | 0.284 | 0.850 | 1.000 | -3.053 | Likely Benign | 0.682 | Likely Pathogenic | Likely Benign | 0.332 | Likely Benign | -2.04 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 5.57 | Benign | 0.08 | Tolerated | 0.2082 | 0.3956 | 2 | 1 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.3433A>T | N1145Y 2D  AIThe SynGAP1 missense variant N1145Y is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include ESM1b, FATHMM, and AlphaMissense‑Optimized, whereas REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), and SIFT uniformly predict a pathogenic impact. AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields benign, while Foldetta results are unavailable. Overall, the majority of conventional tools predict pathogenicity, but the high‑accuracy consensus leans benign. Based on the collective evidence, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.922952 | Disordered | 0.722723 | Binding | 0.284 | 0.850 | 1.000 | -2.945 | Likely Benign | 0.451 | Ambiguous | Likely Benign | 0.543 | Likely Pathogenic | -4.07 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 5.40 | Benign | 0.01 | Affected | 0.0622 | 0.6337 | -2 | -2 | 2.2 | 49.07 | ||||||||||||||||||||||||||||||||||||||||
| c.3434A>T | N1145I 2D  AIThe SynGAP1 missense variant N1145I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Functional prediction tools cluster into two groups: pathogenic predictions come from REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; benign predictions come from ESM1b, FATHMM, and AlphaMissense‑Optimized. AlphaMissense‑Default is uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) resolves to benign, while Foldetta results are unavailable. Overall, the majority of conventional predictors indicate pathogenicity, whereas the high‑accuracy subset leans benign. Based on the aggregate evidence, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar annotation (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.922952 | Disordered | 0.722723 | Binding | 0.284 | 0.850 | 1.000 | -3.172 | Likely Benign | 0.378 | Ambiguous | Likely Benign | 0.504 | Likely Pathogenic | -4.19 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 5.41 | Benign | 0.00 | Affected | 0.0720 | 0.6145 | -2 | -3 | 8.0 | -0.94 | ||||||||||||||||||||||||||||||||||||||||
| c.3435C>A | N1145K 2D  AIThe SynGAP1 missense variant N1145K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain” and the SGM‑Consensus as “Likely Benign”; Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this conclusion does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.922952 | Disordered | 0.722723 | Binding | 0.284 | 0.850 | 1.000 | -2.545 | Likely Benign | 0.814 | Likely Pathogenic | Ambiguous | 0.440 | Likely Benign | -2.40 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 5.50 | Benign | 0.03 | Affected | 0.1959 | 0.6018 | 1 | 0 | -0.4 | 14.07 | |||||||||||||||||||||||||||||||||||||||
| c.3435C>G | N1145K 2D  AIThe SynGAP1 missense variant N1145K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which collectively suggest a likely benign impact. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized result is uncertain, and Foldetta stability analysis is unavailable. Overall, the balance of evidence leans toward a benign interpretation, with no conflict with ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.922952 | Disordered | 0.722723 | Binding | 0.284 | 0.850 | 1.000 | -2.545 | Likely Benign | 0.814 | Likely Pathogenic | Ambiguous | 0.440 | Likely Benign | -2.40 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 5.50 | Benign | 0.03 | Affected | 0.1959 | 0.6018 | 1 | 0 | -0.4 | 14.07 | |||||||||||||||||||||||||||||||||||||||
| c.3443T>C | M1148T 2D  AIThe SynGAP1 missense variant M1148T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.774279 | Binding | 0.343 | 0.835 | 0.875 | -0.972 | Likely Benign | 0.159 | Likely Benign | Likely Benign | 0.151 | Likely Benign | -1.50 | Neutral | 0.001 | Benign | 0.002 | Benign | 2.56 | Benign | 0.00 | Affected | 0.2063 | 0.2214 | -1 | -1 | -2.6 | -30.09 | |||||||||||||||||||||||||||||||||||||||
| c.3473T>A | V1158D 2D  AIThe SynGAP1 missense variant V1158D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence indicates that V1158D is most likely pathogenic, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.599170 | Disordered | 0.877504 | Binding | 0.369 | 0.847 | 0.250 | -4.076 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.486 | Likely Benign | -4.56 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.29 | Pathogenic | 0.00 | Affected | 0.1502 | 0.1820 | -2 | -3 | -7.7 | 15.96 | |||||||||||||||||||||||||||||||||||||||
| c.34A>T | S12C 2D  AIThe SynGAP1 missense variant S12C is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in‑silico tools largely support a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports it as likely benign. In contrast, two tools—polyPhen‑2 HumDiv and SIFT—predict a pathogenic impact. High‑accuracy methods give a consistent benign signal: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this is not in conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.480142 | Structured | 0.490599 | Uncertain | 0.355 | 0.916 | 0.500 | -5.413 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.101 | Likely Benign | 0.00 | Neutral | 0.872 | Possibly Damaging | 0.206 | Benign | 4.05 | Benign | 0.00 | Affected | 0.1025 | 0.6092 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||||||
| c.3507G>C | E1169D 2D  AIThe SynGAP1 missense variant E1169D is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of consensus tools predict a pathogenic impact, and no ClinVar entry contradicts this assessment. Thus, the variant is most likely pathogenic based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.699094 | Disordered | 0.732455 | Binding | 0.400 | 0.781 | 0.625 | -3.457 | Likely Benign | 0.913 | Likely Pathogenic | Ambiguous | 0.104 | Likely Benign | -1.12 | Neutral | 0.989 | Probably Damaging | 0.924 | Probably Damaging | 2.49 | Pathogenic | 0.00 | Affected | 0.1597 | 0.3968 | 3 | 2 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||||||||||||
| c.3507G>T | E1169D 2D  AIThe SynGAP1 missense variant E1169D is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of consensus tools predict a pathogenic impact, and no ClinVar entry contradicts this assessment. Thus, the variant is most likely pathogenic based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.699094 | Disordered | 0.732455 | Binding | 0.400 | 0.781 | 0.625 | -3.457 | Likely Benign | 0.913 | Likely Pathogenic | Ambiguous | 0.103 | Likely Benign | -1.12 | Neutral | 0.989 | Probably Damaging | 0.924 | Probably Damaging | 2.49 | Pathogenic | 0.00 | Affected | 0.1597 | 0.3968 | 3 | 2 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||||||||||||
| c.3508A>C | S1170R 2D  AIThe SynGAP1 missense variant S1170R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of conventional predictors lean toward pathogenicity, but the high‑accuracy consensus is split. Thus, the variant is most likely pathogenic based on the preponderance of predictions, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.622677 | Disordered | 0.719138 | Binding | 0.417 | 0.767 | 0.500 | -1.451 | Likely Benign | 0.957 | Likely Pathogenic | Likely Pathogenic | 0.475 | Likely Benign | -1.89 | Neutral | 0.998 | Probably Damaging | 0.966 | Probably Damaging | 5.39 | Benign | 0.04 | Affected | 0.0770 | 0.3444 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||||||||
| c.3508A>G | S1170G 2D  AIThe SynGAP1 missense variant S1170G is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on benign impact include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool in the dataset predicts pathogenicity, so the pathogenic group is empty. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also predicts benign, while Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.622677 | Disordered | 0.719138 | Binding | 0.417 | 0.767 | 0.500 | Uncertain | 1 | -4.288 | Likely Benign | 0.221 | Likely Benign | Likely Benign | 0.349 | Likely Benign | -0.81 | Neutral | 0.241 | Benign | 0.229 | Benign | 5.31 | Benign | 0.54 | Tolerated | 4.32 | 4 | 0.2705 | 0.4558 | 1 | 0 | 0.4 | -30.03 | ||||||||||||||||||||||||||||||||||
| c.3508A>T | S1170C 2D  AIThe S1170C missense change occurs in a coiled‑coil region of SynGAP1. ClinVar has no entry for this variant, and it is not reported in gnomAD. Prediction tools that agree on a benign effect include PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized; those that predict a pathogenic effect are REVEL, polyPhen‑2 (HumDiv and HumVar), and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” status. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign, while Foldetta results are unavailable. Overall, the balance of evidence, particularly from the high‑accuracy tools, points to a benign impact for S1170C. This conclusion is not contradicted by ClinVar, which currently contains no classification for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.622677 | Disordered | 0.719138 | Binding | 0.417 | 0.767 | 0.500 | -6.393 | Likely Benign | 0.416 | Ambiguous | Likely Benign | 0.566 | Likely Pathogenic | -2.07 | Neutral | 0.999 | Probably Damaging | 0.992 | Probably Damaging | 5.30 | Benign | 0.02 | Affected | 0.0872 | 0.5850 | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||||
| c.3509G>C | S1170T 2D  AIThe SynGAP1 missense variant S1170T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates “Likely Benign.” In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect for S1170T, and this conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.622677 | Disordered | 0.719138 | Binding | 0.417 | 0.767 | 0.500 | -3.771 | Likely Benign | 0.163 | Likely Benign | Likely Benign | 0.338 | Likely Benign | -1.13 | Neutral | 0.992 | Probably Damaging | 0.925 | Probably Damaging | 5.37 | Benign | 0.08 | Tolerated | 0.1258 | 0.5897 | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||||||||||
| c.3509G>T | S1170I 2D  AIThe SynGAP1 missense variant S1170I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while those that predict a pathogenic effect are REVEL, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, the SGM‑Consensus (majority vote) also predicts benign, and the Foldetta protein‑folding stability analysis is unavailable. Taken together, the preponderance of high‑confidence predictions indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.622677 | Disordered | 0.719138 | Binding | 0.417 | 0.767 | 0.500 | -3.813 | Likely Benign | 0.635 | Likely Pathogenic | Likely Benign | 0.600 | Likely Pathogenic | -2.16 | Neutral | 0.998 | Probably Damaging | 0.990 | Probably Damaging | 5.29 | Benign | 0.01 | Affected | 0.0754 | 0.5329 | -1 | -2 | 5.3 | 26.08 | ||||||||||||||||||||||||||||||||||||||
| c.350G>A | S117N 2D  AIThe SynGAP1 missense variant S117N is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from multiple prediction algorithms and consensus analyses indicates that the variant is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.672422 | Binding | 0.357 | 0.877 | 0.625 | -5.704 | Likely Benign | 0.489 | Ambiguous | Likely Benign | 0.067 | Likely Benign | -1.06 | Neutral | 0.005 | Benign | 0.003 | Benign | 3.71 | Benign | 0.01 | Affected | 0.1491 | 0.4530 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||||||
| c.350G>C | S117T 2D  AIThe SynGAP1 missense variant S117T is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports it as likely benign. In contrast, polyPhen‑2 HumDiv and SIFT predict a pathogenic outcome. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.672422 | Binding | 0.357 | 0.877 | 0.625 | -4.602 | Likely Benign | 0.142 | Likely Benign | Likely Benign | 0.089 | Likely Benign | -0.98 | Neutral | 0.608 | Possibly Damaging | 0.092 | Benign | 3.79 | Benign | 0.02 | Affected | 0.1527 | 0.5505 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.350G>T | S117I 2D  AIThe SynGAP1 missense variant S117I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default remains uncertain. The high‑accuracy consensus (SGM‑Consensus) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.672422 | Binding | 0.357 | 0.877 | 0.625 | -5.483 | Likely Benign | 0.559 | Ambiguous | Likely Benign | 0.137 | Likely Benign | -2.33 | Neutral | 0.971 | Probably Damaging | 0.598 | Possibly Damaging | 3.70 | Benign | 0.00 | Affected | 0.1060 | 0.5048 | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||||||
| c.3510T>A | S1170R 2D  AIThe SynGAP1 missense variant S1170R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, whereas tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, SGM‑Consensus as likely benign, and Foldetta results are unavailable. Overall, the majority of individual predictors lean toward pathogenicity, with the SGM‑Consensus providing a counter‑signal; the evidence is therefore inconclusive. The variant is most likely pathogenic according to the prevailing predictions, and this does not contradict ClinVar status, which has no entry for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.622677 | Disordered | 0.719138 | Binding | 0.417 | 0.767 | 0.500 | -1.451 | Likely Benign | 0.957 | Likely Pathogenic | Likely Pathogenic | 0.452 | Likely Benign | -1.89 | Neutral | 0.998 | Probably Damaging | 0.966 | Probably Damaging | 5.39 | Benign | 0.04 | Affected | 0.0770 | 0.3444 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||||||||
| c.3510T>G | S1170R 2D  AIThe SynGAP1 missense variant S1170R is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized model classifies the variant as pathogenic, whereas the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of conventional predictors lean toward pathogenicity, and the most accurate tools also favor a pathogenic classification, with no conflict from ClinVar or gnomAD data. Thus, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any existing ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.622677 | Disordered | 0.719138 | Binding | 0.417 | 0.767 | 0.500 | -1.451 | Likely Benign | 0.957 | Likely Pathogenic | Likely Pathogenic | 0.452 | Likely Benign | -1.89 | Neutral | 0.998 | Probably Damaging | 0.966 | Probably Damaging | 5.39 | Benign | 0.04 | Affected | 0.0770 | 0.3444 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||||||||
| c.3511G>A | A1171T 2D  AIThe SynGAP1 missense variant A1171T is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. All available in‑silico predictors classify the change as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a “Likely Benign” outcome. No tool predicts pathogenicity. High‑accuracy assessments confirm the benign prediction: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.599170 | Disordered | 0.702689 | Binding | 0.472 | 0.775 | 0.500 | Uncertain | 1 | -3.658 | Likely Benign | 0.149 | Likely Benign | Likely Benign | 0.201 | Likely Benign | -0.48 | Neutral | 0.245 | Benign | 0.138 | Benign | 5.45 | Benign | 0.07 | Tolerated | 4.32 | 4 | 0.1421 | 0.5465 | 1 | 0 | -2.5 | 30.03 | ||||||||||||||||||||||||||||||||||
| c.3506A>T | E1169V 2D  AIThe SynGAP1 E1169V missense variant is not reported in ClinVar and is absent from gnomAD. Consensus from standard prediction algorithms shows a split: benign predictions come from REVEL, ESM1b, and FATHMM, whereas pathogenic predictions arise from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments further support a pathogenic signal: AlphaMissense‑Optimized is pathogenic, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—remains inconclusive (2 pathogenic vs 2 benign), and Foldetta stability analysis is unavailable. Overall, the preponderance of evidence (seven pathogenic vs three benign predictions) indicates that E1169V is most likely pathogenic. This conclusion is consistent with the absence of a ClinVar entry, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.699094 | Disordered | 0.732455 | Binding | 0.400 | 0.781 | 0.625 | -2.482 | Likely Benign | 0.975 | Likely Pathogenic | Likely Pathogenic | 0.227 | Likely Benign | -2.94 | Deleterious | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 2.51 | Benign | 0.00 | Affected | 0.0581 | 0.7028 | -2 | -2 | 7.7 | -29.98 | ||||||||||||||||||||||||||||||||||||||||
| c.3506A>G | E1169G 2D  AIThe SynGAP1 missense variant E1169G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evaluated predictors (five pathogenic vs. three benign) lean toward a pathogenic classification. This prediction is not contradicted by ClinVar status, as the variant is not yet catalogued there. Thus, based on current computational evidence, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.699094 | Disordered | 0.732455 | Binding | 0.400 | 0.781 | 0.625 | -3.172 | Likely Benign | 0.940 | Likely Pathogenic | Ambiguous | 0.203 | Likely Benign | -2.33 | Neutral | 0.995 | Probably Damaging | 0.963 | Probably Damaging | 2.45 | Pathogenic | 0.00 | Affected | 0.3005 | 0.6019 | 0 | -2 | 3.1 | -72.06 | ||||||||||||||||||||||||||||||||||||||||
| c.3500A>C | D1167A 2D  AIThe SynGAP1 missense variant D1167A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) all indicate likely pathogenicity. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus also reports it as likely pathogenic. Foldetta results are unavailable, so they do not influence the overall assessment. Based on the preponderance of pathogenic predictions and the high‑accuracy tool outputs, the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.599170 | Disordered | 0.783999 | Binding | 0.336 | 0.798 | 0.500 | -1.281 | Likely Benign | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.244 | Likely Benign | -3.11 | Deleterious | 0.986 | Probably Damaging | 0.926 | Probably Damaging | 2.30 | Pathogenic | 0.01 | Affected | 0.4156 | 0.7359 | 0 | -2 | 5.3 | -44.01 | |||||||||||||||||||||||||||||||||||||||
| c.3500A>G | D1167G 2D  AIThe SynGAP1 missense variant D1167G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that classify the variant as benign include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict it to be pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is pathogenic, and the SGM Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also likely pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that D1167G is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.599170 | Disordered | 0.783999 | Binding | 0.336 | 0.798 | 0.500 | -2.967 | Likely Benign | 0.986 | Likely Pathogenic | Likely Pathogenic | 0.246 | Likely Benign | -2.57 | Deleterious | 0.995 | Probably Damaging | 0.949 | Probably Damaging | 2.29 | Pathogenic | 0.01 | Affected | 0.4172 | 0.7305 | 1 | -1 | 3.1 | -58.04 | |||||||||||||||||||||||||||||||||||||||
| c.3500A>T | D1167V 2D  AIThe SynGAP1 missense variant D1167V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is labeled Likely Pathogenic. The Foldetta protein‑folding stability analysis is unavailable for this variant. Based on the preponderance of pathogenic predictions and the high‑accuracy tool results, the variant is most likely pathogenic; this conclusion is not contradicted by any ClinVar status, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.599170 | Disordered | 0.783999 | Binding | 0.336 | 0.798 | 0.500 | -2.750 | Likely Benign | 0.994 | Likely Pathogenic | Likely Pathogenic | 0.288 | Likely Benign | -3.34 | Deleterious | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 2.26 | Pathogenic | 0.00 | Affected | 0.0946 | 0.7515 | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||||||
| c.3501C>A | D1167E 2D  AIThe SynGAP1 missense variant D1167E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, with no conflict with ClinVar status because no ClinVar assertion exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.783999 | Binding | 0.336 | 0.798 | 0.500 | -2.908 | Likely Benign | 0.940 | Likely Pathogenic | Ambiguous | 0.141 | Likely Benign | -1.19 | Neutral | 0.989 | Probably Damaging | 0.924 | Probably Damaging | 2.57 | Benign | 0.24 | Tolerated | 0.1468 | 0.7368 | 3 | 2 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3501C>G | D1167E 2D  AIThe SynGAP1 missense variant D1167E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, with no conflict with ClinVar status because no ClinVar assertion exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.783999 | Binding | 0.336 | 0.798 | 0.500 | -2.908 | Likely Benign | 0.940 | Likely Pathogenic | Ambiguous | 0.142 | Likely Benign | -1.19 | Neutral | 0.989 | Probably Damaging | 0.924 | Probably Damaging | 2.57 | Benign | 0.24 | Tolerated | 0.1468 | 0.7368 | 3 | 2 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3502A>C | I1168L 2D  AIThe SynGAP1 missense variant I1168L is not reported in ClinVar and is absent from gnomAD. Consensus from multiple in‑silico predictors indicates a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM all score the substitution as tolerated, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also classifies it as likely benign. Only AlphaMissense‑Default predicts a pathogenic outcome, but this is an outlier relative to the other tools. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized reports a benign effect, and the SGM‑Consensus (majority vote) concurs. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so it does not influence the overall assessment. Overall, the computational evidence overwhelmingly favors a benign classification, and this is consistent with the absence of a ClinVar pathogenic report. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.763262 | Binding | 0.423 | 0.796 | 0.500 | -1.855 | Likely Benign | 0.604 | Likely Pathogenic | Likely Benign | 0.361 | Likely Benign | -0.60 | Neutral | 0.241 | Benign | 0.286 | Benign | 5.48 | Benign | 0.20 | Tolerated | 0.0956 | 0.4465 | 2 | 2 | -0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.3502A>G | I1168V 2D  AIThe SynGAP1 missense variant I1168V is listed in ClinVar (ID 936001.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PolyPhen‑2 HumDiv and PolyPhen‑2 HumVar. AlphaMissense‑Default is uncertain, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports a “Likely Benign” outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this consensus does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.763262 | Binding | 0.423 | 0.796 | 0.500 | Uncertain | 1 | -3.263 | Likely Benign | 0.524 | Ambiguous | Likely Benign | 0.363 | Likely Benign | -0.14 | Neutral | 0.876 | Possibly Damaging | 0.643 | Possibly Damaging | 5.47 | Benign | 0.84 | Tolerated | 3.88 | 3 | 0.1339 | 0.4374 | 4 | 3 | -0.3 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.3502A>T | I1168F 2D  AIThe SynGAP1 missense variant I1168F is not reported in ClinVar and is absent from gnomAD. Prediction tools show a mixed signal: benign calls come from REVEL, PROVEAN, ESM1b, and FATHMM, while pathogenic calls come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments are limited: AlphaMissense‑Optimized is uncertain, and Foldetta results are unavailable. Considering the majority of individual predictors and the SGM‑Consensus outcome, the variant is most likely benign. This assessment does not contradict ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.763262 | Binding | 0.423 | 0.796 | 0.500 | -3.422 | Likely Benign | 0.879 | Likely Pathogenic | Ambiguous | 0.440 | Likely Benign | -1.21 | Neutral | 0.998 | Probably Damaging | 0.958 | Probably Damaging | 5.45 | Benign | 0.04 | Affected | 0.0616 | 0.3524 | 1 | 0 | -1.7 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.3503T>A | I1168N 2D  AIThe SynGAP1 missense variant I1168N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote) which labels the variant as Likely Benign. In contrast, tools that predict a pathogenic effect are PolyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments further show AlphaMissense‑Optimized as Pathogenic, while the SGM‑Consensus remains Likely Benign; Foldetta results are unavailable. Overall, the evidence is split evenly between benign and pathogenic predictions, with no consensus from the most accurate methods. Consequently, the variant’s pathogenicity is uncertain and does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.763262 | Binding | 0.423 | 0.796 | 0.500 | -4.269 | Likely Benign | 0.971 | Likely Pathogenic | Likely Pathogenic | 0.455 | Likely Benign | -1.79 | Neutral | 0.998 | Probably Damaging | 0.987 | Probably Damaging | 5.53 | Benign | 0.01 | Affected | 0.0943 | 0.0969 | -2 | -3 | -8.0 | 0.94 | |||||||||||||||||||||||||||||||||||||||
| c.3503T>C | I1168T 2D  AIThe SynGAP1 missense variant I1168T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” AlphaMissense‑Optimized is uncertain, and no Foldetta stability result is available. Overall, the high‑accuracy consensus (SGM‑Consensus) points to a benign outcome, whereas AlphaMissense‑Optimized remains inconclusive. Thus, the variant is most likely benign based on the available predictions, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.763262 | Binding | 0.423 | 0.796 | 0.500 | -2.970 | Likely Benign | 0.943 | Likely Pathogenic | Ambiguous | 0.426 | Likely Benign | -1.29 | Neutral | 0.992 | Probably Damaging | 0.933 | Probably Damaging | 5.54 | Benign | 0.04 | Affected | 0.1173 | 0.1647 | 0 | -1 | -5.2 | -12.05 | |||||||||||||||||||||||||||||||||||||||
| c.3503T>G | I1168S 2D  AISynGAP1 missense variant I1168S has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, whereas the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the evidence is split, with an equal number of benign and pathogenic calls, and the high‑accuracy predictions are contradictory. Therefore, the variant is not conclusively predicted to be benign or pathogenic, and there is no conflict with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.763262 | Binding | 0.423 | 0.796 | 0.500 | -2.212 | Likely Benign | 0.972 | Likely Pathogenic | Likely Pathogenic | 0.442 | Likely Benign | -1.46 | Neutral | 0.998 | Probably Damaging | 0.958 | Probably Damaging | 5.67 | Benign | 0.01 | Affected | 0.3247 | 0.1540 | -1 | -2 | -5.3 | -26.08 | |||||||||||||||||||||||||||||||||||||||
| c.3504C>G | I1168M 2D  AIThe SynGAP1 missense variant I1168M is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact for I1168M, and this conclusion does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.763262 | Binding | 0.423 | 0.796 | 0.500 | -3.471 | Likely Benign | 0.568 | Likely Pathogenic | Likely Benign | 0.397 | Likely Benign | -0.79 | Neutral | 0.999 | Probably Damaging | 0.985 | Probably Damaging | 5.45 | Benign | 0.04 | Affected | 0.0744 | 0.3335 | 2 | 1 | -2.6 | 18.03 | |||||||||||||||||||||||||||||||||||||||
| c.3506A>C | E1169A 2D  AIThe SynGAP1 missense variant E1169A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on benign impact include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict pathogenicity are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, a majority‑vote method from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, and Foldetta results are unavailable. Considering the consensus of benign‑predicting tools and the SGM‑Consensus outcome, the variant is most likely benign. This assessment does not contradict ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.732455 | Binding | 0.400 | 0.781 | 0.625 | -2.132 | Likely Benign | 0.901 | Likely Pathogenic | Ambiguous | 0.217 | Likely Benign | -2.46 | Neutral | 0.995 | Probably Damaging | 0.949 | Probably Damaging | 2.50 | Benign | 0.00 | Affected | 0.3714 | 0.6293 | 0 | -1 | 5.3 | -58.04 | |||||||||||||||||||||||||||||||||||||||
| c.3511G>C | A1171P 2D  AIThe SynGAP1 A1171P missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. AlphaMissense‑Optimized is uncertain, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. High‑accuracy tools therefore provide a benign consensus (SGM‑Consensus) with no definitive pathogenic signal, and no evidence from Foldetta. Based on the aggregate predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.599170 | Disordered | 0.702689 | Binding | 0.472 | 0.775 | 0.500 | -3.625 | Likely Benign | 0.897 | Likely Pathogenic | Ambiguous | 0.355 | Likely Benign | -1.00 | Neutral | 0.918 | Possibly Damaging | 0.601 | Possibly Damaging | 5.31 | Benign | 0.05 | Affected | 0.1910 | 0.3810 | 1 | -1 | -3.4 | 26.04 | ||||||||||||||||||||||||||||||||||||||
| c.3511G>T | A1171S 2D  AIThe SynGAP1 missense variant A1171S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly classify the change as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. Foldetta results are not available, so they do not influence the assessment. Overall, the variant is most likely benign, and this conclusion is consistent with the lack of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.599170 | Disordered | 0.702689 | Binding | 0.472 | 0.775 | 0.500 | -2.875 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.156 | Likely Benign | -0.24 | Neutral | 0.009 | Benign | 0.012 | Benign | 5.54 | Benign | 0.08 | Tolerated | 0.2536 | 0.4676 | 1 | 1 | -2.6 | 16.00 | ||||||||||||||||||||||||||||||||||||||
| c.3511_3512delinsTG | A1171C 2D  AIThe SynGAP1 missense variant A1171C (ClinVar ID 1723483.0) is listed as “Uncertain” in ClinVar and is not present in gnomAD. Prediction tools that converge on a benign outcome include PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, all of which report a benign or neutral effect. In contrast, PolyPhen‑2 (HumDiv and HumVar) and SIFT uniformly predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign,” reinforcing the benign signal. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus (majority vote) as benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.599170 | Disordered | 0.702689 | Binding | 0.472 | 0.775 | 0.500 | Uncertain | 1 | -5.363 | Likely Benign | 0.496 | Ambiguous | Likely Benign | -1.16 | Neutral | 0.978 | Probably Damaging | 0.825 | Possibly Damaging | 5.32 | Benign | 0.02 | Affected | 4.32 | 4 | 0.1102 | 0.4921 | -2 | 0 | 0.7 | 32.06 | ||||||||||||||||||||||||||||||||||||
| c.3518T>G | I1173S 2D  AIThe SynGAP1 missense variant I1173S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and polyPhen‑2 HumVar, while those that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for I1173S, and this conclusion does not contradict any ClinVar status, as none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.501700 | Disordered | 0.653145 | Binding | 0.521 | 0.756 | 0.375 | -2.416 | Likely Benign | 0.557 | Ambiguous | Likely Benign | 0.455 | Likely Benign | -1.18 | Neutral | 0.625 | Possibly Damaging | 0.265 | Benign | 5.45 | Benign | 0.02 | Affected | 0.2796 | 0.0512 | -1 | -2 | -5.3 | -26.08 | ||||||||||||||||||||||||||||||||||||||
| c.3519C>G | I1173M 2D  AIThe SynGAP1 missense variant I1173M is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta results are unavailable, so they do not influence the overall assessment. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.501700 | Disordered | 0.653145 | Binding | 0.521 | 0.756 | 0.375 | -3.301 | Likely Benign | 0.196 | Likely Benign | Likely Benign | 0.327 | Likely Benign | -0.61 | Neutral | 0.973 | Probably Damaging | 0.830 | Possibly Damaging | 5.37 | Benign | 0.15 | Tolerated | 0.0676 | 0.2295 | 2 | 1 | -2.6 | 18.03 | ||||||||||||||||||||||||||||||||||||||
| c.351C>A | S117R 2D  AIThe SynGAP1 missense variant S117R has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification (3 benign vs. 1 pathogenic votes). High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, whereas the SGM‑Consensus (majority vote) remains benign; Foldetta results are unavailable. Overall, the majority of tools (six benign vs. four pathogenic) and the consensus evidence lean toward a benign impact. This conclusion does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.672422 | Binding | 0.357 | 0.877 | 0.625 | -4.187 | Likely Benign | 0.971 | Likely Pathogenic | Likely Pathogenic | 0.144 | Likely Benign | -1.81 | Neutral | 0.845 | Possibly Damaging | 0.326 | Benign | 3.70 | Benign | 0.01 | Affected | 3.61 | 5 | 0.1040 | 0.3770 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||
| c.3520G>C | E1174Q 2D  AIThe SynGAP1 missense variant E1174Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which is a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus as benign, and Foldetta results are unavailable. Based on the overall distribution of predictions and the consensus from high‑accuracy tools, the variant is most likely benign. This assessment does not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.483068 | Structured | 0.618958 | Binding | 0.523 | 0.734 | 0.375 | -3.778 | Likely Benign | 0.603 | Likely Pathogenic | Likely Benign | 0.323 | Likely Benign | -1.04 | Neutral | 0.959 | Probably Damaging | 0.681 | Possibly Damaging | 5.43 | Benign | 0.03 | Affected | 0.0936 | 0.6268 | 2 | 2 | 0.0 | -0.98 | ||||||||||||||||||||||||||||||||||||||
| c.3521A>C | E1174A 2D  AIThe SynGAP1 missense variant E1174A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves to a likely benign verdict. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. Foldetta results are not available, so they do not influence the conclusion. Overall, the majority of evidence points to a benign effect, and this assessment does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.483068 | Structured | 0.618958 | Binding | 0.523 | 0.734 | 0.375 | -3.512 | Likely Benign | 0.737 | Likely Pathogenic | Likely Benign | 0.413 | Likely Benign | -2.24 | Neutral | 0.790 | Possibly Damaging | 0.353 | Benign | 5.44 | Benign | 0.02 | Affected | 0.3462 | 0.5889 | 0 | -1 | 5.3 | -58.04 | ||||||||||||||||||||||||||||||||||||||
| c.3521A>G | E1174G 2D  AIThe SynGAP1 E1174G missense change is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves as “Likely Benign” (3 benign vs. 1 pathogenic). High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, SGM‑Consensus is likely benign, and the Foldetta stability analysis is unavailable. Overall, the preponderance of evidence points to a benign effect for E1174G, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.483068 | Structured | 0.618958 | Binding | 0.523 | 0.734 | 0.375 | -4.197 | Likely Benign | 0.714 | Likely Pathogenic | Likely Benign | 0.397 | Likely Benign | -2.20 | Neutral | 0.818 | Possibly Damaging | 0.353 | Benign | 5.42 | Benign | 0.01 | Affected | 0.2665 | 0.5614 | 0 | -2 | 3.1 | -72.06 | ||||||||||||||||||||||||||||||||||||||
| c.3521A>T | E1174V 2D  AIThe SynGAP1 missense variant E1174V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split opinion: benign calls come from PROVEAN, ESM1b, and FATHMM, while pathogenic calls are made by REVEL, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. Grouping by consensus, the majority of tools (four out of six) predict a benign effect, whereas the remaining four predict pathogenicity. High‑accuracy assessments further clarify the picture: AlphaMissense‑Optimized returns an uncertain result; the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates a likely benign outcome. No Foldetta stability analysis is available for this residue. Overall, the preponderance of evidence leans toward a benign impact for E1174V, and this assessment does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.483068 | Structured | 0.618958 | Binding | 0.523 | 0.734 | 0.375 | -4.814 | Likely Benign | 0.877 | Likely Pathogenic | Ambiguous | 0.515 | Likely Pathogenic | -2.41 | Neutral | 0.965 | Probably Damaging | 0.703 | Possibly Damaging | 5.41 | Benign | 0.01 | Affected | 0.0539 | 0.6623 | -2 | -2 | 7.7 | -29.98 | ||||||||||||||||||||||||||||||||||||||
| c.3522G>T | E1174D 2D  AIThe SynGAP1 missense change E1174D is not reported in ClinVar and is absent from gnomAD. In silico prediction tools that assess sequence conservation and structural impact (REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) all classify the variant as benign. No tool predicts pathogenicity. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields a “Likely Benign” verdict, while AlphaMissense‑Optimized also reports benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, every available prediction supports a benign effect, and this conclusion is consistent with the lack of a ClinVar classification. Thus, the variant is most likely benign and does not contradict ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.483068 | Structured | 0.618958 | Binding | 0.523 | 0.734 | 0.375 | -4.257 | Likely Benign | 0.253 | Likely Benign | Likely Benign | 0.234 | Likely Benign | -0.19 | Neutral | 0.002 | Benign | 0.006 | Benign | 5.45 | Benign | 0.36 | Tolerated | 4.32 | 2 | 0.1557 | 0.4226 | 2 | 3 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||||||||
| c.3523C>G | R1175G 2D  AIThe SynGAP1 missense variant R1175G is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, and Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.538167 | Disordered | 0.589347 | Binding | 0.545 | 0.732 | 0.375 | -4.888 | Likely Benign | 0.865 | Likely Pathogenic | Ambiguous | 0.477 | Likely Benign | -1.64 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 5.35 | Benign | 0.00 | Affected | 0.3006 | 0.2350 | -3 | -2 | 4.1 | -99.14 | ||||||||||||||||||||||||||||||||||||||
| c.3524G>C | R1175P 2D  AIThe SynGAP1 missense variant R1175P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show mixed results: benign predictions come from PROVEAN, ESM1b, and FATHMM, while pathogenic predictions are returned by REVEL, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates a likely benign outcome. High‑accuracy assessments further support this: AlphaMissense‑Optimized predicts pathogenic, whereas the SGM‑Consensus (majority vote) predicts benign. No Foldetta stability analysis is available. Overall, the majority of high‑confidence tools lean toward a benign interpretation, and this is consistent with the absence of ClinVar evidence. Therefore, the variant is most likely benign, and there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.538167 | Disordered | 0.589347 | Binding | 0.545 | 0.732 | 0.375 | -4.746 | Likely Benign | 0.970 | Likely Pathogenic | Likely Pathogenic | 0.518 | Likely Pathogenic | -0.80 | Neutral | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 5.37 | Benign | 0.00 | Affected | 0.1715 | 0.3223 | 0 | -2 | 2.9 | -59.07 | ||||||||||||||||||||||||||||||||||||||
| c.3524G>T | R1175L 2D  AISynGAP1 missense variant R1175L is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools show a split: benign predictions come from PROVEAN, ESM1b, and FATHMM, while pathogenic predictions arise from REVEL, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further refine the picture: AlphaMissense‑Optimized is Uncertain, SGM‑Consensus remains Likely Benign, and Foldetta data are unavailable. Overall, the majority of individual predictors lean toward pathogenicity, yet the consensus of the most reliable tools suggests a benign outcome, leaving the variant’s clinical significance ambiguous. Consequently, the variant is most likely pathogenic based on the bulk of predictions, and this assessment does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.538167 | Disordered | 0.589347 | Binding | 0.545 | 0.732 | 0.375 | -2.560 | Likely Benign | 0.876 | Likely Pathogenic | Ambiguous | 0.535 | Likely Pathogenic | -2.37 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 5.38 | Benign | 0.00 | Affected | 0.1240 | 0.2849 | -3 | -2 | 8.3 | -43.03 | ||||||||||||||||||||||||||||||||||||||
| c.3526G>A | E1176K 2D  AIThe SynGAP1 E1176K missense change is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves as Likely Benign. High‑accuracy assessments further show AlphaMissense‑Optimized as Pathogenic, SGM‑Consensus as Likely Benign, and Foldetta (combining FoldX‑MD and Rosetta) is not available for this residue. Because the majority of evidence, including the consensus score, points to a benign effect and no ClinVar entry contradicts this, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.538167 | Disordered | 0.572075 | Binding | 0.525 | 0.715 | 0.250 | -4.240 | Likely Benign | 0.959 | Likely Pathogenic | Likely Pathogenic | 0.400 | Likely Benign | -1.41 | Neutral | 0.995 | Probably Damaging | 0.949 | Probably Damaging | 5.54 | Benign | 0.18 | Tolerated | 0.1658 | 0.6321 | 0 | 1 | -0.4 | -0.94 | ||||||||||||||||||||||||||||||||||||||
| c.3526G>C | E1176Q 2D  AIThe SynGAP1 missense variant E1176Q is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while polyPhen‑2 (HumDiv and HumVar) and AlphaMissense‑Default predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the balance of evidence favors a benign interpretation; this conclusion is not contradicted by ClinVar status, which currently contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.538167 | Disordered | 0.572075 | Binding | 0.525 | 0.715 | 0.250 | -3.881 | Likely Benign | 0.860 | Likely Pathogenic | Ambiguous | 0.372 | Likely Benign | -1.19 | Neutral | 0.995 | Probably Damaging | 0.963 | Probably Damaging | 5.45 | Benign | 0.18 | Tolerated | 0.0788 | 0.6068 | 2 | 2 | 0.0 | -0.98 | ||||||||||||||||||||||||||||||||||||||
| c.3518T>C | I1173T 2D  AIThe SynGAP1 missense variant I1173T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect for I1173T, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.501700 | Disordered | 0.653145 | Binding | 0.521 | 0.756 | 0.375 | -3.162 | Likely Benign | 0.441 | Ambiguous | Likely Benign | 0.405 | Likely Benign | -1.18 | Neutral | 0.451 | Benign | 0.265 | Benign | 5.46 | Benign | 0.05 | Affected | 0.1016 | 0.0821 | 0 | -1 | -5.2 | -12.05 | ||||||||||||||||||||||||||||||||||||||
| c.3518T>A | I1173N 2D  AIThe SynGAP1 missense variant I1173N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates a likely benign effect. High‑accuracy assessments further support this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also leans benign. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Taken together, the preponderance of evidence points to a benign impact for I1173N, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.501700 | Disordered | 0.653145 | Binding | 0.521 | 0.756 | 0.375 | -4.130 | Likely Benign | 0.691 | Likely Pathogenic | Likely Benign | 0.443 | Likely Benign | -1.46 | Neutral | 0.925 | Possibly Damaging | 0.611 | Possibly Damaging | 5.34 | Benign | 0.02 | Affected | 0.0920 | 0.0142 | -2 | -3 | -8.0 | 0.94 | ||||||||||||||||||||||||||||||||||||||
| c.3512C>A | A1171D 2D  AIThe SynGAP1 missense variant A1171D is not reported in ClinVar and has no entries in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain,” and Foldetta results are unavailable. Overall, the balance of evidence from multiple independent predictors and the SGM‑Consensus points to a benign impact for A1171D. This conclusion is consistent with the absence of a ClinVar classification, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.599170 | Disordered | 0.702689 | Binding | 0.472 | 0.775 | 0.500 | -3.897 | Likely Benign | 0.814 | Likely Pathogenic | Ambiguous | 0.312 | Likely Benign | -0.80 | Neutral | 0.611 | Possibly Damaging | 0.326 | Benign | 5.34 | Benign | 0.02 | Affected | 0.1952 | 0.2494 | 0 | -2 | -5.3 | 44.01 | ||||||||||||||||||||||||||||||||||||||
| c.3512C>G | A1171G 2D  AIThe SynGAP1 missense variant A1171G (Ala→Gly at residue 1171) is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly classify it as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate a benign effect, and no tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome, while Foldetta results are unavailable. Consequently, the variant is most likely benign, and this assessment does not contradict any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.599170 | Disordered | 0.702689 | Binding | 0.472 | 0.775 | 0.500 | -3.487 | Likely Benign | 0.286 | Likely Benign | Likely Benign | 0.141 | Likely Benign | -0.60 | Neutral | 0.245 | Benign | 0.138 | Benign | 5.38 | Benign | 0.08 | Tolerated | 0.1957 | 0.3201 | 1 | 0 | -2.2 | -14.03 | ||||||||||||||||||||||||||||||||||||||
| c.3512C>T | A1171V 2D  AIThe SynGAP1 missense variant A1171V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate a benign effect, and no tool predicts pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) is likely benign; Foldetta results are not available. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.599170 | Disordered | 0.702689 | Binding | 0.472 | 0.775 | 0.500 | -3.516 | Likely Benign | 0.325 | Likely Benign | Likely Benign | 0.152 | Likely Benign | -0.72 | Neutral | 0.245 | Benign | 0.138 | Benign | 5.34 | Benign | 0.11 | Tolerated | 0.1019 | 0.4611 | 0 | 0 | 2.4 | 28.05 | ||||||||||||||||||||||||||||||||||||||
| c.3514C>A | H1172N 2D  AIThe SynGAP1 missense variant H1172N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as tolerated or benign. Only two tools—polyPhen‑2 HumDiv and SIFT—suggest a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates a benign outcome. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the available predictions points to a benign effect, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.626927 | Disordered | 0.673805 | Binding | 0.465 | 0.758 | 0.625 | -2.770 | Likely Benign | 0.282 | Likely Benign | Likely Benign | 0.255 | Likely Benign | -1.14 | Neutral | 0.625 | Possibly Damaging | 0.265 | Benign | 5.59 | Benign | 0.04 | Affected | 0.1512 | 0.2158 | 2 | 1 | -0.3 | -23.04 | ||||||||||||||||||||||||||||||||||||||
| c.3514C>G | H1172D 2D  AIThe SynGAP1 missense variant H1172D is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and polyPhen‑2 HumVar. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.626927 | Disordered | 0.673805 | Binding | 0.465 | 0.758 | 0.625 | -2.073 | Likely Benign | 0.710 | Likely Pathogenic | Likely Benign | 0.378 | Likely Benign | -1.29 | Neutral | 0.625 | Possibly Damaging | 0.333 | Benign | 5.46 | Benign | 0.04 | Affected | 0.2296 | 0.1417 | 1 | -1 | -0.3 | -22.05 | ||||||||||||||||||||||||||||||||||||||
| c.3514C>T | H1172Y 2D  AIThe SynGAP1 H1172Y missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports the variant as Likely Benign, while AlphaMissense‑Default remains Uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Benign, and the Foldetta stability analysis is unavailable. Based on the preponderance of benign predictions and the lack of pathogenic evidence, the variant is most likely benign. This conclusion is not contradicted by ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.626927 | Disordered | 0.673805 | Binding | 0.465 | 0.758 | 0.625 | -2.820 | Likely Benign | 0.373 | Ambiguous | Likely Benign | 0.356 | Likely Benign | -1.01 | Neutral | 0.925 | Possibly Damaging | 0.529 | Possibly Damaging | 5.42 | Benign | 0.01 | Affected | 0.0693 | 0.4335 | 0 | 2 | 1.9 | 26.03 | ||||||||||||||||||||||||||||||||||||||
| c.3515A>C | H1172P 2D  AIThe SynGAP1 missense variant H1172P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show mixed results: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. Foldetta, a protein‑folding stability predictor, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.626927 | Disordered | 0.673805 | Binding | 0.465 | 0.758 | 0.625 | -2.871 | Likely Benign | 0.653 | Likely Pathogenic | Likely Benign | 0.403 | Likely Benign | -2.09 | Neutral | 0.925 | Possibly Damaging | 0.529 | Possibly Damaging | 5.42 | Benign | 0.02 | Affected | 0.2033 | 0.4128 | 0 | -2 | 1.6 | -40.02 | ||||||||||||||||||||||||||||||||||||||
| c.3515A>G | H1172R 2D  AIThe SynGAP1 missense variant H1172R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that H1172R is most likely benign, and this conclusion does not contradict any ClinVar status, as none is assigned to the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.626927 | Disordered | 0.673805 | Binding | 0.465 | 0.758 | 0.625 | -0.848 | Likely Benign | 0.285 | Likely Benign | Likely Benign | 0.173 | Likely Benign | -1.08 | Neutral | 0.001 | Benign | 0.004 | Benign | 5.46 | Benign | 0.05 | Affected | 0.1547 | 0.2219 | 2 | 0 | -1.3 | 19.05 | ||||||||||||||||||||||||||||||||||||||
| c.3515A>T | H1172L 2D  AIThe SynGAP1 missense variant H1172L is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect for H1172L, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.626927 | Disordered | 0.673805 | Binding | 0.465 | 0.758 | 0.625 | -0.545 | Likely Benign | 0.446 | Ambiguous | Likely Benign | 0.426 | Likely Benign | -2.30 | Neutral | 0.451 | Benign | 0.265 | Benign | 5.47 | Benign | 0.01 | Affected | 0.0815 | 0.5285 | -2 | -3 | 7.0 | -23.98 | ||||||||||||||||||||||||||||||||||||||
| c.3516C>A | H1172Q 2D  AIThe SynGAP1 missense variant H1172Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. No tool predicts a pathogenic outcome; AlphaMissense‑Default is uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus confirms Likely Benign, and Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.626927 | Disordered | 0.673805 | Binding | 0.465 | 0.758 | 0.625 | -2.169 | Likely Benign | 0.414 | Ambiguous | Likely Benign | 0.227 | Likely Benign | -0.51 | Neutral | 0.451 | Benign | 0.265 | Benign | 5.47 | Benign | 0.39 | Tolerated | 0.1249 | 0.3557 | 3 | 0 | -0.3 | -9.01 | ||||||||||||||||||||||||||||||||||||||
| c.3516C>G | H1172Q 2D  AIThe SynGAP1 missense variant H1172Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. No tool predicts a pathogenic outcome; AlphaMissense‑Default is uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus confirms Likely Benign, and Foldetta (combining FoldX‑MD and Rosetta) has no available result for this variant. Based on the collective predictions, H1172Q is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.626927 | Disordered | 0.673805 | Binding | 0.465 | 0.758 | 0.625 | -2.169 | Likely Benign | 0.414 | Ambiguous | Likely Benign | 0.227 | Likely Benign | -0.51 | Neutral | 0.451 | Benign | 0.265 | Benign | 5.47 | Benign | 0.39 | Tolerated | 0.1249 | 0.3557 | 3 | 0 | -0.3 | -9.01 | ||||||||||||||||||||||||||||||||||||||
| c.3517A>C | I1173L 2D  AIThe SynGAP1 missense variant I1173L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly classify the change as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate a benign effect. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the evidence strongly supports a benign classification, and there is no conflict with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.501700 | Disordered | 0.653145 | Binding | 0.521 | 0.756 | 0.375 | -1.792 | Likely Benign | 0.202 | Likely Benign | Likely Benign | 0.218 | Likely Benign | -0.30 | Neutral | 0.152 | Benign | 0.102 | Benign | 5.41 | Benign | 0.97 | Tolerated | 0.0745 | 0.3004 | 2 | 2 | -0.7 | 0.00 | ||||||||||||||||||||||||||||||||||||||
| c.3517A>T | I1173F 2D  AIThe SynGAP1 missense variant I1173F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (both HumDiv and HumVar) predict a pathogenic outcome. AlphaMissense‑Default remains uncertain, and the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for I1173F, and this conclusion does not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.501700 | Disordered | 0.653145 | Binding | 0.521 | 0.756 | 0.375 | -3.635 | Likely Benign | 0.491 | Ambiguous | Likely Benign | 0.365 | Likely Benign | -0.87 | Neutral | 0.934 | Possibly Damaging | 0.636 | Possibly Damaging | 5.39 | Benign | 0.13 | Tolerated | 0.0567 | 0.2108 | 1 | 0 | -1.7 | 34.02 | ||||||||||||||||||||||||||||||||||||||
| c.3527A>C | E1176A 2D  AIThe SynGAP1 E1176A missense change is not reported in ClinVar and has no gnomAD entry. Consensus from multiple in‑silico predictors shows a split: benign‑oriented tools (REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and the SGM‑Consensus, which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) all indicate a benign effect, whereas pathogenic‑oriented tools (polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default) predict a deleterious impact. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and the Foldetta stability assessment is unavailable. Taking the overall evidence together, the variant is most likely benign; this assessment does not conflict with ClinVar, which contains no entry for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | 0.538167 | Disordered | 0.572075 | Binding | 0.525 | 0.715 | 0.250 | -3.164 | Likely Benign | 0.909 | Likely Pathogenic | Ambiguous | 0.411 | Likely Benign | -1.95 | Neutral | 0.995 | Probably Damaging | 0.924 | Probably Damaging | 5.55 | Benign | 0.19 | Tolerated | 0.3160 | 0.5889 | 0 | -1 | 5.3 | -58.04 | ||||||||||||||||||||||||||||||||||||||
| c.34A>C | S12R 2D  AIThe SynGAP1 missense variant S12R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support this: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority of the four high‑accuracy tools) also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.480142 | Structured | 0.490599 | Uncertain | 0.355 | 0.916 | 0.500 | -4.033 | Likely Benign | 0.500 | Ambiguous | Likely Benign | 0.116 | Likely Benign | -0.30 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.09 | Benign | 0.00 | Affected | 4.32 | 1 | 0.0944 | 0.3678 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||
| c.3473T>C | V1158A 2D  AIThe SynGAP1 missense variant V1158A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b. Tools that agree on a pathogenic effect include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the balance of evidence (six pathogenic vs. three benign predictions) indicates the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.599170 | Disordered | 0.877504 | Binding | 0.369 | 0.847 | 0.250 | -2.726 | Likely Benign | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.232 | Likely Benign | -2.46 | Neutral | 0.992 | Probably Damaging | 0.989 | Probably Damaging | 2.34 | Pathogenic | 0.02 | Affected | 0.2789 | 0.2906 | 0 | 0 | -2.4 | -28.05 | ||||||||||||||||||||||||||||||||||||||||
| c.347A>T | Y116F 2D  AIThe SynGAP1 missense variant Y116F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only polyPhen‑2 HumDiv predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign likelihood. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.670235 | Binding | 0.381 | 0.878 | 0.625 | -2.911 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.048 | Likely Benign | -0.71 | Neutral | 0.539 | Possibly Damaging | 0.042 | Benign | 4.21 | Benign | 0.33 | Tolerated | 0.2632 | 0.3069 | 7 | 3 | 4.1 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.3480C>A | N1160K 2D  AIThe SynGAP1 missense variant N1160K is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split: benign calls come from REVEL and ESM1b, whereas pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. No Foldetta stability analysis is available for this residue. Overall, the majority of evidence points to a pathogenic impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.861611 | Binding | 0.361 | 0.836 | 0.375 | -3.768 | Likely Benign | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.188 | Likely Benign | -3.92 | Deleterious | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 1.83 | Pathogenic | 0.04 | Affected | 0.1919 | 0.5017 | 1 | 0 | -0.4 | 14.07 | |||||||||||||||||||||||||||||||||||||||
| c.3480C>G | N1160K 2D  AIThe SynGAP1 missense variant N1160K is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split: benign calls come from REVEL and ESM1b, whereas pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. No Foldetta stability analysis is available for this residue. Overall, the majority of evidence points to a pathogenic impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.861611 | Binding | 0.361 | 0.836 | 0.375 | -3.768 | Likely Benign | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.189 | Likely Benign | -3.92 | Deleterious | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 1.83 | Pathogenic | 0.04 | Affected | 0.1919 | 0.5017 | 1 | 0 | -0.4 | 14.07 | |||||||||||||||||||||||||||||||||||||||
| c.3481A>C | M1161L 2D  AIThe SynGAP1 missense variant M1161L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and ESM1b. Tools that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool rates the variant as uncertain, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split, and Foldetta results are unavailable. Consequently, the available computational evidence is conflicting, with an equal number of benign and pathogenic predictions and no definitive high‑accuracy support. The variant is most likely of uncertain significance; this lack of consensus is consistent with its absence from ClinVar and gnomAD. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.580690 | Disordered | 0.864109 | Binding | 0.372 | 0.830 | 0.375 | -2.129 | Likely Benign | 0.862 | Likely Pathogenic | Ambiguous | 0.155 | Likely Benign | -1.59 | Neutral | 0.699 | Possibly Damaging | 0.833 | Possibly Damaging | 2.38 | Pathogenic | 0.48 | Tolerated | 0.1614 | 0.4244 | 4 | 2 | 1.9 | -18.03 | ||||||||||||||||||||||||||||||||||||||||
| c.3481A>T | M1161L 2D  AISynGAP1 missense variant M1161L is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and ESM1b, while those that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Default. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive because it yields a 2‑to‑2 split. High‑accuracy methods give mixed results: AlphaMissense‑Optimized is uncertain, and both the SGM Consensus and Foldetta (which would combine FoldX‑MD and Rosetta outputs) are unavailable. Consequently, the overall computational evidence does not strongly favor either outcome. The variant is most likely inconclusive in pathogenicity prediction, and this does not contradict the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.580690 | Disordered | 0.864109 | Binding | 0.372 | 0.830 | 0.375 | -2.129 | Likely Benign | 0.862 | Likely Pathogenic | Ambiguous | 0.156 | Likely Benign | -1.59 | Neutral | 0.699 | Possibly Damaging | 0.833 | Possibly Damaging | 2.38 | Pathogenic | 0.48 | Tolerated | 0.1614 | 0.4244 | 4 | 2 | 1.9 | -18.03 | ||||||||||||||||||||||||||||||||||||||||
| c.3482T>A | M1161K 2D  AIThe SynGAP1 missense variant M1161K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, SIFT, and ESM1b, whereas tools that predict a pathogenic effect include PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic and the SGM‑Consensus labeling it as Likely Pathogenic; Foldetta results are unavailable. Overall, the majority of computational predictions support a pathogenic impact, and this conclusion is not contradicted by any ClinVar annotation. Thus, the variant is most likely pathogenic based on the available predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.580690 | Disordered | 0.864109 | Binding | 0.372 | 0.830 | 0.375 | -3.005 | Likely Benign | 0.994 | Likely Pathogenic | Likely Pathogenic | 0.236 | Likely Benign | -2.91 | Deleterious | 0.968 | Probably Damaging | 0.969 | Probably Damaging | 2.19 | Pathogenic | 0.17 | Tolerated | 0.1629 | 0.0828 | 0 | -1 | -5.8 | -3.02 | |||||||||||||||||||||||||||||||||||||||
| c.3482T>G | M1161R 2D  AIThe SynGAP1 missense variant M1161R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, SIFT, and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN)—predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic and the SGM‑Consensus as likely pathogenic; Foldetta results are unavailable. Based on the overall distribution of predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.580690 | Disordered | 0.864109 | Binding | 0.372 | 0.830 | 0.375 | -2.653 | Likely Benign | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.220 | Likely Benign | -2.97 | Deleterious | 0.968 | Probably Damaging | 0.978 | Probably Damaging | 2.18 | Pathogenic | 0.13 | Tolerated | 0.1689 | 0.0809 | 0 | -1 | -6.4 | 24.99 | |||||||||||||||||||||||||||||||||||||||
| c.3483G>A | M1161I 2D  AIThe SynGAP1 missense variant M1161I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and ESM1b. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of evidence (five pathogenic vs. four benign predictions) indicates that the variant is most likely pathogenic. This conclusion is not contradicted by ClinVar status, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.580690 | Disordered | 0.864109 | Binding | 0.372 | 0.830 | 0.375 | -3.124 | Likely Benign | 0.994 | Likely Pathogenic | Likely Pathogenic | 0.155 | Likely Benign | -1.92 | Neutral | 0.925 | Possibly Damaging | 0.954 | Probably Damaging | 2.27 | Pathogenic | 0.25 | Tolerated | 3.77 | 5 | 0.1437 | 0.3190 | 1 | 2 | 2.6 | -18.03 | ||||||||||||||||||||||||||||||||||||||
| c.3483G>C | M1161I 2D  AIThe SynGAP1 missense variant M1161I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and ESM1b. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs. four benign) indicate that the variant is most likely pathogenic. This conclusion is not contradicted by ClinVar status, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.580690 | Disordered | 0.864109 | Binding | 0.372 | 0.830 | 0.375 | -3.124 | Likely Benign | 0.994 | Likely Pathogenic | Likely Pathogenic | 0.155 | Likely Benign | -1.92 | Neutral | 0.925 | Possibly Damaging | 0.954 | Probably Damaging | 2.27 | Pathogenic | 0.25 | Tolerated | 3.77 | 5 | 0.1437 | 0.3190 | 1 | 2 | 2.6 | -18.03 | ||||||||||||||||||||||||||||||||||||||
| c.3484C>A | P1162T 2D  AIThe SynGAP1 missense variant P1162T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, with no conflict with ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.858809 | Binding | 0.366 | 0.823 | 0.375 | -3.466 | Likely Benign | 0.879 | Likely Pathogenic | Ambiguous | 0.171 | Likely Benign | -2.15 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.71 | Benign | 0.22 | Tolerated | 0.1395 | 0.6097 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.3484C>G | P1162A 2D  AIThe SynGAP1 missense variant P1162A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.858809 | Binding | 0.366 | 0.823 | 0.375 | -2.594 | Likely Benign | 0.878 | Likely Pathogenic | Ambiguous | 0.169 | Likely Benign | -2.33 | Neutral | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.74 | Benign | 0.41 | Tolerated | 0.3437 | 0.5655 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.3484C>T | P1162S 2D  AIThe SynGAP1 missense variant P1162S is listed in ClinVar (ID 2287942.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM. Those that predict a pathogenic effect are PolyPhen‑2 HumDiv, PolyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. AlphaMissense‑Optimized is uncertain, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the majority of high‑accuracy and consensus predictions lean toward a benign impact. Thus, the variant is most likely benign, which is consistent with its ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.858809 | Binding | 0.366 | 0.823 | 0.375 | Uncertain | 1 | -2.118 | Likely Benign | 0.913 | Likely Pathogenic | Ambiguous | 0.215 | Likely Benign | -1.93 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.73 | Benign | 0.55 | Tolerated | 3.88 | 3 | 0.3386 | 0.6055 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||
| c.3485C>A | P1162H 2D  AIThe SynGAP1 missense variant P1162H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as Pathogenic, whereas the SGM‑Consensus (majority vote) indicates Likely Benign; Foldetta results are unavailable. Overall, the majority of tools (six benign vs. four pathogenic) lean toward a benign interpretation, but the high‑accuracy AlphaMissense‑Optimized prediction conflicts with the consensus. Because ClinVar contains no entry, there is no contradiction with clinical database status. Based on the collective predictions, the variant is most likely benign, though the AlphaMissense‑Optimized result suggests caution. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.858809 | Binding | 0.366 | 0.823 | 0.375 | -3.733 | Likely Benign | 0.972 | Likely Pathogenic | Likely Pathogenic | 0.168 | Likely Benign | -2.46 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.67 | Benign | 0.17 | Tolerated | 0.1481 | 0.5001 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.347A>G | Y116C 2D  AIThe SynGAP1 missense variant Y116C is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods indicates that the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.670235 | Binding | 0.381 | 0.878 | 0.625 | -2.822 | Likely Benign | 0.197 | Likely Benign | Likely Benign | 0.160 | Likely Benign | -0.90 | Neutral | 0.804 | Possibly Damaging | 0.187 | Benign | 4.19 | Benign | 0.11 | Tolerated | 0.3208 | 0.2105 | 0 | -2 | 3.8 | -60.04 | |||||||||||||||||||||||||||||||||||||||
| c.347A>C | Y116S 2D  AIThe SynGAP1 missense variant Y116S is not reported in ClinVar and is absent from gnomAD, indicating no documented clinical or population evidence. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this view: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of all available predictions strongly suggests that Y116S is most likely benign, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.670235 | Binding | 0.381 | 0.878 | 0.625 | -0.249 | Likely Benign | 0.269 | Likely Benign | Likely Benign | 0.178 | Likely Benign | -0.05 | Neutral | 0.033 | Benign | 0.013 | Benign | 4.31 | Benign | 0.58 | Tolerated | 0.5111 | 0.1694 | Weaken | -3 | -2 | 0.5 | -76.10 | ||||||||||||||||||||||||||||||||||||||
| c.3473T>G | V1158G 2D  AIThe SynGAP1 missense variant V1158G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect are REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic outcome. High‑accuracy assessments reinforce this pattern: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a pathogenic verdict. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that V1158G is most likely pathogenic, and this conclusion does not contradict the current ClinVar status, which has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.599170 | Disordered | 0.877504 | Binding | 0.369 | 0.847 | 0.250 | -3.058 | Likely Benign | 0.986 | Likely Pathogenic | Likely Pathogenic | 0.433 | Likely Benign | -4.62 | Deleterious | 0.997 | Probably Damaging | 0.999 | Probably Damaging | 2.30 | Pathogenic | 0.00 | Affected | 0.1870 | 0.3289 | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.3475T>A | S1159T 2D  AIThe SynGAP1 missense variant S1159T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. The variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.626927 | Disordered | 0.867068 | Binding | 0.343 | 0.846 | 0.375 | -4.057 | Likely Benign | 0.207 | Likely Benign | Likely Benign | 0.076 | Likely Benign | -0.59 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.68 | Benign | 0.28 | Tolerated | 0.1114 | 0.5715 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3475T>C | S1159P 2D  AIThe SynGAP1 missense variant S1159P is not reported in ClinVar and has no gnomAD entry. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and the SGM‑Consensus score, which is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN. Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote) is benign. No Foldetta stability prediction is available for this residue. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.626927 | Disordered | 0.867068 | Binding | 0.343 | 0.846 | 0.375 | -3.656 | Likely Benign | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.188 | Likely Benign | -2.06 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.64 | Benign | 0.14 | Tolerated | 0.1807 | 0.5111 | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||||||
| c.3475T>G | S1159A 2D  AIThe SynGAP1 missense variant S1159A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports it as likely benign. In contrast, polyPhen‑2 (HumDiv and HumVar) predict the variant to be pathogenic. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.626927 | Disordered | 0.867068 | Binding | 0.343 | 0.846 | 0.375 | -3.653 | Likely Benign | 0.327 | Likely Benign | Likely Benign | 0.095 | Likely Benign | -0.46 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.71 | Benign | 0.44 | Tolerated | 0.4754 | 0.4541 | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.3476C>A | S1159Y 2D  AIThe SynGAP1 missense variant S1159Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of evidence (six benign predictions versus five pathogenic, plus a benign consensus) points to a likely benign impact for S1159Y. This conclusion does not contradict ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.626927 | Disordered | 0.867068 | Binding | 0.343 | 0.846 | 0.375 | -5.665 | Likely Benign | 0.957 | Likely Pathogenic | Likely Pathogenic | 0.195 | Likely Benign | -1.57 | Neutral | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 2.65 | Benign | 0.07 | Tolerated | 0.0567 | 0.4923 | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||||||
| c.3476C>G | S1159C 2D  AIThe SynGAP1 missense variant S1159C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this assessment, so the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.626927 | Disordered | 0.867068 | Binding | 0.343 | 0.846 | 0.375 | -6.650 | Likely Benign | 0.591 | Likely Pathogenic | Likely Benign | 0.172 | Likely Benign | -1.22 | Neutral | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.63 | Benign | 0.06 | Tolerated | 0.0807 | 0.5668 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||||||
| c.3476C>T | S1159F 2D  AIThe SynGAP1 missense variant S1159F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) remains benign; Foldetta stability analysis is unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this conclusion does not contradict the absence of a ClinVar entry. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.626927 | Disordered | 0.867068 | Binding | 0.343 | 0.846 | 0.375 | -5.627 | Likely Benign | 0.976 | Likely Pathogenic | Likely Pathogenic | 0.181 | Likely Benign | -1.11 | Neutral | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 2.66 | Benign | 0.20 | Tolerated | 0.0527 | 0.5211 | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||||||||||||
| c.3478A>C | N1160H 2D  AIThe SynGAP1 missense variant N1160H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas a majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is unavailable for this variant. Overall, the preponderance of evidence from multiple in silico predictors points to a pathogenic effect. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.861611 | Binding | 0.361 | 0.836 | 0.375 | -3.704 | Likely Benign | 0.848 | Likely Pathogenic | Ambiguous | 0.262 | Likely Benign | -3.44 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.79 | Pathogenic | 0.02 | Affected | 0.1152 | 0.6583 | 2 | 1 | 0.3 | 23.04 | |||||||||||||||||||||||||||||||||||||||
| c.3478A>G | N1160D 2D  AIThe SynGAP1 missense variant at position N1160D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, SIFT, and ESM1b, whereas a majority of tools predict a pathogenic outcome: PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is labeled Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.861611 | Binding | 0.361 | 0.836 | 0.375 | -3.222 | Likely Benign | 0.978 | Likely Pathogenic | Likely Pathogenic | 0.225 | Likely Benign | -3.22 | Deleterious | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 1.84 | Pathogenic | 0.08 | Tolerated | 0.1618 | 0.3920 | 2 | 1 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.3478A>T | N1160Y 2D  AIThe SynGAP1 missense variant N1160Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—SGM‑Consensus, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Pathogenic.” No Foldetta stability prediction is available. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.861611 | Binding | 0.361 | 0.836 | 0.375 | -4.074 | Likely Benign | 0.978 | Likely Pathogenic | Likely Pathogenic | 0.421 | Likely Benign | -4.90 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.79 | Pathogenic | 0.01 | Affected | 0.0591 | 0.5681 | -2 | -2 | 2.2 | 49.07 | |||||||||||||||||||||||||||||||||||||||
| c.3479A>C | N1160T 2D  AIThe SynGAP1 missense variant N1160T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, SIFT, and ESM1b, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) is unavailable for this variant. Overall, the majority of evidence points to a pathogenic impact, and this conclusion does not contradict any existing ClinVar annotation, as none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.861611 | Binding | 0.361 | 0.836 | 0.375 | -3.017 | Likely Benign | 0.889 | Likely Pathogenic | Ambiguous | 0.229 | Likely Benign | -3.61 | Deleterious | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 1.81 | Pathogenic | 0.63 | Tolerated | 0.1147 | 0.6759 | 0 | 0 | 2.8 | -13.00 | |||||||||||||||||||||||||||||||||||||||
| c.3479A>G | N1160S 2D  AIThe SynGAP1 missense variant N1160S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) predicts pathogenic. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the variant is most likely pathogenic based on the high‑accuracy consensus, and this assessment does not contradict ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.585406 | Disordered | 0.861611 | Binding | 0.361 | 0.836 | 0.375 | -1.880 | Likely Benign | 0.550 | Ambiguous | Likely Benign | 0.228 | Likely Benign | -3.26 | Deleterious | 0.997 | Probably Damaging | 0.989 | Probably Damaging | 1.83 | Pathogenic | 0.15 | Tolerated | 0.3459 | 0.6304 | 1 | 1 | 2.7 | -27.03 | ||||||||||||||||||||||||||||||||||||||||
| c.3479A>T | N1160I 2D  AIThe SynGAP1 missense variant N1160I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—SGM‑Consensus, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict a pathogenic or likely pathogenic outcome. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as likely pathogenic. No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence from multiple in silico tools indicates that the variant is most likely pathogenic, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.861611 | Binding | 0.361 | 0.836 | 0.375 | -4.996 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.440 | Likely Benign | -5.35 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.79 | Pathogenic | 0.01 | Affected | 0.0618 | 0.5903 | -2 | -3 | 8.0 | -0.94 | |||||||||||||||||||||||||||||||||||||||
| c.3485C>G | P1162R 2D  AIThe SynGAP1 missense variant P1162R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, and FATHMM. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of predictions (5 pathogenic vs 4 benign) and the pathogenic call from AlphaMissense‑Optimized suggest the variant is most likely pathogenic. This conclusion does not contradict ClinVar status, as the variant has no ClinVar entry. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.599170 | Disordered | 0.858809 | Binding | 0.366 | 0.823 | 0.375 | -2.657 | Likely Benign | 0.977 | Likely Pathogenic | Likely Pathogenic | 0.208 | Likely Benign | -2.68 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.70 | Benign | 0.14 | Tolerated | 0.1269 | 0.3439 | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||||||||
| c.3485C>T | P1162L 2D  AIThe SynGAP1 missense variant P1162L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, and FATHMM. Tools that agree on a pathogenic effect include PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenicity. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the balance of evidence (five pathogenic vs. four benign predictions) indicates that the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.599170 | Disordered | 0.858809 | Binding | 0.366 | 0.823 | 0.375 | -3.370 | Likely Benign | 0.962 | Likely Pathogenic | Likely Pathogenic | 0.209 | Likely Benign | -3.48 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.68 | Benign | 0.06 | Tolerated | 0.2153 | 0.7372 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||||||
| c.3487C>A | H1163N 2D  AIThe SynGAP1 missense variant H1163N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for H1163N, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.858469 | Binding | 0.328 | 0.825 | 0.375 | -3.219 | Likely Benign | 0.643 | Likely Pathogenic | Likely Benign | 0.280 | Likely Benign | -1.70 | Neutral | 0.991 | Probably Damaging | 0.988 | Probably Damaging | 5.47 | Benign | 0.17 | Tolerated | 0.1588 | 0.2759 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.3494C>G | S1165W 2D  AIThe SynGAP1 missense variant S1165W has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign); pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome; Foldetta stability analysis is unavailable. Overall, the evidence is split, with an equal number of tools supporting benign versus pathogenic effects. Consequently, the variant is most likely pathogenic based on the current computational predictions, and this assessment does not contradict any ClinVar status because none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.835017 | Binding | 0.308 | 0.807 | 0.375 | -6.419 | Likely Benign | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.195 | Likely Benign | -2.29 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.54 | Benign | 0.02 | Affected | 0.0671 | 0.4807 | -2 | -3 | -0.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.3494C>T | S1165L 2D  AIThe SynGAP1 missense variant S1165L is listed in ClinVar with an uncertain significance (ClinVar ID 225899.0) and is not reported in gnomAD. Functional prediction tools show a split: benign predictions come from REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while pathogenic predictions arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. Grouping by consensus, the benign‑predicted tools outnumber the pathogenic ones. High‑accuracy assessments further clarify the picture: the SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, leans toward benign (Likely Benign); AlphaMissense‑Optimized remains uncertain, and Foldetta data are unavailable. Overall, the majority of evidence points to a benign effect, aligning with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.835017 | Binding | 0.308 | 0.807 | 0.375 | Conflicting | 2 | -2.984 | Likely Benign | 0.793 | Likely Pathogenic | Ambiguous | 0.166 | Likely Benign | -2.01 | Neutral | 0.998 | Probably Damaging | 0.992 | Probably Damaging | 2.60 | Benign | 0.33 | Tolerated | 3.88 | 3 | 0.1133 | 0.4803 | -3 | -2 | 4.6 | 26.08 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||||||||||
| c.3496G>A | A1166T 2D  AIThe SynGAP1 missense variant A1166T is not reported in ClinVar and has no gnomAD entry. Consensus and most in silico predictors classify it as benign: SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate a benign effect. In contrast, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default predict a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports Likely Benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect, and this conclusion is not contradicted by ClinVar data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.811691 | Binding | 0.381 | 0.803 | 0.375 | -3.615 | Likely Benign | 0.748 | Likely Pathogenic | Likely Benign | 0.343 | Likely Benign | -1.13 | Neutral | 0.995 | Probably Damaging | 0.963 | Probably Damaging | 5.37 | Benign | 0.26 | Tolerated | 0.1328 | 0.6418 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3496G>C | A1166P 2D  AIThe SynGAP1 missense variant A1166P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as Pathogenic, whereas the SGM‑Consensus (majority vote) indicates Likely Benign; Foldetta results are unavailable. Overall, the predictions are mixed, with a slight bias toward benign, and there is no ClinVar entry to contradict the current assessment. Thus, the variant is most likely benign based on the collective evidence, though high‑accuracy tools provide conflicting signals. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.811691 | Binding | 0.381 | 0.803 | 0.375 | -2.890 | Likely Benign | 0.972 | Likely Pathogenic | Likely Pathogenic | 0.468 | Likely Benign | -1.09 | Neutral | 0.999 | Probably Damaging | 0.993 | Probably Damaging | 5.29 | Benign | 0.23 | Tolerated | 0.1774 | 0.4237 | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||||||
| c.3496G>T | A1166S 2D  AIThe SynGAP1 missense variant A1166S is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic impact. The AlphaMissense‑Default score is uncertain, and no Foldetta stability assessment is available. High‑accuracy methods that are available—AlphaMissense‑Optimized and the SGM‑Consensus—both support a benign classification. Consequently, the variant is most likely benign according to the majority of predictive evidence, and this assessment does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.811691 | Binding | 0.381 | 0.803 | 0.375 | -2.587 | Likely Benign | 0.396 | Ambiguous | Likely Benign | 0.308 | Likely Benign | -0.59 | Neutral | 0.995 | Probably Damaging | 0.949 | Probably Damaging | 5.41 | Benign | 0.35 | Tolerated | 0.2568 | 0.5239 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.3497C>A | A1166D 2D  AIThe SynGAP1 missense variant A1166D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Pathogenic, while the SGM‑Consensus remains Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence (five benign vs. four pathogenic) and the consensus score lean toward a benign interpretation, with no conflict with ClinVar status. Thus, the variant is most likely benign, although the AlphaMissense‑Optimized prediction introduces some uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.811691 | Binding | 0.381 | 0.803 | 0.375 | -4.204 | Likely Benign | 0.978 | Likely Pathogenic | Likely Pathogenic | 0.417 | Likely Benign | -1.56 | Neutral | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 5.29 | Benign | 0.42 | Tolerated | 0.1709 | 0.2104 | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||||||
| c.3497C>G | A1166G 2D  AISynGAP1 missense variant A1166G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only AlphaMissense‑Default predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the consensus of the majority of tools and the high‑accuracy predictions indicate that the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.811691 | Binding | 0.381 | 0.803 | 0.375 | -3.679 | Likely Benign | 0.745 | Likely Pathogenic | Likely Benign | 0.355 | Likely Benign | -1.17 | Neutral | 0.361 | Benign | 0.307 | Benign | 5.34 | Benign | 0.31 | Tolerated | 0.2244 | 0.3977 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3497C>T | A1166V 2D  AIThe SynGAP1 A1166V missense variant has no ClinVar record and is not listed in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect. The variant’s predicted benign status does not contradict any ClinVar annotation, as none exists. Thus, based on current computational predictions, the A1166V variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.811691 | Binding | 0.381 | 0.803 | 0.375 | -4.305 | Likely Benign | 0.843 | Likely Pathogenic | Ambiguous | 0.291 | Likely Benign | -1.04 | Neutral | 0.995 | Probably Damaging | 0.963 | Probably Damaging | 5.43 | Benign | 0.40 | Tolerated | 0.0967 | 0.5384 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||||||||||||||
| c.3499G>A | D1167N 2D  AIThe SynGAP1 missense variant D1167N is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evaluated predictors (five pathogenic vs. three benign) lean toward a pathogenic interpretation. This prediction does not contradict any ClinVar status, as the variant is not yet catalogued in that database. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.599170 | Disordered | 0.783999 | Binding | 0.336 | 0.798 | 0.500 | -3.671 | Likely Benign | 0.924 | Likely Pathogenic | Ambiguous | 0.180 | Likely Benign | -1.72 | Neutral | 0.995 | Probably Damaging | 0.963 | Probably Damaging | 2.33 | Pathogenic | 0.01 | Affected | 0.1315 | 0.7940 | 2 | 1 | 0.0 | -0.98 | ||||||||||||||||||||||||||||||||||||||||
| c.3499G>C | D1167H 2D  AIThe SynGAP1 missense variant D1167H is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b. Tools that agree on a pathogenic effect include polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic, two benign). Foldetta predictions are unavailable. Overall, the majority of evaluated tools (six pathogenic vs. three benign) indicate a pathogenic effect. This conclusion is consistent with the lack of ClinVar reporting; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.599170 | Disordered | 0.783999 | Binding | 0.336 | 0.798 | 0.500 | -3.774 | Likely Benign | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.257 | Likely Benign | -2.36 | Neutral | 1.000 | Probably Damaging | 0.995 | Probably Damaging | 2.27 | Pathogenic | 0.00 | Affected | 0.1514 | 0.8433 | 1 | -1 | 0.3 | 22.05 | ||||||||||||||||||||||||||||||||||||||||
| c.3499G>T | D1167Y 2D  AIThe SynGAP1 missense variant D1167Y is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a 2‑to‑2 split and is therefore inconclusive. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑confidence predictors (six pathogenic vs. three benign) indicate that D1167Y is most likely pathogenic. This assessment does not contradict any ClinVar status, as no ClinVar entry exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.599170 | Disordered | 0.783999 | Binding | 0.336 | 0.798 | 0.500 | -4.050 | Likely Benign | 0.990 | Likely Pathogenic | Likely Pathogenic | 0.281 | Likely Benign | -2.46 | Neutral | 1.000 | Probably Damaging | 0.995 | Probably Damaging | 2.25 | Pathogenic | 0.00 | Affected | 0.0640 | 0.7307 | -4 | -3 | 2.2 | 48.09 | ||||||||||||||||||||||||||||||||||||||||
| c.349A>C | S117R 2D  AIThe SynGAP1 missense variant S117R has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, whereas the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of predictions lean toward a benign impact, and this does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.672422 | Binding | 0.357 | 0.877 | 0.625 | -4.187 | Likely Benign | 0.971 | Likely Pathogenic | Likely Pathogenic | 0.276 | Likely Benign | -1.81 | Neutral | 0.845 | Possibly Damaging | 0.326 | Benign | 3.70 | Benign | 0.01 | Affected | 3.61 | 5 | 0.1040 | 0.3770 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||
| c.349A>G | S117G 2D  AIThe SynGAP1 missense variant S117G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this conclusion does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.672422 | Binding | 0.357 | 0.877 | 0.625 | -3.280 | Likely Benign | 0.174 | Likely Benign | Likely Benign | 0.122 | Likely Benign | -1.59 | Neutral | 0.390 | Benign | 0.066 | Benign | 3.73 | Benign | 0.01 | Affected | 0.2113 | 0.4627 | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3493T>G | S1165A 2D  AIThe SynGAP1 missense variant S1165A is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign, while the majority of other predictors (polyPhen‑2 HumDiv and HumVar) suggest pathogenic. When predictions are grouped by agreement, the benign‑predicating tools outnumber the pathogenic ones, and the single uncertain call from AlphaMissense‑Default does not alter this balance. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized classifies the variant as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports it as Likely Benign. Foldetta data are unavailable. Overall, the computational evidence points to a benign effect, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.835017 | Binding | 0.308 | 0.807 | 0.375 | -3.308 | Likely Benign | 0.476 | Ambiguous | Likely Benign | 0.106 | Likely Benign | -1.11 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.61 | Benign | 0.60 | Tolerated | 0.5363 | 0.4236 | Weaken | 1 | 1 | 2.6 | -16.00 | ||||||||||||||||||||||||||||||||||||||
| c.3493T>C | S1165P 2D  AIThe SynGAP1 missense variant S1165P is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which is a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Pathogenic, whereas the SGM‑Consensus remains Likely Benign; Foldetta results are unavailable. Overall, the majority of tools (six benign vs. four pathogenic) indicate that the variant is most likely benign. This conclusion is not contradicted by ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.835017 | Binding | 0.308 | 0.807 | 0.375 | -3.476 | Likely Benign | 0.975 | Likely Pathogenic | Likely Pathogenic | 0.147 | Likely Benign | -1.87 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.55 | Benign | 0.23 | Tolerated | 0.2295 | 0.4930 | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||||||
| c.3487C>G | H1163D 2D  AISynGAP1 missense variant H1163D is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, SIFT, ESM1b, and FATHMM, while pathogenic calls come from PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized rates the variant as uncertain, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields a tie and is therefore unavailable, and Foldetta folding‑stability analysis is not provided. With an equal number of benign and pathogenic predictions and no decisive high‑accuracy evidence, the variant remains ambiguous. Thus, it is most likely neither clearly benign nor pathogenic, and this uncertainty aligns with its ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.509769 | Disordered | 0.858469 | Binding | 0.328 | 0.825 | 0.375 | Uncertain | 1 | -2.107 | Likely Benign | 0.949 | Likely Pathogenic | Ambiguous | 0.476 | Likely Benign | -2.60 | Deleterious | 0.991 | Probably Damaging | 0.991 | Probably Damaging | 5.44 | Benign | 0.31 | Tolerated | 3.88 | 3 | 0.2145 | 0.1986 | 1 | -1 | -0.3 | -22.05 | ||||||||||||||||||||||||||||||||||||
| c.3487C>T | H1163Y 2D  AIThe SynGAP1 missense variant H1163Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign and the SGM‑Consensus also indicating a likely benign outcome; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign based on current predictive data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.858469 | Binding | 0.328 | 0.825 | 0.375 | -4.051 | Likely Benign | 0.688 | Likely Pathogenic | Likely Benign | 0.390 | Likely Benign | -1.81 | Neutral | 0.991 | Probably Damaging | 0.988 | Probably Damaging | 5.40 | Benign | 0.08 | Tolerated | 0.0845 | 0.4487 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.3488A>C | H1163P 2D  AIThe SynGAP1 missense variant H1163P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are SIFT, ESM1b, and FATHMM, while those that agree on a pathogenic effect are REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default; AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is a tie (2 pathogenic vs. 2 benign), and Foldetta results are unavailable. Overall, more tools predict pathogenicity than benignity (5 vs. 3), and there is no ClinVar record to contradict this assessment. Thus, the variant is most likely pathogenic based on the available predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.509769 | Disordered | 0.858469 | Binding | 0.328 | 0.825 | 0.375 | -2.023 | Likely Benign | 0.815 | Likely Pathogenic | Ambiguous | 0.578 | Likely Pathogenic | -3.19 | Deleterious | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 5.39 | Benign | 0.10 | Tolerated | 0.2015 | 0.3900 | 0 | -2 | 1.6 | -40.02 | ||||||||||||||||||||||||||||||||||||||||
| c.3488A>G | H1163R 2D  AIThe SynGAP1 missense variant H1163R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as likely benign, and no Foldetta stability data is available. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.858469 | Binding | 0.328 | 0.825 | 0.375 | -2.505 | Likely Benign | 0.942 | Likely Pathogenic | Ambiguous | 0.472 | Likely Benign | -2.08 | Neutral | 0.991 | Probably Damaging | 0.991 | Probably Damaging | 5.43 | Benign | 0.27 | Tolerated | 0.1863 | 0.2402 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||||||
| c.3488A>T | H1163L 2D  AIThe SynGAP1 missense variant H1163L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign) and Foldetta results are unavailable. Overall, the majority of predictions (5 pathogenic vs. 4 benign) lean toward a pathogenic impact. There is no ClinVar entry to contradict this assessment, so the variant is most likely pathogenic based on the available computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.509769 | Disordered | 0.858469 | Binding | 0.328 | 0.825 | 0.375 | -2.401 | Likely Benign | 0.707 | Likely Pathogenic | Likely Benign | 0.568 | Likely Pathogenic | -3.15 | Deleterious | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 5.55 | Benign | 0.10 | Tolerated | 0.0938 | 0.5356 | -2 | -3 | 7.0 | -23.98 | ||||||||||||||||||||||||||||||||||||||||
| c.3489C>A | H1163Q 2D  AIThe SynGAP1 missense variant H1163Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for H1163Q. This conclusion is consistent with the lack of a ClinVar entry, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.858469 | Binding | 0.328 | 0.825 | 0.375 | -2.970 | Likely Benign | 0.899 | Likely Pathogenic | Ambiguous | 0.414 | Likely Benign | -1.41 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 5.43 | Benign | 0.58 | Tolerated | 0.1445 | 0.3424 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.3489C>G | H1163Q 2D  AIThe SynGAP1 missense variant H1163Q is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates a likely benign outcome. AlphaMissense‑Optimized is uncertain, and no Foldetta (FoldX‑MD/Rosetta stability) result is available. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar reporting and gnomAD presence, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.858469 | Binding | 0.328 | 0.825 | 0.375 | -2.970 | Likely Benign | 0.899 | Likely Pathogenic | Ambiguous | 0.414 | Likely Benign | -1.41 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 5.43 | Benign | 0.58 | Tolerated | 0.1445 | 0.3424 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.3490C>A | L1164M 2D  AIThe SynGAP1 missense variant L1164M is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic predictions arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates a likely benign effect. High‑accuracy assessments further support this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also leans benign. No Foldetta (FoldX‑MD/ Rosetta stability) result is available, so it does not influence the interpretation. Overall, the majority of computational evidence points to a benign impact for the variant, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.853935 | Binding | 0.325 | 0.815 | 0.375 | -5.493 | Likely Benign | 0.757 | Likely Pathogenic | Likely Benign | 0.349 | Likely Benign | -0.29 | Neutral | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 5.34 | Benign | 0.13 | Tolerated | 0.0847 | 0.3378 | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||||||
| c.3490C>G | L1164V 2D  AIThe SynGAP1 missense variant L1164V has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and no Foldetta stability data are available. Overall, the balance of evidence leans toward a benign interpretation, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.853935 | Binding | 0.325 | 0.815 | 0.375 | -4.911 | Likely Benign | 0.797 | Likely Pathogenic | Ambiguous | 0.330 | Likely Benign | -0.88 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 5.54 | Benign | 0.14 | Tolerated | 0.1456 | 0.2617 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3491T>A | L1164Q 2D  AIThe SynGAP1 missense variant L1164Q has no ClinVar record and is not present in gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign status. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, whereas the SGM‑Consensus remains benign; Foldetta results are unavailable. Overall, the balance of evidence favors a benign interpretation, and this conclusion does not conflict with ClinVar, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.853935 | Binding | 0.325 | 0.815 | 0.375 | -5.374 | Likely Benign | 0.990 | Likely Pathogenic | Likely Pathogenic | 0.497 | Likely Benign | -0.95 | Neutral | 0.999 | Probably Damaging | 0.999 | Probably Damaging | 5.32 | Benign | 0.18 | Tolerated | 0.1171 | 0.1181 | -2 | -2 | -7.3 | 14.97 | |||||||||||||||||||||||||||||||||||||||
| c.3491T>C | L1164P 2D  AIThe SynGAP1 missense variant L1164P has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic impact are REVEL, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Pathogenic, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the majority of tools (seven pathogenic vs. three benign) predict a deleterious effect, indicating that the variant is most likely pathogenic. This prediction does not contradict ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.853935 | Binding | 0.325 | 0.815 | 0.375 | -3.928 | Likely Benign | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.573 | Likely Pathogenic | -2.16 | Neutral | 0.999 | Probably Damaging | 0.999 | Probably Damaging | 5.41 | Benign | 0.04 | Affected | 0.3173 | 0.1414 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||||||||||
| c.3491T>G | L1164R 2D  AIThe SynGAP1 missense variant L1164R has no ClinVar entry and is absent from gnomAD, so its population frequency is unknown. Functional prediction tools show mixed results: benign calls come from PROVEAN, SIFT, ESM1b, and FATHMM, while pathogenic calls come from REVEL, polyPhen‑2 (HumDiv and HumVar), AlphaMissense‑Default, and AlphaMissense‑Optimized. Grouping by consensus, four tools predict benign and five predict pathogenic. High‑accuracy methods give a more nuanced view: the SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign outcome; AlphaMissense‑Optimized independently predicts Pathogenic; Foldetta’s stability assessment is unavailable. Overall, the majority of standard predictors lean toward pathogenicity, but the SGM Consensus and several benign‑oriented tools counterbalance this. Given the lack of ClinVar annotation, there is no contradiction. The variant is most likely pathogenic based on the preponderance of predictions, though the SGM Consensus suggests a benign interpretation, indicating uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.853935 | Binding | 0.325 | 0.815 | 0.375 | -4.390 | Likely Benign | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.539 | Likely Pathogenic | -1.65 | Neutral | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 5.33 | Benign | 0.08 | Tolerated | 0.1396 | 0.0623 | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||||||
| c.3493T>A | S1165T 2D  AIThe SynGAP1 missense variant S1165T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.509769 | Disordered | 0.835017 | Binding | 0.308 | 0.807 | 0.375 | -3.970 | Likely Benign | 0.572 | Likely Pathogenic | Likely Benign | 0.096 | Likely Benign | -0.85 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.59 | Benign | 0.45 | Tolerated | 0.1660 | 0.5579 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.349A>T | S117C 2D  AIThe SynGAP1 missense variant S117C is not reported in ClinVar (ClinVar status: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.672422 | Binding | 0.357 | 0.877 | 0.625 | -5.980 | Likely Benign | 0.201 | Likely Benign | Likely Benign | 0.246 | Likely Benign | -1.81 | Neutral | 0.992 | Probably Damaging | 0.667 | Possibly Damaging | 3.68 | Benign | 0.00 | Affected | 0.1222 | 0.5057 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||||||
| c.3130C>T | P1044S 2D  AIThe SynGAP1 missense variant P1044S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.979741 | Disordered | 0.952126 | Binding | 0.331 | 0.855 | 0.750 | -4.114 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.311 | Likely Benign | -0.79 | Neutral | 0.011 | Benign | 0.015 | Benign | 5.51 | Benign | 0.14 | Tolerated | 0.3194 | 0.6081 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2797C>G | H933D 2D  AIThe SynGAP1 missense variant H933D is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus, SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves as Likely Pathogenic, matching the majority of individual scores. AlphaMissense‑Optimized returns an Uncertain result, and no Foldetta stability assessment is available. Overall, the preponderance of evidence points to a pathogenic effect for H933D. This conclusion is consistent with the absence of a ClinVar classification, so there is no contradiction with existing database annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.666105 | Disordered | 0.987531 | Binding | 0.305 | 0.862 | 0.625 | -2.888 | Likely Benign | 0.798 | Likely Pathogenic | Ambiguous | 0.320 | Likely Benign | -4.70 | Deleterious | 0.997 | Probably Damaging | 0.994 | Probably Damaging | 2.40 | Pathogenic | 0.04 | Affected | 0.2481 | 0.2717 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2802G>T | M934I 2D  AIThe SynGAP1 missense variant M934I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are FATHMM and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta results are unavailable. Overall, the majority of evidence (seven benign versus two pathogenic predictions) indicates that M934I is most likely benign, and this conclusion does not contradict any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.762850 | Disordered | 0.984677 | Binding | 0.290 | 0.867 | 0.625 | -4.582 | Likely Benign | 0.757 | Likely Pathogenic | Likely Benign | 0.085 | Likely Benign | -1.78 | Neutral | 0.316 | Benign | 0.101 | Benign | 2.49 | Pathogenic | 0.27 | Tolerated | 0.1686 | 0.3100 | 2 | 1 | 2.6 | -18.03 | ||||||||||||||||||||||||||||||||||||||||
| c.2803G>A | A935T 2D  AIThe SynGAP1 missense variant A935T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are not available. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -4.247 | Likely Benign | 0.228 | Likely Benign | Likely Benign | 0.204 | Likely Benign | -1.27 | Neutral | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.33 | Pathogenic | 0.00 | Affected | 0.1670 | 0.6599 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2803G>C | A935P 2D  AIThe SynGAP1 missense variant A935P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also yields a benign prediction, and Foldetta results are unavailable. Overall, the majority of high‑confidence tools predict a benign impact, and there is no conflict with ClinVar status. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -3.239 | Likely Benign | 0.371 | Ambiguous | Likely Benign | 0.269 | Likely Benign | -2.08 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.29 | Pathogenic | 0.00 | Affected | 0.2062 | 0.5466 | 1 | -1 | -3.4 | 26.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2803G>T | A935S 2D  AIThe SynGAP1 missense variant A935S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the overall assessment. Overall, the majority of evidence points to a benign effect for A935S, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -3.361 | Likely Benign | 0.139 | Likely Benign | Likely Benign | 0.167 | Likely Benign | -0.60 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.53 | Benign | 0.00 | Affected | 0.2719 | 0.5496 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2804C>A | A935D 2D  AIThe SynGAP1 missense variant A935D is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is uncertain, but the SGM‑Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—classifies the variant as likely pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence points to a pathogenic impact for A935D, and this conclusion does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -4.089 | Likely Benign | 0.817 | Likely Pathogenic | Ambiguous | 0.247 | Likely Benign | -2.69 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.31 | Pathogenic | 0.00 | Affected | 0.1832 | 0.1860 | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||||||
| c.2804C>G | A935G 2D  AIThe SynGAP1 missense variant A935G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -2.868 | Likely Benign | 0.179 | Likely Benign | Likely Benign | 0.152 | Likely Benign | -1.97 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.32 | Pathogenic | 0.00 | Affected | 0.2318 | 0.4679 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2804C>T | A935V 2D  AIThe SynGAP1 missense variant A935V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also leans benign (2 benign vs. 1 pathogenic votes). Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for A935V, and this conclusion does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -4.750 | Likely Benign | 0.397 | Ambiguous | Likely Benign | 0.194 | Likely Benign | -2.06 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.30 | Pathogenic | 0.00 | Affected | 0.1340 | 0.5941 | 0 | 0 | 2.4 | 28.05 | ||||||||||||||||||||||||||||||||||||||||
| c.2806G>C | A936P 2D  AIThe SynGAP1 missense variant A936P is not reported in ClinVar and is absent from gnomAD. Consensus from multiple in‑silico predictors indicates a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score all classify the change as benign or likely benign. Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is also benign. No Foldetta stability prediction is available. Overall, the computational evidence overwhelmingly points to a benign effect, and this is consistent with the absence of a ClinVar pathogenic classification. Thus, the variant is most likely benign, and this does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.973218 | Binding | 0.319 | 0.874 | 0.625 | -2.782 | Likely Benign | 0.191 | Likely Benign | Likely Benign | 0.014 | Likely Benign | -1.28 | Neutral | 0.012 | Benign | 0.011 | Benign | 2.46 | Pathogenic | 0.06 | Tolerated | 0.2043 | 0.6109 | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2806G>T | A936S 2D  AIThe SynGAP1 missense variant A936S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are unavailable. Overall, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.973218 | Binding | 0.319 | 0.874 | 0.625 | -3.555 | Likely Benign | 0.065 | Likely Benign | Likely Benign | 0.057 | Likely Benign | -0.07 | Neutral | 0.022 | Benign | 0.008 | Benign | 2.53 | Benign | 0.11 | Tolerated | 0.2675 | 0.5300 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2807C>A | A936D 2D  AIThe SynGAP1 missense variant A936D is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 benign vs 2 pathogenic), and Foldetta results are unavailable. Overall, the majority of evidence points toward a benign impact. This conclusion does not contradict any ClinVar annotation, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.812494 | Disordered | 0.973218 | Binding | 0.319 | 0.874 | 0.625 | -4.162 | Likely Benign | 0.686 | Likely Pathogenic | Likely Benign | 0.115 | Likely Benign | -1.63 | Neutral | 0.801 | Possibly Damaging | 0.339 | Benign | 2.48 | Pathogenic | 0.02 | Affected | 0.1778 | 0.1660 | 0 | -2 | -5.3 | 44.01 | ||||||||||||||||||||||||||||||||||||||||
| c.2807C>G | A936G 2D  AIThe SynGAP1 missense variant A936G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.973218 | Binding | 0.319 | 0.874 | 0.625 | -2.720 | Likely Benign | 0.095 | Likely Benign | Likely Benign | 0.065 | Likely Benign | -1.34 | Neutral | 0.454 | Possibly Damaging | 0.192 | Benign | 2.48 | Pathogenic | 0.12 | Tolerated | 0.2380 | 0.4529 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2809G>A | D937N 2D  AIThe SynGAP1 missense variant D937N is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools largely support a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all classify the change as benign, while only polyPhen‑2 HumDiv predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments further corroborate this view: AlphaMissense‑Optimized indicates benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Taken together, the preponderance of evidence points to a benign impact for D937N, and this conclusion does not conflict with the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.819762 | Disordered | 0.963385 | Binding | 0.348 | 0.883 | 0.625 | -4.259 | Likely Benign | 0.348 | Ambiguous | Likely Benign | 0.095 | Likely Benign | -0.22 | Neutral | 0.561 | Possibly Damaging | 0.139 | Benign | 2.72 | Benign | 0.81 | Tolerated | 0.2430 | 0.7825 | 2 | 1 | 0.0 | -0.98 | |||||||||||||||||||||||||||||||||||||||
| c.2809G>C | D937H 2D  AIThe SynGAP1 D937H missense variant (ClinVar ID 2825773.0) is listed as “Uncertain” and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic outcome are PolyPhen‑2 HumDiv, PolyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, the SGM‑Consensus (majority vote) is benign, and Foldetta (protein‑folding stability analysis combining FoldX‑MD and Rosetta) data are unavailable. Based on the preponderance of evidence from both general and high‑accuracy predictors, the variant is most likely benign, which is consistent with its ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.819762 | Disordered | 0.963385 | Binding | 0.348 | 0.883 | 0.625 | Uncertain | 1 | -0.733 | Likely Benign | 0.677 | Likely Pathogenic | Likely Benign | 0.150 | Likely Benign | -1.74 | Neutral | 1.000 | Probably Damaging | 0.975 | Probably Damaging | 2.68 | Benign | 0.13 | Tolerated | 3.77 | 5 | 0.2792 | 0.8228 | -1 | 1 | 0.3 | 22.05 | |||||||||||||||||||||||||||||||||||
| c.2802G>C | M934I 2D  AIThe SynGAP1 missense variant M934I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are FATHMM and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta results are unavailable. Overall, the majority of evidence (seven benign versus two pathogenic predictions) indicates that M934I is most likely benign, and this conclusion does not contradict any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.762850 | Disordered | 0.984677 | Binding | 0.290 | 0.867 | 0.625 | -4.582 | Likely Benign | 0.757 | Likely Pathogenic | Likely Benign | 0.085 | Likely Benign | -1.78 | Neutral | 0.316 | Benign | 0.101 | Benign | 2.49 | Pathogenic | 0.27 | Tolerated | 0.1686 | 0.3100 | 2 | 1 | 2.6 | -18.03 | ||||||||||||||||||||||||||||||||||||||||
| c.2802G>A | M934I 2D  AIThe SynGAP1 missense variant M934I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are FATHMM and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta results are unavailable. Overall, the majority of evidence (seven benign versus two pathogenic predictions) indicates that M934I is most likely benign, and this conclusion does not contradict any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.762850 | Disordered | 0.984677 | Binding | 0.290 | 0.867 | 0.625 | -4.582 | Likely Benign | 0.757 | Likely Pathogenic | Likely Benign | 0.086 | Likely Benign | -1.78 | Neutral | 0.316 | Benign | 0.101 | Benign | 2.49 | Pathogenic | 0.27 | Tolerated | 0.1686 | 0.3100 | 2 | 1 | 2.6 | -18.03 | ||||||||||||||||||||||||||||||||||||||||
| c.2797C>T | H933Y 2D  AIThe SynGAP1 H933Y variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields a pathogenic prediction. Foldetta results are unavailable. Overall, the majority of evidence (five pathogenic vs. three benign predictions) points to a pathogenic impact for H933Y. This conclusion does not contradict ClinVar status, as the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.987531 | Binding | 0.305 | 0.862 | 0.625 | -3.952 | Likely Benign | 0.525 | Ambiguous | Likely Benign | 0.314 | Likely Benign | -3.49 | Deleterious | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.36 | Pathogenic | 0.01 | Affected | 0.0968 | 0.4815 | 0 | 2 | 1.9 | 26.03 | ||||||||||||||||||||||||||||||||||||||||
| c.2798A>C | H933P 2D  AIThe SynGAP1 missense variant H933P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect are ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 benign vs 2 pathogenic). Foldetta results are unavailable. Overall, six tools predict pathogenicity versus three predicting benign, and the high‑accuracy benign prediction is outweighed by the majority of pathogenic calls. Thus, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.987531 | Binding | 0.305 | 0.862 | 0.625 | -3.890 | Likely Benign | 0.332 | Likely Benign | Likely Benign | 0.508 | Likely Pathogenic | -5.39 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.36 | Pathogenic | 0.02 | Affected | 0.1821 | 0.4400 | 0 | -2 | 1.6 | -40.02 | ||||||||||||||||||||||||||||||||||||||||
| c.2798A>G | H933R 2D  AIThe SynGAP1 missense variant H933R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, and AlphaMissense‑Optimized, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Overall, the majority of tools (five pathogenic vs. four benign) predict a pathogenic impact. Thus, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.666105 | Disordered | 0.987531 | Binding | 0.305 | 0.862 | 0.625 | -4.410 | Likely Benign | 0.650 | Likely Pathogenic | Likely Benign | 0.393 | Likely Benign | -3.84 | Deleterious | 0.997 | Probably Damaging | 0.994 | Probably Damaging | 2.43 | Pathogenic | 0.06 | Tolerated | 0.2074 | 0.2922 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||||||
| c.2798A>T | H933L 2D  AIThe SynGAP1 H933L missense variant is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) predicts pathogenic. Foldetta results are unavailable. Overall, the majority of evidence points to a pathogenic impact. This conclusion does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.987531 | Binding | 0.305 | 0.862 | 0.625 | -0.858 | Likely Benign | 0.470 | Ambiguous | Likely Benign | 0.388 | Likely Benign | -6.26 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.38 | Pathogenic | 0.02 | Affected | 0.1003 | 0.5749 | -2 | -3 | 7.0 | -23.98 | ||||||||||||||||||||||||||||||||||||||||
| c.2799C>A | H933Q 2D  AIThe SynGAP1 missense variant H933Q has no ClinVar record and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, and polyPhen‑2 HumVar. AlphaMissense‑Default is uncertain. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves to benign (two benign versus one pathogenic vote). High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus as benign, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) is unavailable for this variant. Overall, the preponderance of evidence points to a benign impact, and this conclusion does not contradict the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.987531 | Binding | 0.305 | 0.862 | 0.625 | -3.042 | Likely Benign | 0.410 | Ambiguous | Likely Benign | 0.211 | Likely Benign | -2.98 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.54 | Benign | 0.53 | Tolerated | 0.1654 | 0.3938 | 3 | 0 | -0.3 | -9.01 | ||||||||||||||||||||||||||||||||||||||||
| c.2799C>G | H933Q 2D  AIThe SynGAP1 H933Q missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, and polyPhen‑2 HumVar. AlphaMissense‑Default is uncertain, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation; there is no contradiction between the predictions and ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.987531 | Binding | 0.305 | 0.862 | 0.625 | -3.042 | Likely Benign | 0.410 | Ambiguous | Likely Benign | 0.210 | Likely Benign | -2.98 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.54 | Benign | 0.53 | Tolerated | 0.1654 | 0.3938 | 3 | 0 | -0.3 | -9.01 | ||||||||||||||||||||||||||||||||||||||||
| c.27T>A | H9Q 2D  AIThe SynGAP1 missense variant H9Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus, SGM‑Consensus, classifies the variant as Likely Benign, and AlphaMissense‑Optimized also reports a benign prediction. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -3.477 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.069 | Likely Benign | -0.05 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.24 | Benign | 0.00 | Affected | 0.2074 | 0.4297 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.27T>G | H9Q 2D  AIThe SynGAP1 missense variant H9Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus, SGM‑Consensus, classifies the variant as Likely Benign, and AlphaMissense‑Optimized also reports a benign prediction. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -3.477 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.071 | Likely Benign | -0.05 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.24 | Benign | 0.00 | Affected | 0.2074 | 0.4297 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2800A>C | M934L 2D  AIThe SynGAP1 missense variant M934L is not reported in ClinVar and is absent from gnomAD. In silico prediction tools that assess pathogenicity uniformly predict a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate benign. No tool in the dataset predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are not available. Overall, the consensus of all available predictions points to a benign impact, and this is consistent with the lack of a ClinVar classification—there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.984677 | Binding | 0.290 | 0.867 | 0.625 | -2.482 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.093 | Likely Benign | -0.73 | Neutral | 0.002 | Benign | 0.002 | Benign | 3.03 | Benign | 0.68 | Tolerated | 0.2004 | 0.4051 | 4 | 2 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2800A>T | M934L 2D  AIThe SynGAP1 missense variant M934L is not reported in ClinVar and is absent from gnomAD. In silico prediction tools that assess pathogenicity uniformly predict a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate benign. No tool in the dataset predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are not available. Consequently, the variant is most likely benign based on the collective predictions, and this conclusion does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.984677 | Binding | 0.290 | 0.867 | 0.625 | -2.482 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.093 | Likely Benign | -0.73 | Neutral | 0.002 | Benign | 0.002 | Benign | 3.03 | Benign | 0.68 | Tolerated | 0.2004 | 0.4051 | 4 | 2 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2801T>A | M934K 2D  AIThe SynGAP1 missense variant M934K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, SIFT, and ESM1b, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) is unavailable for this variant. Overall, the balance of evidence from the majority of tools points to a pathogenic impact. This conclusion is not contradicted by ClinVar status, which currently contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.762850 | Disordered | 0.984677 | Binding | 0.290 | 0.867 | 0.625 | -2.457 | Likely Benign | 0.816 | Likely Pathogenic | Ambiguous | 0.333 | Likely Benign | -3.66 | Deleterious | 0.929 | Possibly Damaging | 0.521 | Possibly Damaging | 2.38 | Pathogenic | 0.13 | Tolerated | 0.1828 | 0.1262 | 0 | -1 | -5.8 | -3.02 | |||||||||||||||||||||||||||||||||||||||
| c.2801T>C | M934T 2D  AIThe SynGAP1 missense variant M934T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, SIFT, and ESM1b, while those that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and the Foldetta stability analysis is unavailable. Based on the majority of predictions and the consensus call, the variant is most likely pathogenic; this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.762850 | Disordered | 0.984677 | Binding | 0.290 | 0.867 | 0.625 | -2.579 | Likely Benign | 0.823 | Likely Pathogenic | Ambiguous | 0.270 | Likely Benign | -3.33 | Deleterious | 0.811 | Possibly Damaging | 0.424 | Benign | 2.39 | Pathogenic | 0.19 | Tolerated | 0.2331 | 0.2267 | -1 | -1 | -2.6 | -30.09 | |||||||||||||||||||||||||||||||||||||||
| c.2801T>G | M934R 2D  AISynGAP1 missense variant M934R is not reported in ClinVar and is absent from gnomAD. Prediction tools that classify the variant as benign include REVEL, SIFT, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic predictions come from PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Overall, the balance of evidence leans toward pathogenicity, with no ClinVar entry to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.762850 | Disordered | 0.984677 | Binding | 0.290 | 0.867 | 0.625 | -1.854 | Likely Benign | 0.771 | Likely Pathogenic | Likely Benign | 0.322 | Likely Benign | -3.54 | Deleterious | 0.969 | Probably Damaging | 0.624 | Possibly Damaging | 2.37 | Pathogenic | 0.11 | Tolerated | 0.1902 | 0.1243 | 0 | -1 | -6.4 | 24.99 | |||||||||||||||||||||||||||||||||||||||
| c.2809G>T | D937Y 2D  AIThe SynGAP1 missense variant D937Y is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split, and Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic versus four benign) lean toward a pathogenic impact. Thus, the variant is most likely pathogenic based on current computational evidence, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.819762 | Disordered | 0.963385 | Binding | 0.348 | 0.883 | 0.625 | -4.720 | Likely Benign | 0.711 | Likely Pathogenic | Likely Benign | 0.137 | Likely Benign | -2.52 | Deleterious | 1.000 | Probably Damaging | 0.983 | Probably Damaging | 2.67 | Benign | 0.05 | Affected | 0.1305 | 0.7494 | -4 | -3 | 2.2 | 48.09 | ||||||||||||||||||||||||||||||||||||||||
| c.280C>G | P94A 2D  AIThe SynGAP1 missense variant P94A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and there is no conflict with ClinVar status, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | -3.450 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.085 | Likely Benign | -2.13 | Neutral | 0.092 | Benign | 0.008 | Benign | 4.14 | Benign | 0.00 | Affected | 0.2825 | 0.4043 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2810A>C | D937A 2D  AIThe SynGAP1 missense variant D937A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.819762 | Disordered | 0.963385 | Binding | 0.348 | 0.883 | 0.625 | -1.652 | Likely Benign | 0.385 | Ambiguous | Likely Benign | 0.096 | Likely Benign | -1.45 | Neutral | 0.995 | Probably Damaging | 0.895 | Possibly Damaging | 2.76 | Benign | 0.10 | Tolerated | 0.4296 | 0.7244 | 0 | -2 | 5.3 | -44.01 | |||||||||||||||||||||||||||||||||||||||
| c.2818G>T | G940C 2D  AIThe SynGAP1 missense variant G940C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this conclusion, so the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.889439 | Disordered | 0.920635 | Binding | 0.383 | 0.902 | 0.625 | -8.158 | Likely Pathogenic | 0.097 | Likely Benign | Likely Benign | 0.089 | Likely Benign | -0.40 | Neutral | 0.996 | Probably Damaging | 0.905 | Possibly Damaging | 2.70 | Benign | 0.11 | Tolerated | 0.1307 | 0.4354 | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||||||
| c.2819G>C | G940A 2D  AIThe SynGAP1 missense variant G940A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of all available predictions is that the variant is most likely benign, and this conclusion is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.889439 | Disordered | 0.920635 | Binding | 0.383 | 0.902 | 0.625 | -5.356 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.163 | Likely Benign | 0.64 | Neutral | 0.004 | Benign | 0.012 | Benign | 2.85 | Benign | 0.89 | Tolerated | 0.3627 | 0.4831 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2819G>T | G940V 2D  AIThe SynGAP1 missense variant G940V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Overall, the consensus of available predictions points to a benign impact, and this is consistent with the lack of ClinVar evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.889439 | Disordered | 0.920635 | Binding | 0.383 | 0.902 | 0.625 | -6.252 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.110 | Likely Benign | 0.36 | Neutral | 0.626 | Possibly Damaging | 0.419 | Benign | 2.92 | Benign | 0.31 | Tolerated | 0.1222 | 0.3822 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.281C>G | P94R 2D  AIThe SynGAP1 missense variant P94R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | -3.272 | Likely Benign | 0.281 | Likely Benign | Likely Benign | 0.104 | Likely Benign | -2.31 | Neutral | 0.110 | Benign | 0.012 | Benign | 4.11 | Benign | 0.00 | Affected | 0.1445 | 0.2780 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.281C>T | P94L 2D  AIThe SynGAP1 missense variant P94L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | -2.721 | Likely Benign | 0.111 | Likely Benign | Likely Benign | 0.074 | Likely Benign | -2.27 | Neutral | 0.198 | Benign | 0.017 | Benign | 4.13 | Benign | 0.00 | Affected | 0.2125 | 0.5862 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2822C>A | P941H 2D  AIThe SynGAP1 missense variant P941H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. In contrast, two tools—polyPhen‑2 HumDiv and SIFT—classify the change as pathogenic. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; no Foldetta stability data are available. Overall, the majority of evidence points to a benign effect, and this assessment does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.874069 | Disordered | 0.900790 | Binding | 0.403 | 0.906 | 0.625 | -4.439 | Likely Benign | 0.098 | Likely Benign | Likely Benign | 0.058 | Likely Benign | -0.09 | Neutral | 0.589 | Possibly Damaging | 0.309 | Benign | 2.71 | Benign | 0.00 | Affected | 0.1621 | 0.4826 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2822C>G | P941R 2D  AIThe SynGAP1 missense variant P941R is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the only pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates a benign outcome. Foldetta results are not available, so they do not influence the assessment. Overall, the majority of evidence points to a benign impact for P941R, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.874069 | Disordered | 0.900790 | Binding | 0.403 | 0.906 | 0.625 | -5.463 | Likely Benign | 0.181 | Likely Benign | Likely Benign | 0.056 | Likely Benign | -0.34 | Neutral | 0.144 | Benign | 0.062 | Benign | 2.75 | Benign | 0.01 | Affected | 0.1487 | 0.3775 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2822C>T | P941L 2D  AIThe SynGAP1 missense variant P941L is listed in ClinVar (ID 3451960.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). The only tool that predicts a pathogenic outcome is SIFT. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates “Likely Benign.” No Foldetta (FoldX‑MD/Rosetta) stability result is available, so it does not influence the assessment. Overall, the majority of predictions, including the high‑accuracy tools, point to a benign effect, which is consistent with the ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.874069 | Disordered | 0.900790 | Binding | 0.403 | 0.906 | 0.625 | Uncertain | 2 | -5.692 | Likely Benign | 0.066 | Likely Benign | Likely Benign | 0.054 | Likely Benign | -0.44 | Neutral | 0.144 | Benign | 0.039 | Benign | 2.76 | Benign | 0.01 | Affected | 0.2196 | 0.5931 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||
| c.2824C>A | P942T 2D  AIThe SynGAP1 missense variant P942T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for P942T, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | -5.745 | Likely Benign | 0.067 | Likely Benign | Likely Benign | 0.027 | Likely Benign | -1.23 | Neutral | 0.224 | Benign | 0.096 | Benign | 2.49 | Pathogenic | 0.00 | Affected | 0.1683 | 0.5599 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2824C>G | P942A 2D  AIThe SynGAP1 missense variant P942A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | -4.404 | Likely Benign | 0.047 | Likely Benign | Likely Benign | 0.023 | Likely Benign | -0.90 | Neutral | 0.059 | Benign | 0.061 | Benign | 2.52 | Benign | 0.00 | Affected | 0.3362 | 0.4621 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2825C>A | P942Q 2D  AIThe SynGAP1 missense variant P942Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for P942Q, and this conclusion does not contradict any ClinVar annotation (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | -6.094 | Likely Benign | 0.080 | Likely Benign | Likely Benign | 0.028 | Likely Benign | -1.12 | Neutral | 0.026 | Benign | 0.015 | Benign | 2.36 | Pathogenic | 0.00 | Affected | 0.1508 | 0.4703 | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||||||||||||||
| c.2825C>G | P942R 2D  AIThe SynGAP1 missense variant P942R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | -5.405 | Likely Benign | 0.159 | Likely Benign | Likely Benign | 0.045 | Likely Benign | -0.78 | Neutral | 0.411 | Benign | 0.139 | Benign | 2.36 | Pathogenic | 0.00 | Affected | 0.1307 | 0.3693 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2827G>A | G943S 2D  AIThe SynGAP1 missense variant G943S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. No pathogenic predictions are present. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign. Foldetta results are unavailable. Consequently, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.973328 | Disordered | 0.860437 | Binding | 0.372 | 0.910 | 0.750 | -5.785 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.295 | Likely Benign | 0.34 | Neutral | 0.059 | Benign | 0.039 | Benign | 2.88 | Benign | 0.51 | Tolerated | 0.2482 | 0.5502 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2816C>T | P939L 2D  AIThe SynGAP1 missense variant P939L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy predictions show AlphaMissense‑Optimized as benign, while SGM Consensus and Foldetta are unavailable. Overall, five tools predict pathogenicity versus four predicting benign, so the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -4.191 | Likely Benign | 0.138 | Likely Benign | Likely Benign | 0.159 | Likely Benign | -3.69 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.18 | Pathogenic | 0.00 | Affected | 0.2113 | 0.5142 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2816C>A | P939Q 2D  AIThe SynGAP1 missense variant P939Q is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign vs. two pathogenic votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus remains inconclusive and Foldetta is unavailable. Overall, five of the nine evaluated tools predict pathogenicity versus four predicting benignity, giving a slight tilt toward a pathogenic interpretation. This prediction does not contradict ClinVar status, as no ClinVar entry exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -4.950 | Likely Benign | 0.131 | Likely Benign | Likely Benign | 0.156 | Likely Benign | -3.45 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.18 | Pathogenic | 0.00 | Affected | 0.1551 | 0.4342 | 0 | -1 | -1.9 | 31.01 | ||||||||||||||||||||||||||||||||||||||||
| c.2810A>G | D937G 2D  AIThe SynGAP1 missense variant D937G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also likely benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence indicates that D937G is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.819762 | Disordered | 0.963385 | Binding | 0.348 | 0.883 | 0.625 | -0.770 | Likely Benign | 0.301 | Likely Benign | Likely Benign | 0.155 | Likely Benign | -0.93 | Neutral | 0.990 | Probably Damaging | 0.817 | Possibly Damaging | 2.82 | Benign | 0.72 | Tolerated | 0.4313 | 0.7006 | 1 | -1 | 3.1 | -58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2810A>T | D937V 2D  AIThe SynGAP1 missense variant D937V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) support a benign classification. This consensus does not contradict ClinVar status, as no ClinVar entry exists for this variant. Thus, based on current computational evidence, the D937V variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.819762 | Disordered | 0.963385 | Binding | 0.348 | 0.883 | 0.625 | -3.418 | Likely Benign | 0.673 | Likely Pathogenic | Likely Benign | 0.141 | Likely Benign | -2.21 | Neutral | 1.000 | Probably Damaging | 0.977 | Probably Damaging | 2.70 | Benign | 0.02 | Affected | 0.1766 | 0.7408 | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||||||
| c.2811T>A | D937E 2D  AIThe SynGAP1 missense variant D937E is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic outcome. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the consensus of the majority of prediction tools indicates that the variant is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.819762 | Disordered | 0.963385 | Binding | 0.348 | 0.883 | 0.625 | -3.342 | Likely Benign | 0.216 | Likely Benign | Likely Benign | 0.051 | Likely Benign | -1.07 | Neutral | 0.990 | Probably Damaging | 0.801 | Possibly Damaging | 2.76 | Benign | 0.15 | Tolerated | 0.2524 | 0.6915 | 3 | 2 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2811T>G | D937E 2D  AIThe SynGAP1 D937E missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the preponderance of benign predictions and the consensus from high‑accuracy methods, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.819762 | Disordered | 0.963385 | Binding | 0.348 | 0.883 | 0.625 | -3.342 | Likely Benign | 0.216 | Likely Benign | Likely Benign | 0.051 | Likely Benign | -1.07 | Neutral | 0.990 | Probably Damaging | 0.801 | Possibly Damaging | 2.76 | Benign | 0.15 | Tolerated | 0.2524 | 0.6915 | 3 | 2 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2812G>A | G938R 2D  AIThe SynGAP1 missense variant G938R is listed in ClinVar (ID 1019898.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence (seven benign versus three pathogenic predictions) supports a benign classification. This consensus does not contradict the ClinVar “Uncertain” designation, which remains unresolved. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.905695 | Disordered | 0.949795 | Binding | 0.318 | 0.883 | 0.625 | Uncertain | 1 | -5.271 | Likely Benign | 0.732 | Likely Pathogenic | Likely Benign | 0.141 | Likely Benign | -1.11 | Neutral | 0.999 | Probably Damaging | 0.985 | Probably Damaging | 2.74 | Benign | 0.36 | Tolerated | 3.77 | 5 | 0.0924 | 0.3614 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.2812G>C | G938R 2D  AIThe SynGAP1 missense variant G938R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus “Likely Benign” call. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of ClinVar annotation. Thus, the variant is most likely benign, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.905695 | Disordered | 0.949795 | Binding | 0.318 | 0.883 | 0.625 | -5.271 | Likely Benign | 0.732 | Likely Pathogenic | Likely Benign | 0.141 | Likely Benign | -1.11 | Neutral | 0.999 | Probably Damaging | 0.985 | Probably Damaging | 2.74 | Benign | 0.36 | Tolerated | 3.77 | 5 | 0.0924 | 0.3614 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||
| c.2812G>T | G938W 2D  AIThe SynGAP1 missense variant G938W is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized predicts a benign outcome, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑vs‑2 split, and Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs. four benign) indicate that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar annotation exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.905695 | Disordered | 0.949795 | Binding | 0.318 | 0.883 | 0.625 | -8.763 | Likely Pathogenic | 0.604 | Likely Pathogenic | Likely Benign | 0.194 | Likely Benign | -1.71 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.70 | Benign | 0.04 | Affected | 0.0762 | 0.4008 | -7 | -2 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||||||||||
| c.2813G>A | G938E 2D  AIThe SynGAP1 missense variant G938E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. The variant’s predicted benign status does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.905695 | Disordered | 0.949795 | Binding | 0.318 | 0.883 | 0.625 | -5.394 | Likely Benign | 0.577 | Likely Pathogenic | Likely Benign | 0.112 | Likely Benign | -1.40 | Neutral | 0.997 | Probably Damaging | 0.979 | Probably Damaging | 2.75 | Benign | 0.28 | Tolerated | 0.1370 | 0.3720 | 0 | -2 | -3.1 | 72.06 | |||||||||||||||||||||||||||||||||||||||
| c.2813G>C | G938A 2D  AIThe SynGAP1 missense variant G938A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta’s protein‑folding stability analysis is not available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.905695 | Disordered | 0.949795 | Binding | 0.318 | 0.883 | 0.625 | -5.068 | Likely Benign | 0.139 | Likely Benign | Likely Benign | 0.095 | Likely Benign | -0.41 | Neutral | 0.979 | Probably Damaging | 0.821 | Possibly Damaging | 2.80 | Benign | 0.82 | Tolerated | 0.3462 | 0.4948 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2813G>T | G938V 2D  AIThe SynGAP1 missense variant G938V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence indicates that G938V is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.905695 | Disordered | 0.949795 | Binding | 0.318 | 0.883 | 0.625 | -6.112 | Likely Benign | 0.153 | Likely Benign | Likely Benign | 0.123 | Likely Benign | -0.81 | Neutral | 0.999 | Probably Damaging | 0.985 | Probably Damaging | 2.83 | Benign | 0.31 | Tolerated | 0.1230 | 0.3811 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.2815C>A | P939T 2D  AIThe SynGAP1 missense variant P939T is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy predictions show AlphaMissense‑Optimized as benign, while the SGM Consensus remains inconclusive and Foldetta is unavailable. Overall, more tools (five) predict pathogenicity than benign (four), suggesting the variant is most likely pathogenic. This assessment does not contradict ClinVar status, as the variant has not yet been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -5.739 | Likely Benign | 0.099 | Likely Benign | Likely Benign | 0.139 | Likely Benign | -3.34 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.22 | Pathogenic | 0.00 | Affected | 0.1788 | 0.5310 | 0 | -1 | 0.9 | 3.99 | ||||||||||||||||||||||||||||||||||||||||
| c.2815C>G | P939A 2D  AIThe SynGAP1 missense variant P939A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign versus two pathogenic votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy tools therefore provide a benign prediction from AlphaMissense‑Optimized, an inconclusive SGM Consensus, and no Foldetta data. Overall, the majority of individual predictors (five pathogenic versus four benign) lean toward a pathogenic interpretation, and this does not contradict the lack of ClinVar annotation. **Thus, the variant is most likely pathogenic based on the current computational predictions.** Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -4.614 | Likely Benign | 0.076 | Likely Benign | Likely Benign | 0.111 | Likely Benign | -3.57 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.40 | Pathogenic | 0.00 | Affected | 0.3565 | 0.4336 | 1 | -1 | 3.4 | -26.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2815C>T | P939S 2D  AIThe SynGAP1 missense variant P939S is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs. 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy tools specifically show AlphaMissense‑Optimized as benign, while SGM Consensus and Foldetta remain unavailable. Overall, the majority of predictions (5 pathogenic vs. 4 benign) indicate that P939S is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -4.331 | Likely Benign | 0.098 | Likely Benign | Likely Benign | 0.101 | Likely Benign | -3.44 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.24 | Pathogenic | 0.00 | Affected | 0.3372 | 0.4517 | 1 | -1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2827G>T | G943C 2D  AIThe SynGAP1 missense variant G943C is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which itself is “Likely Benign”). In contrast, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and ESM1b all predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta stability analysis is unavailable. Overall, the majority of evidence—including the consensus and high‑accuracy tools—points to a benign impact. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.973328 | Disordered | 0.860437 | Binding | 0.372 | 0.910 | 0.750 | -9.822 | Likely Pathogenic | 0.108 | Likely Benign | Likely Benign | 0.356 | Likely Benign | -0.94 | Neutral | 0.938 | Possibly Damaging | 0.649 | Possibly Damaging | 2.84 | Benign | 0.10 | Tolerated | 0.1425 | 0.4439 | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||||||
| c.2797C>A | H933N 2D  AIThe SynGAP1 missense variant H933N is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy predictions show AlphaMissense‑Optimized as benign, while SGM Consensus and Foldetta are unavailable. Overall, five tools predict pathogenicity versus four predicting benignity, suggesting the variant is most likely pathogenic. This assessment does not contradict ClinVar status, as the variant has not yet been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.987531 | Binding | 0.305 | 0.862 | 0.625 | -4.333 | Likely Benign | 0.226 | Likely Benign | Likely Benign | 0.261 | Likely Benign | -3.65 | Deleterious | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.39 | Pathogenic | 0.04 | Affected | 0.1897 | 0.3439 | 2 | 1 | -0.3 | -23.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2771C>G | P924R 2D  AIThe SynGAP1 missense variant P924R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split: benign calls from REVEL and ESM1b, whereas the remaining seven tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) predict pathogenicity. Grouping by consensus, the pathogenic group outweighs the benign group. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized scores the variant as pathogenic, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. Foldetta data are unavailable. Overall, the preponderance of evidence indicates that P924R is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -6.024 | Likely Benign | 0.965 | Likely Pathogenic | Likely Pathogenic | 0.420 | Likely Benign | -6.20 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.66 | Pathogenic | 0.00 | Affected | 0.1314 | 0.2083 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2779T>G | F927V 2D  AIThe SynGAP1 missense variant F927V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable and therefore not considered. Overall, the preponderance of evidence from multiple in silico predictors and high‑accuracy tools indicates that the variant is most likely pathogenic, with no ClinVar annotation to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.919 | Likely Benign | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.470 | Likely Benign | -5.21 | Deleterious | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 1.34 | Pathogenic | 0.00 | Affected | 0.2207 | 0.1206 | -1 | -1 | 1.4 | -48.04 | |||||||||||||||||||||||||||||||||||||||
| c.277C>G | R93G 2D  AIThe SynGAP1 missense variant R93G is listed in ClinVar (ID 2504251.0) with an “Uncertain” clinical significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus (derived from the same set of predictors) labels it “Likely Benign”; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that R93G is most likely benign, which does not contradict the current ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | Uncertain | 1 | -2.674 | Likely Benign | 0.400 | Ambiguous | Likely Benign | 0.093 | Likely Benign | -1.69 | Neutral | 0.103 | Benign | 0.019 | Benign | 3.99 | Benign | 0.00 | Affected | 4.32 | 1 | 0.3809 | 0.3814 | -2 | -3 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||
| c.277C>T | R93W 2D  AIThe SynGAP1 missense variant R93W is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for R93W, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | -5.652 | Likely Benign | 0.514 | Ambiguous | Likely Benign | 0.094 | Likely Benign | -2.22 | Neutral | 0.981 | Probably Damaging | 0.257 | Benign | 3.96 | Benign | 0.00 | Affected | 0.1413 | 0.3835 | 2 | -3 | 3.6 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2780T>A | F927Y 2D  AIThe SynGAP1 missense variant F927Y is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas tools that agree on a pathogenic effect include polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (5 pathogenic vs. 3 benign) lean toward a pathogenic interpretation. Thus, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -5.244 | Likely Benign | 0.954 | Likely Pathogenic | Ambiguous | 0.291 | Likely Benign | -2.29 | Neutral | 0.997 | Probably Damaging | 0.987 | Probably Damaging | 1.40 | Pathogenic | 0.00 | Affected | 0.1551 | 0.1518 | 7 | 3 | -4.1 | 16.00 | ||||||||||||||||||||||||||||||||||||||||
| c.2780T>C | F927S 2D  AIThe SynGAP1 missense variant F927S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The high‑accuracy consensus, SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely pathogenic outcome (3 pathogenic vs. 1 benign). AlphaMissense‑Optimized alone predicts pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Based on the preponderance of pathogenic predictions—including the high‑accuracy consensus—the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -5.480 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.481 | Likely Benign | -5.68 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.32 | Pathogenic | 0.00 | Affected | 0.4748 | 0.0591 | -3 | -2 | -3.6 | -60.10 | |||||||||||||||||||||||||||||||||||||||
| c.2780T>G | F927C 2D  AIThe SynGAP1 missense variant F927C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Functional prediction tools uniformly indicate a deleterious effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as pathogenic. No tool in the dataset predicts a benign outcome. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as Likely Pathogenic. Foldetta results are not available. Based on the consensus of all available predictions, the variant is most likely pathogenic, and this conclusion is consistent with the absence of a ClinVar entry. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -8.298 | Likely Pathogenic | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.523 | Likely Pathogenic | -6.02 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.31 | Pathogenic | 0.00 | Affected | 0.2921 | 0.1291 | -4 | -2 | -0.3 | -44.04 | |||||||||||||||||||||||||||||||||||||||
| c.2781C>A | F927L 2D  AIThe SynGAP1 missense variant F927L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is labeled “Likely Pathogenic”). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely pathogenicity. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that F927L is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.227 | Likely Benign | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.296 | Likely Benign | -4.48 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.39 | Pathogenic | 0.00 | Affected | 0.2214 | 0.2209 | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2781C>G | F927L 2D  AIThe SynGAP1 missense variant F927L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is labeled Likely Pathogenic. Foldetta results are not available. Based on the preponderance of pathogenic predictions and the high‑accuracy tools, the variant is most likely pathogenic; this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.227 | Likely Benign | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.296 | Likely Benign | -4.48 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.39 | Pathogenic | 0.00 | Affected | 0.2214 | 0.2209 | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2782C>A | Q928K 2D  AIThe SynGAP1 missense variant Q928K has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). AlphaMissense‑Optimized yields an uncertain result, and no Foldetta stability assessment is available. High‑accuracy evidence therefore consists of an uncertain AlphaMissense‑Optimized score, a Likely Pathogenic SGM‑Consensus, and an unavailable Foldetta prediction. Overall, the majority of tools predict pathogenicity, and there is no ClinVar status to contradict this assessment. Thus, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -4.941 | Likely Benign | 0.897 | Likely Pathogenic | Ambiguous | 0.259 | Likely Benign | -2.80 | Deleterious | 0.985 | Probably Damaging | 0.981 | Probably Damaging | 1.60 | Pathogenic | 0.00 | Affected | 0.1728 | 0.5306 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.2782C>G | Q928E 2D  AIThe SynGAP1 missense variant Q928E is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized predicting benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2) and Foldetta results are unavailable. Based on the overall distribution of predictions, the variant is most likely pathogenic; this assessment does not contradict the ClinVar status, which currently contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -5.168 | Likely Benign | 0.590 | Likely Pathogenic | Likely Benign | 0.283 | Likely Benign | -2.06 | Neutral | 0.985 | Probably Damaging | 0.981 | Probably Damaging | 1.58 | Pathogenic | 0.00 | Affected | 0.1368 | 0.3477 | 2 | 2 | 0.0 | 0.98 | ||||||||||||||||||||||||||||||||||||||||
| c.2783A>C | Q928P 2D  AIThe SynGAP1 missense variant Q928P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. AlphaMissense‑Optimized returns an uncertain result, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available prediction for this variant. Based on the preponderance of pathogenic predictions and the SGM‑Consensus designation, the variant is most likely pathogenic; this assessment does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -6.275 | Likely Benign | 0.832 | Likely Pathogenic | Ambiguous | 0.441 | Likely Benign | -4.28 | Deleterious | 0.998 | Probably Damaging | 0.995 | Probably Damaging | 1.54 | Pathogenic | 0.00 | Affected | 0.2016 | 0.5768 | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||||||||||||
| c.2783A>T | Q928L 2D  AIThe SynGAP1 missense variant Q928L has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of other in‑silico predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) and the SGM‑Consensus score (Likely Pathogenic) all indicate a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Based on the preponderance of pathogenic predictions and the SGM‑Consensus outcome, the variant is most likely pathogenic. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -6.237 | Likely Benign | 0.919 | Likely Pathogenic | Ambiguous | 0.373 | Likely Benign | -4.57 | Deleterious | 0.994 | Probably Damaging | 0.988 | Probably Damaging | 1.56 | Pathogenic | 0.00 | Affected | 0.0757 | 0.6091 | -2 | -2 | 7.3 | -14.97 | |||||||||||||||||||||||||||||||||||||||
| c.2784G>C | Q928H 2D  AIThe SynGAP1 missense variant Q928H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that classify the variant as benign include REVEL and ESM1b, whereas the majority of tools predict it to be pathogenic: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further support a pathogenic interpretation: AlphaMissense‑Optimized is uncertain, but the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—agrees on a pathogenic outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -4.358 | Likely Benign | 0.929 | Likely Pathogenic | Ambiguous | 0.329 | Likely Benign | -3.65 | Deleterious | 0.998 | Probably Damaging | 0.996 | Probably Damaging | 1.54 | Pathogenic | 0.00 | Affected | 0.1389 | 0.4811 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2779T>C | F927L 2D  AIThe SynGAP1 missense variant F927L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is labeled “Likely Pathogenic”). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely pathogenicity. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that F927L is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.227 | Likely Benign | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.317 | Likely Benign | -4.48 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.39 | Pathogenic | 0.00 | Affected | 0.2214 | 0.2209 | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2779T>A | F927I 2D  AIThe SynGAP1 missense variant F927I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence from multiple in silico tools indicates that F927I is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.793 | Likely Benign | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.370 | Likely Benign | -4.50 | Deleterious | 0.997 | Probably Damaging | 0.994 | Probably Damaging | 1.34 | Pathogenic | 0.00 | Affected | 0.2255 | 0.1238 | 1 | 0 | 1.7 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2771C>T | P924L 2D  AIThe SynGAP1 missense variant P924L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence indicates that P924L is most likely pathogenic, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -6.361 | Likely Benign | 0.971 | Likely Pathogenic | Likely Pathogenic | 0.422 | Likely Benign | -6.89 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.66 | Pathogenic | 0.00 | Affected | 0.1771 | 0.4941 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2773C>A | L925I 2D  AIThe SynGAP1 missense variant L925I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of consensus tools (five pathogenic vs. three benign) lean toward a pathogenic interpretation. This prediction does not contradict ClinVar status, as the variant is not yet catalogued there. Thus, based on current computational evidence, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -5.266 | Likely Benign | 0.828 | Likely Pathogenic | Ambiguous | 0.205 | Likely Benign | -1.38 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 1.37 | Pathogenic | 0.00 | Affected | 0.1192 | 0.3405 | 2 | 2 | 0.7 | 0.00 | ||||||||||||||||||||||||||||||||||||||||
| c.2773C>G | L925V 2D  AIThe SynGAP1 missense variant L925V is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of consensus tools predict a pathogenic impact, and this prediction does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely pathogenic based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -3.977 | Likely Benign | 0.831 | Likely Pathogenic | Ambiguous | 0.244 | Likely Benign | -2.09 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 1.36 | Pathogenic | 0.00 | Affected | 0.1848 | 0.3230 | 2 | 1 | 0.4 | -14.03 | ||||||||||||||||||||||||||||||||||||||||
| c.2773C>T | L925F 2D  AIThe SynGAP1 missense variant L925F is not reported in ClinVar and is absent from gnomAD. Prediction tools that classify it as benign include REVEL and ESM1b, whereas the majority of tools predict it to be pathogenic: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of computational evidence points to a pathogenic impact for the L925F substitution, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -4.147 | Likely Benign | 0.966 | Likely Pathogenic | Likely Pathogenic | 0.242 | Likely Benign | -3.04 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.32 | Pathogenic | 0.00 | Affected | 0.0897 | 0.2881 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2774T>A | L925H 2D  AIThe SynGAP1 missense variant L925H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Pathogenic.” No Foldetta stability result is available. Overall, the majority of evidence points to a pathogenic effect for L925H, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -3.369 | Likely Benign | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.475 | Likely Benign | -5.24 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.28 | Pathogenic | 0.00 | Affected | 0.1219 | 0.1494 | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2774T>C | L925P 2D  AIThe SynGAP1 missense variant L925P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only ESM1b, whereas the remaining tools—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus—predict a pathogenic or likely pathogenic impact. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as likely pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of evidence from multiple in silico predictors indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -2.733 | Likely Benign | 0.985 | Likely Pathogenic | Likely Pathogenic | 0.501 | Likely Pathogenic | -5.12 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.28 | Pathogenic | 0.00 | Affected | 0.3083 | 0.1996 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2774T>G | L925R 2D  AIThe SynGAP1 missense variant L925R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only ESM1b, whereas the remaining tools—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—all predict a pathogenic or likely pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized reports a pathogenic effect, and the SGM‑Consensus indicates a likely pathogenic classification. Foldetta results are not available for this variant. Based on the preponderance of pathogenic predictions and the lack of benign consensus, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -2.340 | Likely Benign | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.519 | Likely Pathogenic | -4.54 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.28 | Pathogenic | 0.00 | Affected | 0.1278 | 0.1294 | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||||||
| c.2776T>A | S926T 2D  AIThe SynGAP1 missense variant S926T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus (SGM Consensus) is inconclusive, with two pathogenic and two benign votes. AlphaMissense‑Optimized, a high‑accuracy model, predicts benign, while Foldetta stability analysis is unavailable. Overall, the majority of standard tools favor a pathogenic effect, whereas the single high‑accuracy model suggests benign. Thus, the variant is most likely pathogenic based on the collective predictions, and this assessment does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -2.917 | Likely Benign | 0.651 | Likely Pathogenic | Likely Benign | 0.226 | Likely Benign | -2.34 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.55 | Pathogenic | 0.00 | Affected | 0.1174 | 0.5663 | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||||||||||||
| c.2776T>C | S926P 2D  AIThe SynGAP1 missense variant S926P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic outcome: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The high‑accuracy consensus, SGM‑Consensus, is derived from a majority vote of AlphaMissense‑Default (pathogenic), ESM1b (benign), FATHMM (pathogenic), and PROVEAN (pathogenic), yielding a Likely Pathogenic classification. AlphaMissense‑Optimized is uncertain, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a pathogenic effect for S926P. This conclusion is not contradicted by ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -3.873 | Likely Benign | 0.916 | Likely Pathogenic | Ambiguous | 0.424 | Likely Benign | -3.79 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.51 | Pathogenic | 0.00 | Affected | 0.1738 | 0.5058 | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2776T>G | S926A 2D  AIThe SynGAP1 missense variant S926A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of reliable predictions indicate a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -3.042 | Likely Benign | 0.463 | Ambiguous | Likely Benign | 0.186 | Likely Benign | -2.13 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.59 | Pathogenic | 0.00 | Affected | 0.4657 | 0.4488 | 1 | 1 | 2.6 | -16.00 | ||||||||||||||||||||||||||||||||||||||||
| c.2777C>A | S926Y 2D  AIThe SynGAP1 missense variant S926Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict a pathogenic outcome. High‑accuracy assessments reinforce this trend: AlphaMissense‑Optimized classifies the variant as pathogenic; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports it as Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect for S926Y, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -6.602 | Likely Benign | 0.968 | Likely Pathogenic | Likely Pathogenic | 0.444 | Likely Benign | -4.53 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | 0.0602 | 0.4932 | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||||||
| c.2777C>G | S926C 2D  AIThe SynGAP1 missense variant S926C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is unavailable for this variant. Overall, the majority of predictions lean toward pathogenicity, with the high‑accuracy AlphaMissense‑Optimized result providing a conflicting benign signal. Thus, the variant is most likely pathogenic based on the collective evidence, and this assessment does not contradict any ClinVar status because none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -6.546 | Likely Benign | 0.680 | Likely Pathogenic | Likely Benign | 0.414 | Likely Benign | -3.81 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | 0.0815 | 0.5487 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||||||
| c.2777C>T | S926F 2D  AIThe SynGAP1 missense variant S926F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is labeled Likely Pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that S926F is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -6.157 | Likely Benign | 0.980 | Likely Pathogenic | Likely Pathogenic | 0.458 | Likely Benign | -4.57 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | 0.0585 | 0.5220 | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||||||||||||
| c.2784G>T | Q928H 2D  AIThe SynGAP1 missense variant Q928H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that classify the variant as benign include REVEL and ESM1b, whereas the majority of tools predict it to be pathogenic: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further support a pathogenic interpretation: AlphaMissense‑Optimized is uncertain, but the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—agrees on a pathogenic outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -4.358 | Likely Benign | 0.929 | Likely Pathogenic | Ambiguous | 0.330 | Likely Benign | -3.65 | Deleterious | 0.998 | Probably Damaging | 0.996 | Probably Damaging | 1.54 | Pathogenic | 0.00 | Affected | 0.1389 | 0.4811 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2785A>C | N929H 2D  AIThe SynGAP1 missense variant N929H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. ESM1b is uncertain, and Foldetta results are unavailable. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Pathogenic. With no conflicting ClinVar annotation, the collective evidence indicates that the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -7.070 | In-Between | 0.967 | Likely Pathogenic | Likely Pathogenic | 0.342 | Likely Benign | -3.52 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.45 | Pathogenic | 0.00 | Affected | 0.1507 | 0.7659 | 2 | 1 | 0.3 | 23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2785A>G | N929D 2D  AIThe SynGAP1 missense variant N929D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which reports “Likely Pathogenic”). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely pathogenicity. Foldetta results are unavailable. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -6.856 | Likely Benign | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.365 | Likely Benign | -3.86 | Deleterious | 0.999 | Probably Damaging | 0.995 | Probably Damaging | 1.49 | Pathogenic | 0.00 | Affected | 0.1991 | 0.4605 | 2 | 1 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.2791C>A | L931I 2D  AIThe SynGAP1 missense variant L931I is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 HumDiv and polyPhen‑2 HumVar both predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from multiple prediction algorithms indicates that the variant is most likely benign, and this conclusion does not contradict the lack of ClinVar reporting. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.549308 | Disordered | 0.989212 | Binding | 0.335 | 0.856 | 0.375 | -3.813 | Likely Benign | 0.296 | Likely Benign | Likely Benign | 0.120 | Likely Benign | 0.42 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 2.58 | Benign | 0.14 | Tolerated | 0.1076 | 0.3540 | 2 | 2 | 0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.2791C>G | L931V 2D  AIThe SynGAP1 missense variant L931V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic effect. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for the L931V variant, and this conclusion does not contradict any ClinVar annotation, as none exists for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.549308 | Disordered | 0.989212 | Binding | 0.335 | 0.856 | 0.375 | -1.512 | Likely Benign | 0.234 | Likely Benign | Likely Benign | 0.182 | Likely Benign | 0.71 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 3.03 | Benign | 0.70 | Tolerated | 0.1690 | 0.3164 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2791C>T | L931F 2D  AIThe SynGAP1 missense variant L931F is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, and ESM1b, while pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain. A high‑accuracy consensus (SGM) that aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN yields a 2‑to‑2 split, leaving the consensus inconclusive. Foldetta, which assesses protein‑folding stability via FoldX‑MD and Rosetta, has no available result for this variant. Consequently, the overall evidence leans toward pathogenicity, driven by the majority of standard predictors, and does not conflict with the absence of a ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.549308 | Disordered | 0.989212 | Binding | 0.335 | 0.856 | 0.375 | -5.101 | Likely Benign | 0.790 | Likely Pathogenic | Ambiguous | 0.167 | Likely Benign | -2.00 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.41 | Pathogenic | 0.04 | Affected | 0.0718 | 0.3208 | 2 | 0 | -1.0 | 34.02 | ||||||||||||||||||||||||||||||||||||||||
| c.2792T>A | L931H 2D  AIThe SynGAP1 missense variant L931H has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include only REVEL, whereas the majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic impact. ESM1b and AlphaMissense‑Optimized are uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Based on the preponderance of pathogenic predictions and the SGM‑Consensus outcome, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.549308 | Disordered | 0.989212 | Binding | 0.335 | 0.856 | 0.375 | -7.495 | In-Between | 0.954 | Likely Pathogenic | Ambiguous | 0.301 | Likely Benign | -3.76 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.38 | Pathogenic | 0.00 | Affected | 0.1198 | 0.1205 | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2792T>C | L931P 2D  AIThe SynGAP1 missense variant L931P is not reported in ClinVar and is absent from gnomAD. Prediction tools show a strong bias toward pathogenicity: SIFT, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, PROVEAN, and AlphaMissense‑Default all predict a deleterious effect, whereas only REVEL indicates a benign outcome. High‑accuracy assessments reinforce this trend: the SGM consensus—derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—classifies the variant as likely pathogenic, and AlphaMissense‑Optimized returns an uncertain result. Foldetta predictions are unavailable. Overall, the preponderance of evidence points to a pathogenic impact for L931P, and this conclusion is consistent with the absence of any ClinVar annotation, so there is no contradiction with existing clinical databases. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.549308 | Disordered | 0.989212 | Binding | 0.335 | 0.856 | 0.375 | -8.624 | Likely Pathogenic | 0.950 | Likely Pathogenic | Ambiguous | 0.376 | Likely Benign | -3.43 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.38 | Pathogenic | 0.01 | Affected | 0.3203 | 0.1698 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2792T>G | L931R 2D  AIThe SynGAP1 missense variant L931R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, while the SGM‑Consensus remains pathogenic; Foldetta results are not available. Overall, the preponderance of evidence from multiple in‑silico predictors indicates that the variant is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.549308 | Disordered | 0.989212 | Binding | 0.335 | 0.856 | 0.375 | -8.606 | Likely Pathogenic | 0.933 | Likely Pathogenic | Ambiguous | 0.344 | Likely Benign | -3.48 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.39 | Pathogenic | 0.01 | Affected | 0.1241 | 0.1205 | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||||||
| c.2794T>A | F932I 2D  AIThe SynGAP1 missense variant F932I is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves as Likely Pathogenic because three of the four constituent tools predict pathogenicity. High‑accuracy assessments further support this: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus (majority vote) is also pathogenic; Foldetta results are unavailable. Consequently, the collective evidence points to a pathogenic effect for F932I. This conclusion is consistent with the absence of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -4.963 | Likely Benign | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.348 | Likely Benign | -3.45 | Deleterious | 0.997 | Probably Damaging | 0.994 | Probably Damaging | 2.32 | Pathogenic | 0.01 | Affected | 0.2202 | 0.2914 | 1 | 0 | 1.7 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2794T>C | F932L 2D  AIThe SynGAP1 missense variant F932L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the consensus of the available predictions points to a pathogenic effect for F932L, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -3.390 | Likely Benign | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.309 | Likely Benign | -3.50 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.36 | Pathogenic | 0.03 | Affected | 0.2373 | 0.3895 | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2794T>G | F932V 2D  AIThe SynGAP1 missense variant F932V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict a pathogenic outcome. High‑accuracy assessments reinforce this trend: AlphaMissense‑Optimized classifies the variant as pathogenic; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely pathogenic status. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -5.728 | Likely Benign | 0.976 | Likely Pathogenic | Likely Pathogenic | 0.392 | Likely Benign | -3.68 | Deleterious | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.32 | Pathogenic | 0.01 | Affected | 0.2137 | 0.3082 | -1 | -1 | 1.4 | -48.04 | |||||||||||||||||||||||||||||||||||||||
| c.2795T>A | F932Y 2D  AIThe SynGAP1 missense variant F932Y is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign, while the majority of other predictors (polyPhen‑2 HumDiv and HumVar) suggest pathogenic. When predictions are grouped by agreement, the benign‑predicating tools outnumber the pathogenic ones, and the single uncertain call from AlphaMissense‑Default does not alter this balance. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized classifies the variant as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports it as Likely Benign. Foldetta data are unavailable. Overall, the computational evidence overwhelmingly supports a benign classification, and this is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -5.248 | Likely Benign | 0.540 | Ambiguous | Likely Benign | 0.180 | Likely Benign | 1.58 | Neutral | 0.997 | Probably Damaging | 0.987 | Probably Damaging | 3.13 | Benign | 0.74 | Tolerated | 0.1473 | 0.2003 | 7 | 3 | -4.1 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2795T>C | F932S 2D  AIThe SynGAP1 missense variant F932S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. In contrast, the majority of tools predict a pathogenic effect: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as pathogenic. ESM1b is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from multiple prediction algorithms and high‑accuracy tools indicates that F932S is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -7.196 | In-Between | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.260 | Likely Benign | -4.45 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.31 | Pathogenic | 0.00 | Affected | 0.4257 | 0.0584 | -3 | -2 | -3.6 | -60.10 | |||||||||||||||||||||||||||||||||||||||
| c.2795T>G | F932C 2D  AIThe SynGAP1 missense variant F932C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts Pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from multiple in silico predictors and high‑accuracy tools indicates that the F932C variant is most likely pathogenic, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -8.601 | Likely Pathogenic | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.309 | Likely Benign | -4.62 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.30 | Pathogenic | 0.00 | Affected | 0.2446 | 0.1506 | -4 | -2 | -0.3 | -44.04 | |||||||||||||||||||||||||||||||||||||||
| c.2796C>A | F932L 2D  AIThe SynGAP1 missense variant F932L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the consensus of the available predictions points to a pathogenic effect for F932L, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -3.390 | Likely Benign | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.170 | Likely Benign | -3.50 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.36 | Pathogenic | 0.03 | Affected | 0.2373 | 0.3895 | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.278G>T | R93L 2D  AIThe SynGAP1 R93L missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus indicates Likely Benign, and Foldetta results are unavailable. Overall, the preponderance of evidence points to the variant being most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | -2.850 | Likely Benign | 0.425 | Ambiguous | Likely Benign | 0.064 | Likely Benign | -1.72 | Neutral | 0.103 | Benign | 0.019 | Benign | 4.00 | Benign | 0.00 | Affected | 0.2197 | 0.4861 | -3 | -2 | 8.3 | -43.03 | |||||||||||||||||||||||||||||||||||||||
| c.278G>C | R93P 2D  AIThe SynGAP1 missense variant R93P is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus indicates Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | -3.164 | Likely Benign | 0.473 | Ambiguous | Likely Benign | 0.121 | Likely Benign | -0.32 | Neutral | 0.361 | Benign | 0.038 | Benign | 3.99 | Benign | 0.00 | Affected | 0.2200 | 0.4844 | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2785A>T | N929Y 2D  AIThe SynGAP1 missense variant N929Y is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL predicts benign, whereas the remaining eleven predictors—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized—predict pathogenicity. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as Likely Pathogenic. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus result is consistent with a likely pathogenic classification. Foldetta predictions are unavailable. Taken together, the preponderance of evidence indicates that the variant is most likely pathogenic, and this assessment does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -10.327 | Likely Pathogenic | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.391 | Likely Benign | -6.02 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.45 | Pathogenic | 0.00 | Affected | 0.0656 | 0.6489 | -2 | -2 | 2.2 | 49.07 | |||||||||||||||||||||||||||||||||||||||
| c.2786A>C | N929T 2D  AIThe SynGAP1 missense variant N929T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining 11 predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) all indicate pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts a pathogenic change, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability analysis is available. Taken together, the overwhelming majority of evidence supports a pathogenic classification for N929T, and this conclusion is consistent with the absence of a ClinVar entry (i.e., there is no conflicting ClinVar status). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -9.245 | Likely Pathogenic | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.257 | Likely Benign | -4.68 | Deleterious | 0.999 | Probably Damaging | 0.995 | Probably Damaging | 1.47 | Pathogenic | 0.00 | Affected | 0.1469 | 0.8140 | 0 | 0 | 2.8 | -13.00 | |||||||||||||||||||||||||||||||||||||||
| c.2786A>G | N929S 2D  AIThe SynGAP1 missense variant N929S is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools show a consensus toward pathogenicity: SIFT, polyPhen‑2 (HumDiv and HumVar), PROVEAN, FATHMM, and AlphaMissense‑Default all predict a deleterious effect, while REVEL uniquely predicts a benign outcome. The remaining tools, ESM1b and AlphaMissense‑Optimized, return uncertain results. High‑accuracy assessments further support a damaging interpretation: the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic, and AlphaMissense‑Optimized remains uncertain; Foldetta data are unavailable. Taken together, the preponderance of evidence points to a pathogenic effect for N929S. This conclusion does not conflict with ClinVar, which currently contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -7.100 | In-Between | 0.948 | Likely Pathogenic | Ambiguous | 0.249 | Likely Benign | -3.89 | Deleterious | 0.999 | Probably Damaging | 0.992 | Probably Damaging | 1.48 | Pathogenic | 0.00 | Affected | 0.4117 | 0.7639 | 1 | 1 | 2.7 | -27.03 | |||||||||||||||||||||||||||||||||||||||
| c.2786A>T | N929I 2D  AIThe SynGAP1 missense variant N929I is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining eight tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) predict pathogenicity. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as Likely Pathogenic. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus result is consistent. Foldetta, a protein‑folding stability method that combines FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, and this conclusion does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -11.799 | Likely Pathogenic | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.297 | Likely Benign | -6.82 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.45 | Pathogenic | 0.00 | Affected | 0.0780 | 0.6486 | -2 | -3 | 8.0 | -0.94 | |||||||||||||||||||||||||||||||||||||||
| c.2787C>A | N929K 2D  AIThe SynGAP1 missense variant N929K is not reported in ClinVar and is absent from gnomAD. Prediction tools cluster into two groups: benign—REVEL—and pathogenic—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely pathogenic outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus confirms a likely pathogenic status; Foldetta results are not available. Taken together, the preponderance of evidence supports a pathogenic interpretation for N929K, and this conclusion is consistent with the absence of a ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -10.041 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.182 | Likely Benign | -4.35 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.48 | Pathogenic | 0.00 | Affected | 0.2098 | 0.6046 | 1 | 0 | -0.4 | 14.07 | |||||||||||||||||||||||||||||||||||||||
| c.2787C>G | N929K 2D  AIThe SynGAP1 missense variant N929K is not reported in ClinVar and is absent from gnomAD. Prediction tools cluster into two groups: benign—REVEL—and pathogenic—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely pathogenic outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus confirms a likely pathogenic status; Foldetta results are not available. Taken together, the preponderance of evidence supports a pathogenic interpretation for N929K, and this conclusion is consistent with the absence of a ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -10.041 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.182 | Likely Benign | -4.35 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.48 | Pathogenic | 0.00 | Affected | 0.2098 | 0.6046 | 1 | 0 | -0.4 | 14.07 | |||||||||||||||||||||||||||||||||||||||
| c.2788C>A | P930T 2D  AIThe SynGAP1 missense variant P930T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining 11 predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) all indicate pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts a pathogenic change, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability analysis is available. Overall, the consensus of the majority of tools supports a pathogenic classification, and this is consistent with the absence of any ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -9.558 | Likely Pathogenic | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.454 | Likely Benign | -5.97 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.67 | Pathogenic | 0.00 | Affected | 0.1666 | 0.5593 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2788C>G | P930A 2D  AIThe SynGAP1 missense variant P930A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that P930A is most likely pathogenic, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -8.938 | Likely Pathogenic | 0.963 | Likely Pathogenic | Likely Pathogenic | 0.392 | Likely Benign | -6.03 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 0.68 | Pathogenic | 0.00 | Affected | 0.3376 | 0.4983 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2788C>T | P930S 2D  AIThe SynGAP1 missense variant P930S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are limited to REVEL, which scores the variant as benign. In contrast, the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is Likely Pathogenic; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that P930S is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -8.439 | Likely Pathogenic | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.379 | Likely Benign | -5.84 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.68 | Pathogenic | 0.00 | Affected | 0.3301 | 0.5457 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2789C>A | P930H 2D  AIThe SynGAP1 missense variant P930H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. All other evaluated predictors—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—classify the variant as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from multiple independent predictors and high‑accuracy tools indicates that P930H is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -9.824 | Likely Pathogenic | 0.983 | Likely Pathogenic | Likely Pathogenic | 0.497 | Likely Benign | -6.44 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 0.65 | Pathogenic | 0.00 | Affected | 0.1942 | 0.4392 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2789C>G | P930R 2D  AIThe SynGAP1 missense variant P930R is not reported in ClinVar and is absent from gnomAD. Prediction tools that assess pathogenicity all converge on a deleterious effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict pathogenicity. No tool in the dataset predicts a benign outcome. High‑accuracy assessments further support this view: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the consensus of available predictions indicates that P930R is most likely pathogenic, and this conclusion is consistent with the absence of a ClinVar entry (i.e., no contradictory status). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -9.474 | Likely Pathogenic | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.505 | Likely Pathogenic | -6.67 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.66 | Pathogenic | 0.00 | Affected | 0.1460 | 0.3515 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2789C>T | P930L 2D  AIThe SynGAP1 missense variant P930L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts Pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from multiple in silico predictors points to a pathogenic effect for P930L, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -10.690 | Likely Pathogenic | 0.988 | Likely Pathogenic | Likely Pathogenic | 0.440 | Likely Benign | -7.25 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.66 | Pathogenic | 0.00 | Affected | 0.2275 | 0.6113 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||||
| c.278G>A | R93Q 2D  AIThe SynGAP1 missense variant R93Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect; there is no conflict with ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | -3.938 | Likely Benign | 0.167 | Likely Benign | Likely Benign | 0.074 | Likely Benign | -0.38 | Neutral | 0.203 | Benign | 0.006 | Benign | 4.14 | Benign | 0.00 | Affected | 0.3568 | 0.2669 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||||||||||||||
| c.2796C>G | F932L 2D  AIThe SynGAP1 missense variant F932L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the consensus of the available predictions points to a pathogenic effect for F932L, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -3.390 | Likely Benign | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.170 | Likely Benign | -3.50 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.36 | Pathogenic | 0.03 | Affected | 0.2373 | 0.3895 | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2828G>A | G943D 2D  AIThe SynGAP1 missense variant G943D is not reported in ClinVar and is absent from gnomAD. In silico predictors that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts a pathogenic outcome; ESM1b is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Taken together, the overwhelming majority of predictions indicate a benign effect, and this conclusion does not contradict any ClinVar status (none is assigned). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.973328 | Disordered | 0.860437 | Binding | 0.372 | 0.910 | 0.750 | -7.951 | In-Between | 0.304 | Likely Benign | Likely Benign | 0.296 | Likely Benign | -0.28 | Neutral | 0.224 | Benign | 0.113 | Benign | 2.85 | Benign | 0.11 | Tolerated | 0.1904 | 0.2826 | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2863T>G | S955A 2D  AIThe SynGAP1 missense variant S955A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | -4.258 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.091 | Likely Benign | -0.71 | Neutral | 0.004 | Benign | 0.006 | Benign | 2.41 | Pathogenic | 0.00 | Affected | 0.4262 | 0.5038 | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2871T>G | H957Q 2D  AIThe SynGAP1 missense variant H957Q is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is not contradicted by any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.968874 | Binding | 0.362 | 0.915 | 0.750 | -6.304 | Likely Benign | 0.130 | Likely Benign | Likely Benign | 0.113 | Likely Benign | -0.87 | Neutral | 0.255 | Benign | 0.105 | Benign | 2.54 | Benign | 0.56 | Tolerated | 0.2227 | 0.4114 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2872C>A | H958N 2D  AIThe SynGAP1 missense variant H958N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.976011 | Binding | 0.371 | 0.913 | 0.750 | -8.644 | Likely Pathogenic | 0.097 | Likely Benign | Likely Benign | 0.110 | Likely Benign | -0.56 | Neutral | 0.836 | Possibly Damaging | 0.232 | Benign | 4.17 | Benign | 1.00 | Tolerated | 0.2358 | 0.3638 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2872C>G | H958D 2D  AIThe SynGAP1 missense variant H958D is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and ESM1b—suggest a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.976011 | Binding | 0.371 | 0.913 | 0.750 | -11.494 | Likely Pathogenic | 0.227 | Likely Benign | Likely Benign | 0.200 | Likely Benign | -0.55 | Neutral | 0.925 | Possibly Damaging | 0.232 | Benign | 4.16 | Benign | 0.55 | Tolerated | 0.2732 | 0.2866 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2872C>T | H958Y 2D  AIThe SynGAP1 missense variant H958Y is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and ESM1b—suggest a pathogenic impact. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not conflict with the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.976011 | Binding | 0.371 | 0.913 | 0.750 | -8.393 | Likely Pathogenic | 0.132 | Likely Benign | Likely Benign | 0.129 | Likely Benign | -1.03 | Neutral | 0.836 | Possibly Damaging | 0.232 | Benign | 4.14 | Benign | 0.06 | Tolerated | 0.1792 | 0.4706 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.2873A>G | H958R 2D  AIThe SynGAP1 missense variant H958R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.976011 | Binding | 0.371 | 0.913 | 0.750 | -9.188 | Likely Pathogenic | 0.169 | Likely Benign | Likely Benign | 0.134 | Likely Benign | -1.29 | Neutral | 0.836 | Possibly Damaging | 0.232 | Benign | 4.17 | Benign | 0.09 | Tolerated | 0.2440 | 0.3410 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||||||
| c.2873A>T | H958L 2D  AIThe SynGAP1 missense variant H958L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as benign. Only two tools, SIFT and ESM1b, predict a pathogenic outcome. When the high‑accuracy consensus is considered, the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a likely benign verdict, and AlphaMissense‑Optimized also reports benign. Foldetta predictions are unavailable. Overall, the majority of evidence supports a benign interpretation, and this is consistent with the lack of ClinVar annotation. Thus, the variant is most likely benign and does not contradict existing ClinVar data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.976011 | Binding | 0.371 | 0.913 | 0.750 | -8.422 | Likely Pathogenic | 0.087 | Likely Benign | Likely Benign | 0.181 | Likely Benign | -1.28 | Neutral | 0.001 | Benign | 0.001 | Benign | 4.25 | Benign | 0.03 | Affected | 0.1723 | 0.5338 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2874C>A | H958Q 2D  AIThe SynGAP1 missense variant H958Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. The predictions do not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.976011 | Binding | 0.371 | 0.913 | 0.750 | -8.625 | Likely Pathogenic | 0.117 | Likely Benign | Likely Benign | 0.144 | Likely Benign | -0.97 | Neutral | 0.925 | Possibly Damaging | 0.316 | Benign | 4.18 | Benign | 0.11 | Tolerated | 0.2281 | 0.3736 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2874C>G | H958Q 2D  AIThe SynGAP1 missense variant H958Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. The predictions do not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.976011 | Binding | 0.371 | 0.913 | 0.750 | -8.625 | Likely Pathogenic | 0.117 | Likely Benign | Likely Benign | 0.144 | Likely Benign | -0.97 | Neutral | 0.925 | Possibly Damaging | 0.316 | Benign | 4.18 | Benign | 0.11 | Tolerated | 0.2281 | 0.3736 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2875C>A | H959N 2D  AIThe SynGAP1 missense variant H959N is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only ESM1b predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from multiple independent predictors and high‑accuracy tools indicates that H959N is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.980566 | Binding | 0.333 | 0.905 | 0.750 | -8.811 | Likely Pathogenic | 0.104 | Likely Benign | Likely Benign | 0.115 | Likely Benign | -0.10 | Neutral | 0.144 | Benign | 0.058 | Benign | 4.15 | Benign | 0.21 | Tolerated | 0.2298 | 0.3838 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2875C>T | H959Y 2D  AIThe SynGAP1 missense variant H959Y is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in‑silico tools cluster around a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate a tolerated change, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also classifies it as Likely Benign. In contrast, polyPhen‑2 HumDiv, SIFT, and ESM1b predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect for H959Y, and this conclusion does not conflict with ClinVar, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.980566 | Binding | 0.333 | 0.905 | 0.750 | -8.367 | Likely Pathogenic | 0.146 | Likely Benign | Likely Benign | 0.140 | Likely Benign | -1.09 | Neutral | 0.510 | Possibly Damaging | 0.147 | Benign | 4.09 | Benign | 0.05 | Affected | 0.1672 | 0.4906 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.2876A>C | H959P 2D  AIThe SynGAP1 missense variant H959P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.980566 | Binding | 0.333 | 0.905 | 0.750 | -8.902 | Likely Pathogenic | 0.070 | Likely Benign | Likely Benign | 0.232 | Likely Benign | -0.61 | Neutral | 0.453 | Possibly Damaging | 0.105 | Benign | 4.14 | Benign | 0.38 | Tolerated | 0.2284 | 0.4901 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2876A>G | H959R 2D  AIThe SynGAP1 missense variant H959R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only ESM1b predicts a pathogenic outcome, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as likely benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates likely benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the preponderance of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.980566 | Binding | 0.333 | 0.905 | 0.750 | -9.459 | Likely Pathogenic | 0.182 | Likely Benign | Likely Benign | 0.162 | Likely Benign | -1.11 | Neutral | 0.144 | Benign | 0.078 | Benign | 4.14 | Benign | 0.15 | Tolerated | 0.2416 | 0.3610 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||||||
| c.2876A>T | H959L 2D  AIThe SynGAP1 missense variant H959L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only ESM1b predicts a pathogenic outcome, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as likely benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that H959L is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.980566 | Binding | 0.333 | 0.905 | 0.750 | -8.515 | Likely Pathogenic | 0.100 | Likely Benign | Likely Benign | 0.236 | Likely Benign | -1.38 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.14 | Benign | 0.09 | Tolerated | 0.1698 | 0.5538 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2871T>A | H957Q 2D  AIThe SynGAP1 missense variant H957Q is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign.” Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is not contradicted by any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.968874 | Binding | 0.362 | 0.915 | 0.750 | -6.304 | Likely Benign | 0.130 | Likely Benign | Likely Benign | 0.113 | Likely Benign | -0.87 | Neutral | 0.255 | Benign | 0.105 | Benign | 2.54 | Benign | 0.56 | Tolerated | 0.2227 | 0.4114 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2870A>T | H957L 2D  AIThe SynGAP1 missense variant H957L is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only FATHMM predicts a pathogenic outcome. When predictions are grouped by consensus, the benign group contains eight tools, whereas the pathogenic group contains only FATHMM. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a Likely Benign result. Foldetta, a protein‑folding stability method, has no available output for this variant. Overall, the preponderance of evidence indicates that H957L is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.968874 | Binding | 0.362 | 0.915 | 0.750 | -6.598 | Likely Benign | 0.090 | Likely Benign | Likely Benign | 0.296 | Likely Benign | -0.38 | Neutral | 0.000 | Benign | 0.000 | Benign | 2.44 | Pathogenic | 0.09 | Tolerated | 0.1525 | 0.5516 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2864C>A | S955Y 2D  AIThe SynGAP1 missense variant S955Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) support a benign classification. There is no ClinVar entry to contradict this assessment, so the variant is most likely benign based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | -6.212 | Likely Benign | 0.216 | Likely Benign | Likely Benign | 0.077 | Likely Benign | -1.62 | Neutral | 0.977 | Probably Damaging | 0.721 | Possibly Damaging | 2.32 | Pathogenic | 0.00 | Affected | 0.1321 | 0.4932 | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||||||
| c.2864C>G | S955C 2D  AIThe SynGAP1 missense variant S955C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs. 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (5 pathogenic vs. 4 benign) indicate a likely pathogenic impact. This conclusion is not contradicted by ClinVar status, as the variant has no existing ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | -8.675 | Likely Pathogenic | 0.117 | Likely Benign | Likely Benign | 0.064 | Likely Benign | -1.48 | Neutral | 0.977 | Probably Damaging | 0.796 | Possibly Damaging | 2.32 | Pathogenic | 0.00 | Affected | 0.1741 | 0.5470 | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||||||
| c.2866T>A | S956T 2D  AIThe SynGAP1 missense variant S956T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign impact for S956T, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.957345 | Binding | 0.364 | 0.917 | 0.750 | -5.404 | Likely Benign | 0.097 | Likely Benign | Likely Benign | 0.079 | Likely Benign | -0.45 | Neutral | 0.369 | Benign | 0.159 | Benign | 2.00 | Pathogenic | 0.08 | Tolerated | 0.2244 | 0.5727 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2866T>C | S956P 2D  AIThe SynGAP1 missense variant S956P is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.957345 | Binding | 0.364 | 0.917 | 0.750 | -3.526 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.157 | Likely Benign | -0.55 | Neutral | 0.000 | Benign | 0.001 | Benign | 1.95 | Pathogenic | 0.05 | Affected | 0.2670 | 0.5337 | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2866T>G | S956A 2D  AIThe SynGAP1 missense variant S956A is not reported in ClinVar or gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify it as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as likely benign. Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta stability analysis is unavailable. Overall, the collective evidence indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.957345 | Binding | 0.364 | 0.917 | 0.750 | -4.468 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.111 | Likely Benign | -0.47 | Neutral | 0.112 | Benign | 0.039 | Benign | 2.18 | Pathogenic | 0.13 | Tolerated | 0.4239 | 0.4838 | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2867C>A | S956Y 2D  AIThe SynGAP1 missense variant S956Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the lack of ClinVar annotation. Thus, the variant is most likely benign, with no contradiction from ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.957345 | Binding | 0.364 | 0.917 | 0.750 | -4.920 | Likely Benign | 0.233 | Likely Benign | Likely Benign | 0.085 | Likely Benign | -0.90 | Neutral | 0.411 | Benign | 0.097 | Benign | 1.93 | Pathogenic | 0.22 | Tolerated | 0.1449 | 0.4932 | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||||||
| c.2867C>G | S956C 2D  AIThe SynGAP1 missense variant S956C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign vs. two pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (five pathogenic vs. four benign) indicate a likely pathogenic impact. This conclusion is not contradicted by ClinVar status, as the variant has no existing ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.984871 | Disordered | 0.957345 | Binding | 0.364 | 0.917 | 0.750 | -9.292 | Likely Pathogenic | 0.108 | Likely Benign | Likely Benign | 0.107 | Likely Benign | -0.34 | Neutral | 0.938 | Possibly Damaging | 0.665 | Possibly Damaging | 1.94 | Pathogenic | 0.03 | Affected | 0.1833 | 0.5470 | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||||||
| c.2867C>T | S956F 2D  AIThe SynGAP1 missense variant S956F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. The predictions do not contradict any ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.957345 | Binding | 0.364 | 0.917 | 0.750 | -6.654 | Likely Benign | 0.226 | Likely Benign | Likely Benign | 0.101 | Likely Benign | -1.04 | Neutral | 0.832 | Possibly Damaging | 0.398 | Benign | 1.93 | Pathogenic | 0.39 | Tolerated | 0.1315 | 0.4943 | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||||||||||||
| c.2869C>A | H957N 2D  AIThe SynGAP1 missense variant H957N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as benign. Only FATHMM predicts a pathogenic outcome. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign status. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.968874 | Binding | 0.362 | 0.915 | 0.750 | -6.804 | Likely Benign | 0.090 | Likely Benign | Likely Benign | 0.053 | Likely Benign | -0.45 | Neutral | 0.144 | Benign | 0.058 | Benign | 2.45 | Pathogenic | 0.50 | Tolerated | 0.2343 | 0.3788 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2869C>G | H957D 2D  AIThe SynGAP1 missense variant H957D is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are ESM1b and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 benign vs 2 pathogenic), and Foldetta results are unavailable. Overall, the majority of evidence (7 benign vs 2 pathogenic) supports a benign classification. This prediction is consistent with the lack of ClinVar annotation and gnomAD presence, indicating no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.985964 | Disordered | 0.968874 | Binding | 0.362 | 0.915 | 0.750 | -8.777 | Likely Pathogenic | 0.237 | Likely Benign | Likely Benign | 0.149 | Likely Benign | -0.77 | Neutral | 0.144 | Benign | 0.058 | Benign | 2.45 | Pathogenic | 0.44 | Tolerated | 0.2646 | 0.3066 | 1 | -1 | -0.3 | -22.05 | ||||||||||||||||||||||||||||||||||||||||
| c.286G>C | G96R 2D  AIThe SynGAP1 missense variant G96R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G96R, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -3.872 | Likely Benign | 0.349 | Ambiguous | Likely Benign | 0.059 | Likely Benign | -0.91 | Neutral | 0.687 | Possibly Damaging | 0.062 | Benign | 4.19 | Benign | 0.00 | Affected | 0.1300 | 0.5031 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.286G>T | G96C 2D  AIThe SynGAP1 missense variant G96C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The high‑accuracy consensus methods give a benign verdict: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign.” No result is available from Foldetta, so its folding‑stability assessment is not considered. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of any ClinVar classification. Thus, the variant is most likely benign and does not contradict ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -5.140 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.112 | Likely Benign | -1.87 | Neutral | 0.981 | Probably Damaging | 0.216 | Benign | 4.12 | Benign | 0.00 | Affected | 0.1645 | 0.4142 | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||||||
| c.2870A>C | H957P 2D  AIThe SynGAP1 H957P missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and FATHMM. The high‑accuracy consensus (SGM‑Consensus) is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yielding a Likely Benign classification (3 benign vs. 1 pathogenic). AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.968874 | Binding | 0.362 | 0.915 | 0.750 | -6.347 | Likely Benign | 0.061 | Likely Benign | Likely Benign | 0.244 | Likely Benign | -0.61 | Neutral | 0.453 | Possibly Damaging | 0.105 | Benign | 2.42 | Pathogenic | 0.13 | Tolerated | 0.2300 | 0.4701 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2877C>A | H959Q 2D  AIThe SynGAP1 missense variant H959Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as likely benign. Only ESM1b predicts a pathogenic outcome, representing the sole discordant signal. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that H959Q is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.980566 | Binding | 0.333 | 0.905 | 0.750 | -8.657 | Likely Pathogenic | 0.126 | Likely Benign | Likely Benign | 0.182 | Likely Benign | -0.77 | Neutral | 0.255 | Benign | 0.105 | Benign | 4.15 | Benign | 0.10 | Tolerated | 0.2264 | 0.3936 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2877C>G | H959Q 2D  AIThe SynGAP1 missense variant H959Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign, while the single pathogenic prediction comes from ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus itself is benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that H959Q is most likely benign, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.980566 | Binding | 0.333 | 0.905 | 0.750 | -8.657 | Likely Pathogenic | 0.126 | Likely Benign | Likely Benign | 0.182 | Likely Benign | -0.77 | Neutral | 0.255 | Benign | 0.105 | Benign | 4.15 | Benign | 0.10 | Tolerated | 0.2264 | 0.3936 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2878C>A | H960N 2D  AIThe SynGAP1 missense variant H960N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987911 | Disordered | 0.983385 | Binding | 0.380 | 0.901 | 0.750 | -8.822 | Likely Pathogenic | 0.101 | Likely Benign | Likely Benign | 0.130 | Likely Benign | -0.57 | Neutral | 0.494 | Possibly Damaging | 0.129 | Benign | 4.19 | Benign | 0.40 | Tolerated | 0.2137 | 0.3695 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2884C>A | H962N 2D  AIThe SynGAP1 missense variant H962N is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only ESM1b predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.984483 | Binding | 0.369 | 0.886 | 0.750 | -8.481 | Likely Pathogenic | 0.098 | Likely Benign | Likely Benign | 0.058 | Likely Benign | -0.61 | Neutral | 0.174 | Benign | 0.045 | Benign | 4.18 | Benign | 0.24 | Tolerated | 0.1957 | 0.3216 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2884C>G | H962D 2D  AIThe SynGAP1 missense variant H962D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only ESM1b predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect. There is no ClinVar entry to contradict this conclusion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.984483 | Binding | 0.369 | 0.886 | 0.750 | -12.472 | Likely Pathogenic | 0.237 | Likely Benign | Likely Benign | 0.178 | Likely Benign | -1.08 | Neutral | 0.001 | Benign | 0.002 | Benign | 4.20 | Benign | 0.20 | Tolerated | 0.2402 | 0.2643 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2884C>T | H962Y 2D  AIThe SynGAP1 missense variant H962Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for H962Y, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.984483 | Binding | 0.369 | 0.886 | 0.750 | -7.735 | In-Between | 0.167 | Likely Benign | Likely Benign | 0.093 | Likely Benign | -1.27 | Neutral | 0.878 | Possibly Damaging | 0.232 | Benign | 4.12 | Benign | 0.03 | Affected | 0.1741 | 0.4411 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.2885A>C | H962P 2D  AIThe SynGAP1 missense variant H962P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and ESM1b—suggest a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized reports benign, and the SGM‑Consensus (majority vote) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.984483 | Binding | 0.369 | 0.886 | 0.750 | -8.419 | Likely Pathogenic | 0.066 | Likely Benign | Likely Benign | 0.197 | Likely Benign | -0.94 | Neutral | 0.748 | Possibly Damaging | 0.170 | Benign | 4.16 | Benign | 0.07 | Tolerated | 0.2011 | 0.4352 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2885A>G | H962R 2D  AIThe SynGAP1 missense variant H962R is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” status. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the lack of any ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.984483 | Binding | 0.369 | 0.886 | 0.750 | -9.166 | Likely Pathogenic | 0.212 | Likely Benign | Likely Benign | 0.117 | Likely Benign | -1.04 | Neutral | 0.325 | Benign | 0.129 | Benign | 4.18 | Benign | 0.07 | Tolerated | 0.2276 | 0.3337 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||||||
| c.2885A>T | H962L 2D  AIThe SynGAP1 missense variant H962L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact for H962L, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.984483 | Binding | 0.369 | 0.886 | 0.750 | -8.478 | Likely Pathogenic | 0.108 | Likely Benign | Likely Benign | 0.151 | Likely Benign | -1.49 | Neutral | 0.494 | Possibly Damaging | 0.170 | Benign | 4.15 | Benign | 0.03 | Affected | 0.1738 | 0.5487 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2886C>A | H962Q 2D  AIThe SynGAP1 missense variant H962Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only ESM1b predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.984483 | Binding | 0.369 | 0.886 | 0.750 | -8.161 | Likely Pathogenic | 0.130 | Likely Benign | Likely Benign | 0.114 | Likely Benign | -1.04 | Neutral | 0.325 | Benign | 0.045 | Benign | 4.19 | Benign | 0.06 | Tolerated | 0.1943 | 0.3844 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2886C>G | H962Q 2D  AIThe SynGAP1 missense variant H962Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only ESM1b predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect. There is no ClinVar entry to contradict this conclusion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.984483 | Binding | 0.369 | 0.886 | 0.750 | -8.161 | Likely Pathogenic | 0.130 | Likely Benign | Likely Benign | 0.114 | Likely Benign | -1.04 | Neutral | 0.325 | Benign | 0.045 | Benign | 4.19 | Benign | 0.06 | Tolerated | 0.1943 | 0.3844 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2887C>A | H963N 2D  AIThe SynGAP1 missense variant H963N is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only ESM1b predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical databases. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.983973 | Binding | 0.325 | 0.886 | 0.750 | -8.274 | Likely Pathogenic | 0.099 | Likely Benign | Likely Benign | 0.089 | Likely Benign | -0.21 | Neutral | 0.369 | Benign | 0.120 | Benign | 4.18 | Benign | 0.16 | Tolerated | 0.2094 | 0.3596 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2887C>G | H963D 2D  AIThe SynGAP1 missense variant H963D is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the only pathogenic prediction comes from ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” status. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates benign; Foldetta results are not available. Overall, the consensus of the majority of tools and the high‑accuracy predictions point to a benign impact for H963D, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.983973 | Binding | 0.325 | 0.886 | 0.750 | -12.082 | Likely Pathogenic | 0.204 | Likely Benign | Likely Benign | 0.167 | Likely Benign | -0.81 | Neutral | 0.369 | Benign | 0.159 | Benign | 4.16 | Benign | 0.46 | Tolerated | 0.2482 | 0.3023 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2888A>C | H963P 2D  AIThe SynGAP1 missense variant H963P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as benign. Only ESM1b predicts a pathogenic outcome, while the consensus score from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized reports benign, and the SGM Consensus also indicates Likely Benign; Foldetta data are not available. Overall, the majority of evidence points to a benign effect, and this conclusion is not in conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.983973 | Binding | 0.325 | 0.886 | 0.750 | -8.158 | Likely Pathogenic | 0.074 | Likely Benign | Likely Benign | 0.223 | Likely Benign | -1.10 | Neutral | 0.000 | Benign | 0.001 | Benign | 4.10 | Benign | 0.14 | Tolerated | 0.2101 | 0.4487 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2888A>T | H963L 2D  AIThe SynGAP1 missense variant H963L is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only ESM1b predicts a pathogenic outcome, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as likely benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized indicates benign, and the SGM‑Consensus likewise suggests a benign effect; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for H963L, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.983973 | Binding | 0.325 | 0.886 | 0.750 | -8.110 | Likely Pathogenic | 0.109 | Likely Benign | Likely Benign | 0.172 | Likely Benign | -1.58 | Neutral | 0.224 | Benign | 0.091 | Benign | 4.13 | Benign | 0.43 | Tolerated | 0.1626 | 0.5680 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2889T>A | H963Q 2D  AIThe SynGAP1 missense variant H963Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts a pathogenic outcome; the only uncertain result comes from ESM1b. The high‑accuracy consensus methods also support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta, a protein‑folding stability predictor, has no available result for this variant. Overall, the evidence overwhelmingly indicates that H963Q is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.983973 | Binding | 0.325 | 0.886 | 0.750 | -7.798 | In-Between | 0.116 | Likely Benign | Likely Benign | 0.104 | Likely Benign | -0.75 | Neutral | 0.411 | Benign | 0.132 | Benign | 4.17 | Benign | 0.29 | Tolerated | 0.2112 | 0.3979 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2883C>G | H961Q 2D  AIThe SynGAP1 missense variant H961Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT and ESM1b predict a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a majority‑benign vote and is reported as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote) is benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the available predictions indicates that the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.989835 | Disordered | 0.984562 | Binding | 0.323 | 0.893 | 0.750 | -8.368 | Likely Pathogenic | 0.118 | Likely Benign | Likely Benign | 0.088 | Likely Benign | -0.49 | Neutral | 0.255 | Benign | 0.105 | Benign | 4.17 | Benign | 0.02 | Affected | 0.2022 | 0.3743 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2883C>A | H961Q 2D  AIThe SynGAP1 missense variant H961Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only SIFT and ESM1b predict pathogenicity. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a “Likely Benign” verdict (3 benign vs. 1 pathogenic). High‑accuracy assessments reinforce this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote) is benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.989835 | Disordered | 0.984562 | Binding | 0.323 | 0.893 | 0.750 | -8.368 | Likely Pathogenic | 0.118 | Likely Benign | Likely Benign | 0.088 | Likely Benign | -0.49 | Neutral | 0.255 | Benign | 0.105 | Benign | 4.17 | Benign | 0.02 | Affected | 0.2022 | 0.3743 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2878C>G | H960D 2D  AIThe SynGAP1 missense variant H960D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. The predictions do not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987911 | Disordered | 0.983385 | Binding | 0.380 | 0.901 | 0.750 | -12.235 | Likely Pathogenic | 0.243 | Likely Benign | Likely Benign | 0.147 | Likely Benign | -1.09 | Neutral | 0.494 | Possibly Damaging | 0.170 | Benign | 4.19 | Benign | 0.31 | Tolerated | 0.2504 | 0.2923 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2879A>C | H960P 2D  AIThe SynGAP1 missense variant H960P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987911 | Disordered | 0.983385 | Binding | 0.380 | 0.901 | 0.750 | -8.672 | Likely Pathogenic | 0.064 | Likely Benign | Likely Benign | 0.119 | Likely Benign | -0.95 | Neutral | 0.494 | Possibly Damaging | 0.170 | Benign | 4.18 | Benign | 0.28 | Tolerated | 0.2157 | 0.4901 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2879A>T | H960L 2D  AIThe SynGAP1 missense variant H960L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the only pathogenic prediction comes from ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987911 | Disordered | 0.983385 | Binding | 0.380 | 0.901 | 0.750 | -8.386 | Likely Pathogenic | 0.108 | Likely Benign | Likely Benign | 0.130 | Likely Benign | -1.30 | Neutral | 0.174 | Benign | 0.043 | Benign | 4.17 | Benign | 0.33 | Tolerated | 0.1465 | 0.5595 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.287G>A | G96D 2D  AIThe SynGAP1 missense variant G96D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -3.699 | Likely Benign | 0.249 | Likely Benign | Likely Benign | 0.099 | Likely Benign | -0.95 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.18 | Benign | 0.00 | Affected | 0.2283 | 0.3150 | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||||||
| c.287G>C | G96A 2D  AIThe SynGAP1 missense variant G96A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -3.669 | Likely Benign | 0.066 | Likely Benign | Likely Benign | 0.064 | Likely Benign | -1.00 | Neutral | 0.092 | Benign | 0.007 | Benign | 4.23 | Benign | 0.00 | Affected | 0.4098 | 0.4212 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.287G>T | G96V 2D  AIThe SynGAP1 missense variant G96V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -4.266 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.109 | Likely Benign | -1.43 | Neutral | 0.334 | Benign | 0.029 | Benign | 4.18 | Benign | 0.00 | Affected | 0.1575 | 0.3833 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.2880C>A | H960Q 2D  AIThe SynGAP1 missense variant H960Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. The predictions do not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987911 | Disordered | 0.983385 | Binding | 0.380 | 0.901 | 0.750 | -8.551 | Likely Pathogenic | 0.124 | Likely Benign | Likely Benign | 0.109 | Likely Benign | -0.79 | Neutral | 0.748 | Possibly Damaging | 0.170 | Benign | 4.20 | Benign | 0.21 | Tolerated | 0.2045 | 0.3993 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2880C>G | H960Q 2D  AIThe SynGAP1 missense variant H960Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987911 | Disordered | 0.983385 | Binding | 0.380 | 0.901 | 0.750 | -8.551 | Likely Pathogenic | 0.124 | Likely Benign | Likely Benign | 0.109 | Likely Benign | -0.79 | Neutral | 0.748 | Possibly Damaging | 0.170 | Benign | 4.20 | Benign | 0.21 | Tolerated | 0.2045 | 0.3993 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2881C>A | H961N 2D  AIThe SynGAP1 missense variant H961N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only ESM1b predicts a pathogenic outcome, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as likely benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that H961N is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.989835 | Disordered | 0.984562 | Binding | 0.323 | 0.893 | 0.750 | -8.561 | Likely Pathogenic | 0.096 | Likely Benign | Likely Benign | 0.084 | Likely Benign | -0.32 | Neutral | 0.069 | Benign | 0.036 | Benign | 4.17 | Benign | 0.81 | Tolerated | 0.2074 | 0.3695 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2881C>G | H961D 2D  AIThe SynGAP1 missense variant H961D is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only ESM1b predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.989835 | Disordered | 0.984562 | Binding | 0.323 | 0.893 | 0.750 | -12.522 | Likely Pathogenic | 0.224 | Likely Benign | Likely Benign | 0.115 | Likely Benign | -0.98 | Neutral | 0.069 | Benign | 0.036 | Benign | 4.19 | Benign | 0.09 | Tolerated | 0.2487 | 0.2723 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2882A>C | H961P 2D  AIThe SynGAP1 missense variant H961P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as benign, while only SIFT and ESM1b predict pathogenicity. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a Likely Benign verdict (3 benign vs. 1 pathogenic). High‑accuracy assessments reinforce this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Consequently, the collective evidence indicates that H961P is most likely benign, and this conclusion is not in conflict with ClinVar, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.989835 | Disordered | 0.984562 | Binding | 0.323 | 0.893 | 0.750 | -8.434 | Likely Pathogenic | 0.071 | Likely Benign | Likely Benign | 0.210 | Likely Benign | -0.58 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.15 | Benign | 0.02 | Affected | 0.2061 | 0.4701 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2882A>G | H961R 2D  AIThe SynGAP1 missense variant H961R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and ESM1b. The SGM‑Consensus, which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign (3 benign vs. 1 pathogenic). High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.989835 | Disordered | 0.984562 | Binding | 0.323 | 0.893 | 0.750 | -9.258 | Likely Pathogenic | 0.189 | Likely Benign | Likely Benign | 0.101 | Likely Benign | -0.90 | Neutral | 0.144 | Benign | 0.078 | Benign | 4.16 | Benign | 0.02 | Affected | 0.2201 | 0.3267 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||||||
| c.2882A>T | H961L 2D  AIThe SynGAP1 missense variant H961L is not reported in ClinVar and is absent from gnomAD, indicating no known population frequency data. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only SIFT and ESM1b predict pathogenicity, but these are outliers among the consensus. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as likely benign. High‑accuracy assessments reinforce this: AlphaMissense‑Optimized is benign, SGM‑Consensus is benign, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the preponderance of evidence indicates the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.989835 | Disordered | 0.984562 | Binding | 0.323 | 0.893 | 0.750 | -8.547 | Likely Pathogenic | 0.109 | Likely Benign | Likely Benign | 0.155 | Likely Benign | -1.21 | Neutral | 0.144 | Benign | 0.078 | Benign | 4.13 | Benign | 0.01 | Affected | 0.1465 | 0.5344 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2889T>G | H963Q 2D  AIThe SynGAP1 missense variant H963Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity; the only uncertain result comes from ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the collective evidence strongly supports a benign classification, and there is no ClinVar annotation to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.991070 | Disordered | 0.983973 | Binding | 0.325 | 0.886 | 0.750 | -7.798 | In-Between | 0.116 | Likely Benign | Likely Benign | 0.104 | Likely Benign | -0.75 | Neutral | 0.411 | Benign | 0.132 | Benign | 4.17 | Benign | 0.29 | Tolerated | 0.2112 | 0.3979 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2863T>A | S955T 2D  AIThe SynGAP1 missense variant S955T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for S955T, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | -4.717 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.070 | Likely Benign | -0.96 | Neutral | 0.451 | Benign | 0.265 | Benign | 2.38 | Pathogenic | 0.00 | Affected | 0.2184 | 0.5927 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2828G>T | G943V 2D  AIThe SynGAP1 missense variant G943V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as benign. The only tool with an uncertain outcome is ESM1b, which does not alter the overall consensus. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign classification. High‑accuracy predictors corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign; Foldetta results are not available. Taken together, the evidence overwhelmingly supports a benign interpretation, and there is no conflict with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.973328 | Disordered | 0.860437 | Binding | 0.372 | 0.910 | 0.750 | -7.658 | In-Between | 0.092 | Likely Benign | Likely Benign | 0.265 | Likely Benign | -0.43 | Neutral | 0.224 | Benign | 0.062 | Benign | 2.85 | Benign | 0.16 | Tolerated | 0.1399 | 0.3507 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.283C>T | H95Y 2D  AIThe SynGAP1 missense variant H95Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence indicates that H95Y is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -3.610 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.090 | Likely Benign | -1.45 | Neutral | 0.219 | Benign | 0.014 | Benign | 4.14 | Benign | 0.00 | Affected | 0.0880 | 0.4122 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.2840G>T | G947V 2D  AIThe SynGAP1 missense variant G947V is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate benign, whereas polyPhen‑2 HumDiv and SIFT predict pathogenicity; ESM1b remains uncertain. High‑accuracy methods reinforce the benign assessment: AlphaMissense‑Optimized scores benign, the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign,” and Foldetta data are not available. Overall, the preponderance of evidence points to a benign impact for G947V, and this conclusion is consistent with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988695 | Disordered | 0.850554 | Binding | 0.358 | 0.919 | 0.750 | -7.171 | In-Between | 0.105 | Likely Benign | Likely Benign | 0.296 | Likely Benign | -1.11 | Neutral | 0.586 | Possibly Damaging | 0.303 | Benign | 4.93 | Benign | 0.01 | Affected | 0.1443 | 0.3507 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.2842G>A | G948S 2D  AIThe SynGAP1 missense variant G948S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools that assess sequence conservation and structural impact uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this benign assessment: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign.” Foldetta, a protein‑folding stability predictor, has no available result for this variant. Overall, the collective evidence indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988505 | Disordered | 0.862121 | Binding | 0.365 | 0.919 | 0.750 | -4.760 | Likely Benign | 0.080 | Likely Benign | Likely Benign | 0.177 | Likely Benign | 1.09 | Neutral | 0.068 | Benign | 0.026 | Benign | 4.57 | Benign | 1.00 | Tolerated | 0.2466 | 0.5502 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2842G>C | G948R 2D  AIThe SynGAP1 missense variant G948R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and SIFT—suggest a pathogenic impact. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988505 | Disordered | 0.862121 | Binding | 0.365 | 0.919 | 0.750 | -6.782 | Likely Benign | 0.315 | Likely Benign | Likely Benign | 0.266 | Likely Benign | -0.60 | Neutral | 0.818 | Possibly Damaging | 0.435 | Benign | 4.58 | Benign | 0.04 | Affected | 0.1069 | 0.4933 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2842G>T | G948C 2D  AIThe SynGAP1 missense variant G948C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and ESM1b. The high‑accuracy consensus (SGM‑Consensus) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the majority of evidence points to a benign impact. The predictions do not contradict ClinVar status, as no ClinVar assertion exists for this variant. Thus, based on the available computational predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988505 | Disordered | 0.862121 | Binding | 0.365 | 0.919 | 0.750 | -10.254 | Likely Pathogenic | 0.102 | Likely Benign | Likely Benign | 0.247 | Likely Benign | -0.77 | Neutral | 0.997 | Probably Damaging | 0.840 | Possibly Damaging | 4.50 | Benign | 0.04 | Affected | 0.1422 | 0.4439 | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||||||
| c.2843G>A | G948D 2D  AIThe SynGAP1 missense variant G948D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988505 | Disordered | 0.862121 | Binding | 0.365 | 0.919 | 0.750 | -8.423 | Likely Pathogenic | 0.267 | Likely Benign | Likely Benign | 0.279 | Likely Benign | 0.15 | Neutral | 0.818 | Possibly Damaging | 0.266 | Benign | 4.55 | Benign | 0.11 | Tolerated | 0.1871 | 0.2826 | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2843G>C | G948A 2D  AIThe SynGAP1 missense variant G948A is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988505 | Disordered | 0.862121 | Binding | 0.365 | 0.919 | 0.750 | -5.627 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.189 | Likely Benign | 0.04 | Neutral | 0.288 | Benign | 0.103 | Benign | 4.64 | Benign | 0.39 | Tolerated | 0.3350 | 0.4957 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2843G>T | G948V 2D  AIThe SynGAP1 missense variant G948V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also reports Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods indicates that G948V is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988505 | Disordered | 0.862121 | Binding | 0.365 | 0.919 | 0.750 | -6.541 | Likely Benign | 0.102 | Likely Benign | Likely Benign | 0.256 | Likely Benign | -0.48 | Neutral | 0.901 | Possibly Damaging | 0.435 | Benign | 4.52 | Benign | 0.06 | Tolerated | 0.1464 | 0.3507 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.2845G>C | G949R 2D  AIThe SynGAP1 missense variant G949R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Consensus from standard in silico predictors shows a split: benign calls come from REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic calls come from polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; ESM1b is uncertain. High‑accuracy assessment further supports a benign interpretation: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields a benign outcome, and Foldetta data are unavailable. Overall, the preponderance of evidence indicates the variant is most likely benign, and this conclusion does not conflict with any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.988861 | Disordered | 0.874971 | Binding | 0.365 | 0.923 | 0.750 | -7.650 | In-Between | 0.301 | Likely Benign | Likely Benign | 0.317 | Likely Benign | -0.14 | Neutral | 0.997 | Probably Damaging | 0.934 | Probably Damaging | 2.21 | Pathogenic | 0.01 | Affected | 0.1095 | 0.4569 | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||||||
| c.2845G>T | G949C 2D  AIThe SynGAP1 missense variant G949C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign vs. two pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (five pathogenic vs. four benign) indicate that the variant is most likely pathogenic. This assessment does not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.988861 | Disordered | 0.874971 | Binding | 0.365 | 0.923 | 0.750 | -10.580 | Likely Pathogenic | 0.091 | Likely Benign | Likely Benign | 0.345 | Likely Benign | -1.02 | Neutral | 1.000 | Probably Damaging | 0.975 | Probably Damaging | 2.21 | Pathogenic | 0.01 | Affected | 0.1480 | 0.4097 | -3 | -3 | 2.9 | 46.09 | ||||||||||||||||||||||||||||||||||||||||
| c.2846G>A | G949D 2D  AIThe SynGAP1 missense variant G949D is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and FATHMM. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs. 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑confidence predictors (5 pathogenic vs. 4 benign) indicate a pathogenic impact. This conclusion is not contradicted by ClinVar status, as the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.988861 | Disordered | 0.874971 | Binding | 0.365 | 0.923 | 0.750 | -8.692 | Likely Pathogenic | 0.239 | Likely Benign | Likely Benign | 0.316 | Likely Benign | 0.25 | Neutral | 0.945 | Possibly Damaging | 0.753 | Possibly Damaging | 2.21 | Pathogenic | 0.02 | Affected | 0.1927 | 0.2826 | 1 | -1 | -3.1 | 58.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2846G>C | G949A 2D  AIThe SynGAP1 missense variant G949A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign” (3 benign vs. 1 pathogenic votes). High‑accuracy methods confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988861 | Disordered | 0.874971 | Binding | 0.365 | 0.923 | 0.750 | -6.838 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.274 | Likely Benign | -0.08 | Neutral | 0.779 | Possibly Damaging | 0.425 | Benign | 2.28 | Pathogenic | 0.08 | Tolerated | 0.3385 | 0.4414 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2846G>T | G949V 2D  AIThe SynGAP1 missense variant G949V is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also favors a benign outcome. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.988861 | Disordered | 0.874971 | Binding | 0.365 | 0.923 | 0.750 | -7.364 | In-Between | 0.102 | Likely Benign | Likely Benign | 0.328 | Likely Benign | -0.55 | Neutral | 0.992 | Probably Damaging | 0.834 | Possibly Damaging | 2.21 | Pathogenic | 0.01 | Affected | 0.1493 | 0.3165 | -1 | -3 | 4.6 | 42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.283C>A | H95N 2D  AIThe SynGAP1 missense variant H95N is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the only pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) indicates likely benign. Foldetta results are unavailable, so they do not influence the assessment. Overall, the computational evidence overwhelmingly supports a benign classification for H95N, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -3.454 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.089 | Likely Benign | -1.12 | Neutral | 0.219 | Benign | 0.009 | Benign | 4.20 | Benign | 0.00 | Affected | 0.1821 | 0.2491 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2839G>T | G947W 2D  AIThe SynGAP1 missense variant G947W has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988695 | Disordered | 0.850554 | Binding | 0.358 | 0.919 | 0.750 | -10.819 | Likely Pathogenic | 0.285 | Likely Benign | Likely Benign | 0.349 | Likely Benign | -1.21 | Neutral | 0.983 | Probably Damaging | 0.868 | Possibly Damaging | 4.90 | Benign | 0.01 | Affected | 0.0914 | 0.4059 | -7 | -2 | -0.5 | 129.16 | |||||||||||||||||||||||||||||||||||||||
| c.2830G>C | G944R 2D  AIThe SynGAP1 missense variant G944R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G944R, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | -6.577 | Likely Benign | 0.359 | Ambiguous | Likely Benign | 0.459 | Likely Benign | -1.82 | Neutral | 0.639 | Possibly Damaging | 0.299 | Benign | 3.73 | Benign | 0.00 | Affected | 0.1030 | 0.4733 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2831G>A | G944D 2D  AIThe SynGAP1 missense variant G944D is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools largely support a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the majority‑vote SGM‑Consensus also indicates a likely benign outcome. Only SIFT predicts a pathogenic effect, and ESM1b remains uncertain. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports a likely benign classification. Foldetta stability analysis is not available for this residue. Overall, the preponderance of evidence points to a benign impact, and this assessment does not conflict with the absence of a ClinVar claim. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | -7.684 | In-Between | 0.296 | Likely Benign | Likely Benign | 0.421 | Likely Benign | -1.42 | Neutral | 0.224 | Benign | 0.131 | Benign | 3.70 | Benign | 0.00 | Affected | 0.1833 | 0.2826 | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2831G>C | G944A 2D  AIThe SynGAP1 missense variant G944A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | -6.397 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.314 | Likely Benign | -0.61 | Neutral | 0.059 | Benign | 0.042 | Benign | 3.81 | Benign | 0.00 | Affected | 0.3352 | 0.4957 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2833C>A | H945N 2D  AIThe SynGAP1 missense variant H945N is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available. Overall, the majority of evidence points to a benign effect for H945N, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.982235 | Disordered | 0.849210 | Binding | 0.386 | 0.923 | 0.750 | -5.709 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.266 | Likely Benign | -0.29 | Neutral | 0.982 | Probably Damaging | 0.870 | Possibly Damaging | 5.03 | Benign | 0.05 | Affected | 0.2556 | 0.2997 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2833C>G | H945D 2D  AIThe SynGAP1 missense variant H945D is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for H945D, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.982235 | Disordered | 0.849210 | Binding | 0.386 | 0.923 | 0.750 | -6.572 | Likely Benign | 0.191 | Likely Benign | Likely Benign | 0.396 | Likely Benign | -0.18 | Neutral | 0.982 | Probably Damaging | 0.870 | Possibly Damaging | 5.02 | Benign | 0.04 | Affected | 0.2803 | 0.2275 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2833C>T | H945Y 2D  AIThe SynGAP1 missense variant H945Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available. Overall, the majority of evidence points to a benign effect for H945Y, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.982235 | Disordered | 0.849210 | Binding | 0.386 | 0.923 | 0.750 | -6.036 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.335 | Likely Benign | -0.07 | Neutral | 0.982 | Probably Damaging | 0.903 | Possibly Damaging | 5.27 | Benign | 0.05 | Affected | 0.2102 | 0.3912 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.2834A>C | H945P 2D  AIThe SynGAP1 missense variant H945P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the preponderance of benign predictions and the lack of pathogenic evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.982235 | Disordered | 0.849210 | Binding | 0.386 | 0.923 | 0.750 | -1.962 | Likely Benign | 0.055 | Likely Benign | Likely Benign | 0.420 | Likely Benign | -0.34 | Neutral | 0.012 | Benign | 0.047 | Benign | 5.04 | Benign | 0.05 | Affected | 0.2730 | 0.3907 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2835T>G | H945Q 2D  AIThe SynGAP1 missense variant H945Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. Only two tools, polyPhen‑2 HumDiv and polyPhen‑2 HumVar, predict a pathogenic effect. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact for H945Q, and this conclusion is not contradicted by any ClinVar classification (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.982235 | Disordered | 0.849210 | Binding | 0.386 | 0.923 | 0.750 | -5.248 | Likely Benign | 0.091 | Likely Benign | Likely Benign | 0.343 | Likely Benign | -0.36 | Neutral | 0.995 | Probably Damaging | 0.939 | Probably Damaging | 5.03 | Benign | 0.06 | Tolerated | 4.32 | 4 | 0.2671 | 0.3128 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||
| c.2836G>A | G946R 2D  AIThe SynGAP1 missense variant G946R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while ESM1b is uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus itself is Likely Benign. Foldetta results are unavailable. Overall, the majority of computational evidence indicates that G946R is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | -7.127 | In-Between | 0.308 | Likely Benign | Likely Benign | 0.296 | Likely Benign | -0.69 | Neutral | 0.818 | Possibly Damaging | 0.435 | Benign | 4.65 | Benign | 0.00 | Affected | 0.1157 | 0.5133 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2836G>C | G946R 2D  AIThe SynGAP1 missense variant G946R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while ESM1b is uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus itself is Likely Benign. Foldetta results are unavailable. Overall, the majority of computational evidence indicates that G946R is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | -7.127 | In-Between | 0.308 | Likely Benign | Likely Benign | 0.296 | Likely Benign | -0.69 | Neutral | 0.818 | Possibly Damaging | 0.435 | Benign | 4.65 | Benign | 0.00 | Affected | 0.1157 | 0.5133 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2837G>C | G946A 2D  AIThe SynGAP1 missense variant G946A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G946A, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | -7.004 | In-Between | 0.079 | Likely Benign | Likely Benign | 0.191 | Likely Benign | 0.33 | Neutral | 0.649 | Possibly Damaging | 0.209 | Benign | 4.77 | Benign | 0.00 | Affected | 0.3378 | 0.4957 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2837G>T | G946V 2D  AIThe SynGAP1 missense variant G946V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. ESM1b is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G946V, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | -7.528 | In-Between | 0.109 | Likely Benign | Likely Benign | 0.368 | Likely Benign | -0.30 | Neutral | 0.901 | Possibly Damaging | 0.516 | Possibly Damaging | 4.57 | Benign | 0.00 | Affected | 0.1524 | 0.3707 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.2839G>C | G947R 2D  AIThe SynGAP1 missense variant G947R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while ESM1b and AlphaMissense‑Default are uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive, and Foldetta stability analysis is unavailable. Overall, the balance of evidence favors a benign classification, and this conclusion does not contradict the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.988695 | Disordered | 0.850554 | Binding | 0.358 | 0.919 | 0.750 | -7.481 | In-Between | 0.343 | Ambiguous | Likely Benign | 0.273 | Likely Benign | -0.60 | Neutral | 0.411 | Benign | 0.140 | Benign | 4.94 | Benign | 0.04 | Affected | 4.32 | 4 | 0.1034 | 0.4733 | -2 | -3 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||||
| c.2848G>A | G950S 2D  AIThe SynGAP1 missense variant G950S is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987317 | Disordered | 0.888649 | Binding | 0.368 | 0.923 | 0.750 | -5.286 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.288 | Likely Benign | 0.46 | Neutral | 0.004 | Benign | 0.008 | Benign | 2.32 | Pathogenic | 0.52 | Tolerated | 0.2459 | 0.5502 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2848G>C | G950R 2D  AIThe SynGAP1 missense variant G950R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for G950R, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987317 | Disordered | 0.888649 | Binding | 0.368 | 0.923 | 0.750 | -6.929 | Likely Benign | 0.318 | Likely Benign | Likely Benign | 0.388 | Likely Benign | -1.10 | Neutral | 0.002 | Benign | 0.005 | Benign | 2.26 | Pathogenic | 0.01 | Affected | 0.1009 | 0.4733 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2848G>T | G950C 2D  AIThe SynGAP1 missense variant G950C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and FATHMM. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs. 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑confidence predictors (five pathogenic vs. four benign) indicate a pathogenic impact. This conclusion is not contradicted by ClinVar status, as the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.987317 | Disordered | 0.888649 | Binding | 0.368 | 0.923 | 0.750 | -9.589 | Likely Pathogenic | 0.092 | Likely Benign | Likely Benign | 0.395 | Likely Benign | -0.35 | Neutral | 0.983 | Probably Damaging | 0.750 | Possibly Damaging | 2.26 | Pathogenic | 0.01 | Affected | 0.1406 | 0.4439 | -3 | -3 | 2.9 | 46.09 | ||||||||||||||||||||||||||||||||||||||||
| c.2855G>A | G952D 2D  AIThe SynGAP1 missense variant G952D is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in‑silico tools overwhelmingly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all score benign, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is labeled Likely Benign. Only SIFT predicts a pathogenic outcome, and ESM1b remains uncertain. High‑accuracy assessments corroborate the benign trend: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are not available. Overall, the computational evidence strongly supports a benign classification, and this is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.910621 | Binding | 0.341 | 0.926 | 0.750 | -7.299 | In-Between | 0.274 | Likely Benign | Likely Benign | 0.241 | Likely Benign | -0.75 | Neutral | 0.033 | Benign | 0.015 | Benign | 3.20 | Benign | 0.05 | Affected | 0.1928 | 0.2826 | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2855G>C | G952A 2D  AIThe SynGAP1 missense variant G952A is predicted to be benign by every evaluated in‑silico tool. Benign predictions are reported by REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity, so the pathogenic group is empty. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta, a protein‑folding stability method, has no available result for this variant. ClinVar contains no entry for G952A, so there is no conflicting clinical annotation. Overall, the computational evidence indicates the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.910621 | Binding | 0.341 | 0.926 | 0.750 | -5.796 | Likely Benign | 0.075 | Likely Benign | Likely Benign | 0.273 | Likely Benign | -0.20 | Neutral | 0.000 | Benign | 0.000 | Benign | 3.26 | Benign | 0.08 | Tolerated | 0.3309 | 0.4957 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2855G>T | G952V 2D  AIThe SynGAP1 missense variant G952V is listed in ClinVar (ID 2055482.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while ESM1b remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that G952V is most likely benign, and this conclusion does not contradict the current ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.910621 | Binding | 0.341 | 0.926 | 0.750 | Uncertain | 1 | -7.074 | In-Between | 0.078 | Likely Benign | Likely Benign | 0.231 | Likely Benign | -0.33 | Neutral | 0.000 | Benign | 0.000 | Benign | 3.20 | Benign | 0.02 | Affected | 3.77 | 5 | 0.1538 | 0.3507 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.2857C>A | P953T 2D  AIThe SynGAP1 missense variant P953T is predicted to be benign by all evaluated in silico tools. Consensus predictions from SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) classify the variant as Likely Benign. High‑accuracy predictors AlphaMissense‑Optimized also report a benign effect. Other pathogenicity predictors—REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM)—uniformly predict benign. No tools predict pathogenicity. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so its stability impact remains unknown. The variant is not listed in ClinVar and has no entry in gnomAD, so no population frequency or clinical classification is available. Based on the unanimous benign predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.920633 | Binding | 0.403 | 0.926 | 0.750 | -6.646 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.075 | Likely Benign | -0.80 | Neutral | 0.009 | Benign | 0.015 | Benign | 2.88 | Benign | 0.24 | Tolerated | 0.2261 | 0.6004 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2857C>G | P953A 2D  AIThe SynGAP1 missense variant P953A is not reported in ClinVar and is absent from gnomAD. In silico prediction tools that assess functional impact all converge on a benign outcome: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. No tool in the dataset indicates pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are not available, so they do not alter the overall assessment. Consequently, the variant is most likely benign based on the collective predictions, and this conclusion is not contradicted by any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.920633 | Binding | 0.403 | 0.926 | 0.750 | -4.879 | Likely Benign | 0.059 | Likely Benign | Likely Benign | 0.082 | Likely Benign | -0.98 | Neutral | 0.124 | Benign | 0.061 | Benign | 2.81 | Benign | 0.41 | Tolerated | 0.3476 | 0.5203 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2857C>T | P953S 2D  AIThe SynGAP1 missense variant P953S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. No pathogenic predictions are present among the evaluated tools. High‑accuracy assessments confirm this trend: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign. Foldetta results are unavailable, so they do not influence the overall assessment. **Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status, as no ClinVar entry exists.** Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.920633 | Binding | 0.403 | 0.926 | 0.750 | -5.430 | Likely Benign | 0.061 | Likely Benign | Likely Benign | 0.073 | Likely Benign | -0.35 | Neutral | 0.009 | Benign | 0.008 | Benign | 2.96 | Benign | 0.37 | Tolerated | 0.3359 | 0.5184 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2858C>A | P953Q 2D  AIThe SynGAP1 missense variant P953Q is listed in ClinVar with an uncertain significance (ClinVar ID 1176820.0) and is not found in gnomAD. Functional prediction tools uniformly classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report benign effects, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also reports likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of all available predictions points to a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.920633 | Binding | 0.403 | 0.926 | 0.750 | Uncertain | 1 | -6.038 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.086 | Likely Benign | -0.78 | Neutral | 0.058 | Benign | 0.015 | Benign | 2.78 | Benign | 0.29 | Tolerated | 3.77 | 5 | 0.2105 | 0.5108 | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||||||||||
| c.2858C>G | P953R 2D  AIThe SynGAP1 missense variant P953R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic outcome, creating a single discordant signal. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also reports Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence supports a benign classification, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.920633 | Binding | 0.403 | 0.926 | 0.750 | -6.036 | Likely Benign | 0.174 | Likely Benign | Likely Benign | 0.083 | Likely Benign | -1.50 | Neutral | 0.611 | Possibly Damaging | 0.185 | Benign | 2.78 | Benign | 0.31 | Tolerated | 0.1771 | 0.4525 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.285C>G | H95Q 2D  AIThe SynGAP1 missense variant H95Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 HumDiv and SIFT predict pathogenicity, but these two tools are in minority. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized scores benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -3.355 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.070 | Likely Benign | -0.97 | Neutral | 0.633 | Possibly Damaging | 0.017 | Benign | 4.21 | Benign | 0.00 | Affected | 4.32 | 1 | 0.1551 | 0.3375 | 0 | 3 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||
| c.2860C>A | P954T 2D  AIThe SynGAP1 missense variant P954T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. The variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984159 | Disordered | 0.932268 | Binding | 0.465 | 0.926 | 0.750 | -5.657 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.089 | Likely Benign | -0.77 | Neutral | 0.977 | Probably Damaging | 0.856 | Possibly Damaging | 2.79 | Benign | 0.48 | Tolerated | 0.1860 | 0.6324 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2860C>G | P954A 2D  AIThe SynGAP1 missense variant P954A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar classification to contradict this conclusion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984159 | Disordered | 0.932268 | Binding | 0.465 | 0.926 | 0.750 | -4.985 | Likely Benign | 0.051 | Likely Benign | Likely Benign | 0.072 | Likely Benign | -0.21 | Neutral | 0.856 | Possibly Damaging | 0.652 | Possibly Damaging | 2.91 | Benign | 0.90 | Tolerated | 0.3103 | 0.5550 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2861C>A | P954H 2D  AIThe SynGAP1 missense variant P954H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of ClinVar or gnomAD entries—there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984159 | Disordered | 0.932268 | Binding | 0.465 | 0.926 | 0.750 | -3.670 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.098 | Likely Benign | -0.50 | Neutral | 0.041 | Benign | 0.067 | Benign | 2.75 | Benign | 0.04 | Affected | 0.2054 | 0.4756 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2861C>G | P954R 2D  AIThe SynGAP1 missense variant P954R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also likely benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence indicates that P954R is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984159 | Disordered | 0.932268 | Binding | 0.465 | 0.926 | 0.750 | -6.329 | Likely Benign | 0.160 | Likely Benign | Likely Benign | 0.097 | Likely Benign | -1.19 | Neutral | 0.954 | Possibly Damaging | 0.826 | Possibly Damaging | 2.78 | Benign | 0.11 | Tolerated | 0.1548 | 0.3940 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2854G>T | G952C 2D  AIThe SynGAP1 missense variant G952C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.910621 | Binding | 0.341 | 0.926 | 0.750 | -8.599 | Likely Pathogenic | 0.088 | Likely Benign | Likely Benign | 0.272 | Likely Benign | -0.18 | Neutral | 0.371 | Benign | 0.169 | Benign | 3.20 | Benign | 0.01 | Affected | 0.1458 | 0.4439 | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||||||
| c.2854G>C | G952R 2D  AIThe SynGAP1 missense variant G952R is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that G952R is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985964 | Disordered | 0.910621 | Binding | 0.341 | 0.926 | 0.750 | -5.974 | Likely Benign | 0.295 | Likely Benign | Likely Benign | 0.139 | Likely Benign | -0.93 | Neutral | 0.077 | Benign | 0.011 | Benign | 3.20 | Benign | 0.02 | Affected | 0.1145 | 0.4933 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2849G>A | G950D 2D  AIThe SynGAP1 missense variant G950D is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM, while ESM1b remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a benign outcome. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the preponderance of evidence indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.987317 | Disordered | 0.888649 | Binding | 0.368 | 0.923 | 0.750 | -7.515 | In-Between | 0.245 | Likely Benign | Likely Benign | 0.402 | Likely Benign | -0.61 | Neutral | 0.411 | Benign | 0.131 | Benign | 2.26 | Pathogenic | 0.02 | Affected | 0.1874 | 0.2826 | 1 | -1 | -3.1 | 58.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2849G>C | G950A 2D  AIThe SynGAP1 missense variant G950A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only FATHMM predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a “Likely Benign” verdict, reflecting the majority of benign calls. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote) is benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of available predictions indicates that the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.987317 | Disordered | 0.888649 | Binding | 0.368 | 0.923 | 0.750 | -6.557 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.339 | Likely Benign | -0.13 | Neutral | 0.059 | Benign | 0.061 | Benign | 2.28 | Pathogenic | 0.08 | Tolerated | 0.3343 | 0.4957 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2849G>T | G950V 2D  AIThe SynGAP1 missense variant G950V is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM, while ESM1b remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a benign prediction. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the preponderance of evidence indicates that G950V is most likely benign, and this conclusion does not contradict the ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.987317 | Disordered | 0.888649 | Binding | 0.368 | 0.923 | 0.750 | -7.796 | In-Between | 0.093 | Likely Benign | Likely Benign | 0.428 | Likely Benign | -0.91 | Neutral | 0.411 | Benign | 0.239 | Benign | 2.26 | Pathogenic | 0.01 | Affected | 0.1392 | 0.3507 | -1 | -3 | 4.6 | 42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.284A>C | H95P 2D  AIThe SynGAP1 missense variant H95P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign, while the single pathogenic call comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) confirms a benign outcome. Foldetta results are unavailable, so they do not influence the assessment. Overall, the majority of evidence indicates that H95P is most likely benign, and this conclusion is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -1.611 | Likely Benign | 0.045 | Likely Benign | Likely Benign | 0.164 | Likely Benign | -1.66 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.19 | Benign | 0.00 | Affected | 0.2244 | 0.3704 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.284A>T | H95L 2D  AIThe SynGAP1 missense variant H95L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -1.967 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.085 | Likely Benign | -2.31 | Neutral | 0.084 | Benign | 0.007 | Benign | 4.17 | Benign | 0.00 | Affected | 0.1017 | 0.5074 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2851C>A | H951N 2D  AIThe SynGAP1 missense variant H951N is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the evidence strongly supports a benign classification, and this conclusion is consistent with the lack of a ClinVar entry, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988291 | Disordered | 0.901477 | Binding | 0.415 | 0.925 | 0.750 | -5.833 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.140 | Likely Benign | -0.41 | Neutral | 0.011 | Benign | 0.018 | Benign | 5.43 | Benign | 0.16 | Tolerated | 0.2590 | 0.3197 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.2851C>G | H951D 2D  AIThe SynGAP1 missense variant H951D is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. No pathogenic predictions are present among the evaluated methods. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign; Foldetta results are unavailable. Consequently, the variant is most likely benign based on current predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988291 | Disordered | 0.901477 | Binding | 0.415 | 0.925 | 0.750 | -5.901 | Likely Benign | 0.188 | Likely Benign | Likely Benign | 0.186 | Likely Benign | -0.33 | Neutral | 0.000 | Benign | 0.001 | Benign | 5.43 | Benign | 0.46 | Tolerated | 0.2851 | 0.2475 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2851C>T | H951Y 2D  AIThe SynGAP1 missense variant H951Y is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. No pathogenic predictions are present among the evaluated tools. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the consensus of high‑accuracy predictors (AlphaMissense‑Optimized and SGM‑Consensus) supports a benign interpretation. Consequently, the variant is most likely benign, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988291 | Disordered | 0.901477 | Binding | 0.415 | 0.925 | 0.750 | -5.165 | Likely Benign | 0.113 | Likely Benign | Likely Benign | 0.142 | Likely Benign | -1.03 | Neutral | 0.000 | Benign | 0.001 | Benign | 5.61 | Benign | 0.10 | Tolerated | 0.2247 | 0.4112 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.2852A>C | H951P 2D  AIThe SynGAP1 missense variant H951P is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the collective evidence strongly supports a benign classification, and this conclusion is consistent with the lack of a ClinVar entry, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988291 | Disordered | 0.901477 | Binding | 0.415 | 0.925 | 0.750 | -2.798 | Likely Benign | 0.051 | Likely Benign | Likely Benign | 0.312 | Likely Benign | -0.06 | Neutral | 0.000 | Benign | 0.001 | Benign | 5.43 | Benign | 0.14 | Tolerated | 0.2745 | 0.3707 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2852A>G | H951R 2D  AIThe SynGAP1 missense variant H951R is listed in ClinVar as Pathogenic (ClinVar ID 1003739.0) and is not reported in gnomAD. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign. The Foldetta protein‑folding stability analysis is unavailable for this variant. Based on the collective predictions, the variant is most likely benign, which contradicts its ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988291 | Disordered | 0.901477 | Binding | 0.415 | 0.925 | 0.750 | Likely Pathogenic | 1 | -4.964 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.185 | Likely Benign | -1.08 | Neutral | 0.048 | Benign | 0.029 | Benign | 5.46 | Benign | 0.24 | Tolerated | 3.77 | 5 | 0.2808 | 0.3220 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||
| c.2852A>T | H951L 2D  AIThe SynGAP1 missense variant H951L is not reported in ClinVar and is absent from gnomAD, indicating no documented clinical or population evidence. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification. Foldetta results are not available, so they do not influence the assessment. Overall, the variant is most likely benign, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988291 | Disordered | 0.901477 | Binding | 0.415 | 0.925 | 0.750 | -4.822 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.192 | Likely Benign | -0.99 | Neutral | 0.022 | Benign | 0.018 | Benign | 5.47 | Benign | 0.43 | Tolerated | 0.2224 | 0.4526 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2853T>A | H951Q 2D  AIThe SynGAP1 missense variant H951Q is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. No pathogenic predictions are present among the evaluated tools. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign; Foldetta results are unavailable. Consequently, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988291 | Disordered | 0.901477 | Binding | 0.415 | 0.925 | 0.750 | -4.755 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.172 | Likely Benign | -0.51 | Neutral | 0.001 | Benign | 0.002 | Benign | 5.43 | Benign | 0.29 | Tolerated | 0.2757 | 0.3328 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2853T>G | H951Q 2D  AIThe SynGAP1 missense variant H951Q is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. No pathogenic predictions are present among the evaluated tools. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign; Foldetta results are unavailable. Consequently, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988291 | Disordered | 0.901477 | Binding | 0.415 | 0.925 | 0.750 | -4.755 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.171 | Likely Benign | -0.51 | Neutral | 0.001 | Benign | 0.002 | Benign | 5.43 | Benign | 0.29 | Tolerated | 0.2757 | 0.3328 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2861C>T | P954L 2D  AIThe SynGAP1 missense variant P954L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign. In contrast, polyPhen‑2 (HumDiv and HumVar) predict it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984159 | Disordered | 0.932268 | Binding | 0.465 | 0.926 | 0.750 | -5.607 | Likely Benign | 0.092 | Likely Benign | Likely Benign | 0.097 | Likely Benign | -0.43 | Neutral | 0.977 | Probably Damaging | 0.812 | Possibly Damaging | 2.78 | Benign | 0.55 | Tolerated | 0.2346 | 0.5867 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2675C>T | S892F 2D  AIThe SynGAP1 missense variant S892F is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2). Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs four benign) lean toward pathogenicity. Thus, the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.754692 | Disordered | 0.473390 | Uncertain | 0.319 | 0.926 | 0.875 | -4.709 | Likely Benign | 0.605 | Likely Pathogenic | Likely Benign | 0.090 | Likely Benign | -3.07 | Deleterious | 0.998 | Probably Damaging | 0.959 | Probably Damaging | 2.55 | Benign | 0.00 | Affected | 0.0715 | 0.5124 | -3 | -2 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||||||||||||
| c.2685T>G | S895R 2D  AIThe SynGAP1 missense variant S895R is not reported in ClinVar and has no gnomAD entry. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and the SGM‑Consensus score (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign impact, and this is consistent with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.414977 | Uncertain | 0.294 | 0.925 | 0.750 | -4.746 | Likely Benign | 0.973 | Likely Pathogenic | Likely Pathogenic | 0.123 | Likely Benign | -1.72 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.64 | Benign | 0.10 | Tolerated | 4.32 | 4 | 0.0949 | 0.4046 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||
| c.2686G>A | G896S 2D  AIThe SynGAP1 missense variant G896S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence supports a benign impact, and there is no conflict with ClinVar status (which has no entry). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.412816 | Uncertain | 0.314 | 0.923 | 0.625 | -2.712 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.103 | Likely Benign | -0.63 | Neutral | 0.896 | Possibly Damaging | 0.334 | Benign | 2.59 | Benign | 0.41 | Tolerated | 0.2684 | 0.4911 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2686G>C | G896R 2D  AIThe SynGAP1 missense variant G896R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and ESM1b, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑to‑2 split. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Consequently, the evidence is evenly split between benign and pathogenic predictions, with no decisive support from the high‑accuracy or folding‑stability analyses. The variant is therefore most likely of uncertain significance; it does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.675549 | Disordered | 0.412816 | Uncertain | 0.314 | 0.923 | 0.625 | -4.511 | Likely Benign | 0.897 | Likely Pathogenic | Ambiguous | 0.218 | Likely Benign | -2.45 | Neutral | 0.999 | Probably Damaging | 0.967 | Probably Damaging | 2.44 | Pathogenic | 0.14 | Tolerated | 0.0931 | 0.4228 | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||||||
| c.2686G>T | G896C 2D  AIThe SynGAP1 missense variant G896C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.412816 | Uncertain | 0.314 | 0.923 | 0.625 | -5.607 | Likely Benign | 0.245 | Likely Benign | Likely Benign | 0.193 | Likely Benign | -2.59 | Deleterious | 1.000 | Probably Damaging | 0.983 | Probably Damaging | 2.50 | Benign | 0.10 | Tolerated | 0.1270 | 0.4369 | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||||||
| c.2687G>A | G896D 2D  AIThe SynGAP1 missense variant G896D is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, and ESM1b, whereas polyPhen‑2 (HumDiv and HumVar), FATHMM, and AlphaMissense‑Default all predict a pathogenic outcome. The high‑accuracy AlphaMissense‑Optimized score is uncertain, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive, and Foldetta stability analysis is unavailable. Consequently, the evidence is evenly split between benign and pathogenic predictions, with no decisive support from the most reliable methods. The variant is therefore classified as of uncertain significance; it does not contradict any ClinVar annotation because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.675549 | Disordered | 0.412816 | Uncertain | 0.314 | 0.923 | 0.625 | -4.500 | Likely Benign | 0.905 | Likely Pathogenic | Ambiguous | 0.159 | Likely Benign | -2.39 | Neutral | 0.997 | Probably Damaging | 0.934 | Probably Damaging | 2.44 | Pathogenic | 0.16 | Tolerated | 0.1707 | 0.1842 | 1 | -1 | -3.1 | 58.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2687G>C | G896A 2D  AIThe SynGAP1 missense variant G896A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only polyPhen‑2 HumDiv predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and there is no conflict with ClinVar status because no ClinVar claim exists. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.412816 | Uncertain | 0.314 | 0.923 | 0.625 | -3.562 | Likely Benign | 0.147 | Likely Benign | Likely Benign | 0.077 | Likely Benign | -0.95 | Neutral | 0.561 | Possibly Damaging | 0.139 | Benign | 2.54 | Benign | 0.79 | Tolerated | 0.3799 | 0.4963 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2689T>A | S897T 2D  AIThe SynGAP1 missense variant S897T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.418474 | Uncertain | 0.292 | 0.928 | 0.500 | -4.930 | Likely Benign | 0.145 | Likely Benign | Likely Benign | 0.086 | Likely Benign | -0.69 | Neutral | 0.790 | Possibly Damaging | 0.535 | Possibly Damaging | 2.68 | Benign | 0.04 | Affected | 0.1480 | 0.6392 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2689T>C | S897P 2D  AIThe SynGAP1 missense variant S897P is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.418474 | Uncertain | 0.292 | 0.928 | 0.500 | -4.088 | Likely Benign | 0.216 | Likely Benign | Likely Benign | 0.097 | Likely Benign | -0.21 | Neutral | 0.990 | Probably Damaging | 0.856 | Possibly Damaging | 2.64 | Benign | 0.04 | Affected | 0.2014 | 0.5933 | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2689T>G | S897A 2D  AIThe SynGAP1 missense variant S897A is not reported in ClinVar and is absent from gnomAD. All evaluated in‑silico predictors—including REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods likewise support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are unavailable. Consequently, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.418474 | Uncertain | 0.292 | 0.928 | 0.500 | -3.959 | Likely Benign | 0.122 | Likely Benign | Likely Benign | 0.060 | Likely Benign | -0.48 | Neutral | 0.288 | Benign | 0.208 | Benign | 2.71 | Benign | 0.81 | Tolerated | 0.4539 | 0.5684 | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.268G>C | V90L 2D  AIThe SynGAP1 missense variant V90L is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all classify the change as benign, while SIFT uniquely predicts it as pathogenic. The consensus from the SGM framework, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a Likely Benign verdict. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized reports a benign outcome, the SGM Consensus also indicates Likely Benign, and Foldetta data are unavailable. With the majority of evidence pointing to a benign effect and no conflicting ClinVar annotation, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | -3.676 | Likely Benign | 0.522 | Ambiguous | Likely Benign | 0.056 | Likely Benign | -0.24 | Neutral | 0.103 | Benign | 0.015 | Benign | 4.02 | Benign | 0.00 | Affected | 0.1334 | 0.4487 | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.268G>T | V90L 2D  AIThe SynGAP1 missense variant V90L is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus indicates Likely Benign, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect for V90L, and this conclusion does not contradict any ClinVar status, as none is assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | -3.676 | Likely Benign | 0.522 | Ambiguous | Likely Benign | 0.056 | Likely Benign | -0.24 | Neutral | 0.103 | Benign | 0.015 | Benign | 4.02 | Benign | 0.00 | Affected | 0.1334 | 0.4487 | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2690C>T | S897L 2D  AIThe SynGAP1 missense variant S897L is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (derived from the same four high‑accuracy tools) also as benign. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not conflict with the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.418474 | Uncertain | 0.292 | 0.928 | 0.500 | Uncertain | 1 | -4.034 | Likely Benign | 0.299 | Likely Benign | Likely Benign | 0.028 | Likely Benign | -1.71 | Neutral | 0.901 | Possibly Damaging | 0.636 | Possibly Damaging | 2.66 | Benign | 0.01 | Affected | 0.1280 | 0.5770 | -3 | -2 | 4.6 | 26.08 | |||||||||||||||||||||||||||||||||||||
| c.2692T>A | S898T 2D  AIThe SynGAP1 missense variant S898T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.426070 | Uncertain | 0.305 | 0.922 | 0.500 | -4.910 | Likely Benign | 0.166 | Likely Benign | Likely Benign | 0.097 | Likely Benign | -0.33 | Neutral | 0.992 | Probably Damaging | 0.814 | Possibly Damaging | 2.50 | Benign | 0.78 | Tolerated | 0.1833 | 0.6209 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2685T>A | S895R 2D  AIThe SynGAP1 missense variant S895R is not reported in ClinVar and has no gnomAD entry. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and the SGM‑Consensus score (Likely Benign). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect, and this is consistent with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.414977 | Uncertain | 0.294 | 0.925 | 0.750 | -4.746 | Likely Benign | 0.973 | Likely Pathogenic | Likely Pathogenic | 0.123 | Likely Benign | -1.72 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.64 | Benign | 0.10 | Tolerated | 4.32 | 4 | 0.0949 | 0.4046 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||
| c.2684G>T | S895I 2D  AIThe SynGAP1 missense variant S895I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also favors a benign outcome; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for S895I, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.414977 | Uncertain | 0.294 | 0.925 | 0.750 | -6.315 | Likely Benign | 0.697 | Likely Pathogenic | Likely Benign | 0.144 | Likely Benign | -2.34 | Neutral | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 2.65 | Benign | 0.04 | Affected | 0.1010 | 0.6248 | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||||||
| c.2677C>A | Q893K 2D  AIThe SynGAP1 missense variant Q893K is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM all score the substitution as benign, and AlphaMissense‑Optimized also predicts a benign outcome. No tool predicts pathogenicity; AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as likely benign. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the computational evidence strongly supports a benign classification, and this conclusion does not contradict ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.447267 | Uncertain | 0.310 | 0.925 | 0.750 | -5.622 | Likely Benign | 0.496 | Ambiguous | Likely Benign | 0.053 | Likely Benign | -1.45 | Neutral | 0.451 | Benign | 0.265 | Benign | 2.78 | Benign | 0.10 | Tolerated | 0.1901 | 0.4381 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.2677C>G | Q893E 2D  AIThe SynGAP1 missense variant Q893E is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that assess sequence conservation and structural impact all converge on a benign interpretation: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. No tool in the dataset indicates pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized reports benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is labeled “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the computational evidence strongly supports a benign effect, and this conclusion is consistent with the absence of any ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.447267 | Uncertain | 0.310 | 0.925 | 0.750 | -3.888 | Likely Benign | 0.296 | Likely Benign | Likely Benign | 0.088 | Likely Benign | -0.67 | Neutral | 0.421 | Benign | 0.181 | Benign | 2.76 | Benign | 0.06 | Tolerated | 0.1535 | 0.2555 | 2 | 2 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.2678A>G | Q893R 2D  AIThe SynGAP1 missense variant Q893R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Only polyPhen‑2 HumDiv predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.447267 | Uncertain | 0.310 | 0.925 | 0.750 | -3.338 | Likely Benign | 0.392 | Ambiguous | Likely Benign | 0.056 | Likely Benign | -1.43 | Neutral | 0.802 | Possibly Damaging | 0.333 | Benign | 2.79 | Benign | 0.09 | Tolerated | 0.1565 | 0.2412 | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||||||||||
| c.2678A>T | Q893L 2D  AIThe SynGAP1 missense variant Q893L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of all available predictions is benign, and this conclusion does not contradict any ClinVar annotation (none present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.447267 | Uncertain | 0.310 | 0.925 | 0.750 | -1.964 | Likely Benign | 0.204 | Likely Benign | Likely Benign | 0.078 | Likely Benign | -1.92 | Neutral | 0.451 | Benign | 0.209 | Benign | 2.82 | Benign | 1.00 | Tolerated | 0.0904 | 0.5643 | -2 | -2 | 7.3 | -14.97 | |||||||||||||||||||||||||||||||||||||||
| c.2679G>C | Q893H 2D  AIThe SynGAP1 missense variant Q893H is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for Q893H, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.447267 | Uncertain | 0.310 | 0.925 | 0.750 | -3.783 | Likely Benign | 0.306 | Likely Benign | Likely Benign | 0.060 | Likely Benign | -1.67 | Neutral | 0.977 | Probably Damaging | 0.796 | Possibly Damaging | 2.70 | Benign | 0.03 | Affected | 0.1708 | 0.3879 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2679G>T | Q893H 2D  AIThe SynGAP1 missense variant Q893H is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for Q893H, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.447267 | Uncertain | 0.310 | 0.925 | 0.750 | -3.783 | Likely Benign | 0.306 | Likely Benign | Likely Benign | 0.059 | Likely Benign | -1.67 | Neutral | 0.977 | Probably Damaging | 0.796 | Possibly Damaging | 2.70 | Benign | 0.03 | Affected | 0.1708 | 0.3879 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2680G>A | G894R 2D  AISynGAP1 missense variant G894R has no ClinVar record and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, and Foldetta results are unavailable. Considering the consensus from high‑accuracy tools and the balance of individual predictions, the variant is most likely benign, and this assessment does not contradict the ClinVar status, which currently has no entry for G894R. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.425700 | Uncertain | 0.310 | 0.925 | 0.750 | -5.222 | Likely Benign | 0.922 | Likely Pathogenic | Ambiguous | 0.216 | Likely Benign | -1.68 | Neutral | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.86 | Benign | 0.01 | Affected | 0.0982 | 0.4332 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2680G>C | G894R 2D  AISynGAP1 missense variant G894R has no ClinVar record and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, and Foldetta results are unavailable. Considering the consensus from high‑accuracy tools and the balance of individual predictions, the variant is most likely benign, and this assessment does not contradict the ClinVar status, which currently has no entry for G894R. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.425700 | Uncertain | 0.310 | 0.925 | 0.750 | -5.222 | Likely Benign | 0.922 | Likely Pathogenic | Ambiguous | 0.216 | Likely Benign | -1.68 | Neutral | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.86 | Benign | 0.01 | Affected | 0.0982 | 0.4332 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2680G>T | G894W 2D  AIThe SynGAP1 missense variant G894W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (two pathogenic vs. two benign votes). Foldetta, which assesses protein‑folding stability, has no available output for this variant. Overall, the majority of evaluated predictors (five pathogenic vs. three benign) lean toward a pathogenic interpretation. Because there is no ClinVar annotation to contradict this assessment, the variant is most likely pathogenic based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.788093 | Disordered | 0.425700 | Uncertain | 0.310 | 0.925 | 0.750 | -6.927 | Likely Benign | 0.814 | Likely Pathogenic | Ambiguous | 0.177 | Likely Benign | -2.70 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.59 | Benign | 0.00 | Affected | 0.0698 | 0.4184 | -7 | -2 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||||||||||
| c.2681G>T | G894V 2D  AIThe SynGAP1 missense variant G894V is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also leans benign, and Foldetta results are unavailable. Overall, the majority of high‑confidence tools predict a benign impact, and there is no conflict with the ClinVar status. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.788093 | Disordered | 0.425700 | Uncertain | 0.310 | 0.925 | 0.750 | -5.047 | Likely Benign | 0.395 | Ambiguous | Likely Benign | 0.188 | Likely Benign | -2.61 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.64 | Benign | 0.02 | Affected | 0.1187 | 0.4091 | -1 | -3 | 4.6 | 42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.2683A>G | S895G 2D  AIThe SynGAP1 missense variant S895G is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate a benign outcome, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also classifies it as likely benign. In contrast, two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this assessment does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.414977 | Uncertain | 0.294 | 0.925 | 0.750 | -3.728 | Likely Benign | 0.150 | Likely Benign | Likely Benign | 0.149 | Likely Benign | -1.25 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.67 | Benign | 0.90 | Tolerated | 0.2675 | 0.5087 | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2683A>T | S895C 2D  AIThe SynGAP1 missense variant S895C is reported in ClinVar as “None” and is not present in gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.414977 | Uncertain | 0.294 | 0.925 | 0.750 | -8.006 | Likely Pathogenic | 0.259 | Likely Benign | Likely Benign | 0.182 | Likely Benign | -2.44 | Neutral | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.60 | Benign | 0.08 | Tolerated | 0.1072 | 0.6350 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||||||
| c.2684G>A | S895N 2D  AIThe SynGAP1 missense variant S895N is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (majority vote) also leans benign. No Foldetta (protein‑folding stability) result is available, so it does not influence the assessment. Overall, the preponderance of predictions indicates the variant is most likely benign, which is consistent with its ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.414977 | Uncertain | 0.294 | 0.925 | 0.750 | Uncertain | 1 | -6.399 | Likely Benign | 0.604 | Likely Pathogenic | Likely Benign | 0.118 | Likely Benign | -0.85 | Neutral | 0.991 | Probably Damaging | 0.988 | Probably Damaging | 2.64 | Benign | 0.30 | Tolerated | 4.32 | 4 | 0.1271 | 0.4984 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||
| c.2692T>C | S898P 2D  AIThe SynGAP1 missense variant S898P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are not available. Overall, the majority of predictions (six benign vs. four pathogenic) support a benign classification. There is no ClinVar entry to contradict this assessment, so the variant is most likely benign based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.426070 | Uncertain | 0.305 | 0.922 | 0.500 | -4.192 | Likely Benign | 0.307 | Likely Benign | Likely Benign | 0.149 | Likely Benign | 0.44 | Neutral | 0.998 | Probably Damaging | 0.939 | Probably Damaging | 2.45 | Pathogenic | 0.01 | Affected | 0.2400 | 0.6555 | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2692T>G | S898A 2D  AIThe SynGAP1 missense variant S898A is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) all indicate a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports a likely benign classification. No Foldetta stability analysis is available for this residue. Overall, the preponderance of evidence from both general and high‑accuracy predictors suggests that S898A is most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.426070 | Uncertain | 0.305 | 0.922 | 0.500 | -3.932 | Likely Benign | 0.146 | Likely Benign | Likely Benign | 0.052 | Likely Benign | -0.98 | Neutral | 0.942 | Possibly Damaging | 0.748 | Possibly Damaging | 2.63 | Benign | 0.03 | Affected | 0.4552 | 0.5516 | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2693C>A | S898Y 2D  AISynGAP1 missense variant S898Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that classify the variant as benign include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Overall, the majority of tools and the consensus prediction indicate a pathogenic effect, and this conclusion does not contradict any ClinVar annotation because none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.690604 | Disordered | 0.426070 | Uncertain | 0.305 | 0.922 | 0.500 | -5.927 | Likely Benign | 0.712 | Likely Pathogenic | Likely Benign | 0.182 | Likely Benign | -2.69 | Deleterious | 0.998 | Probably Damaging | 0.959 | Probably Damaging | 2.44 | Pathogenic | 0.00 | Affected | 0.1244 | 0.6838 | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||||||
| c.26A>T | H9L 2D  AIThe SynGAP1 missense variant H9L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -2.603 | Likely Benign | 0.135 | Likely Benign | Likely Benign | 0.151 | Likely Benign | 0.48 | Neutral | 0.024 | Benign | 0.002 | Benign | 4.25 | Benign | 0.00 | Affected | 0.1255 | 0.6285 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2701G>A | A901T 2D  AIThe SynGAP1 missense variant A901T is reported as “Likely Benign” in SGM‑Consensus and has no ClinVar entry or gnomAD allele. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool in the dataset predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also benign. Foldetta results are not available. Overall, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.595080 | Disordered | 0.489838 | Uncertain | 0.306 | 0.917 | 0.375 | -4.708 | Likely Benign | 0.116 | Likely Benign | Likely Benign | 0.046 | Likely Benign | -0.95 | Neutral | 0.245 | Benign | 0.096 | Benign | 2.70 | Benign | 0.16 | Tolerated | 0.1587 | 0.7680 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2701G>C | A901P 2D  AIThe SynGAP1 missense variant A901P is not reported in ClinVar and is absent from gnomAD. Consensus from multiple in silico predictors indicates a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while only the two polyPhen‑2 implementations (HumDiv and HumVar) predict pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy tools reinforce this view: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta stability analysis is not available. Overall, the majority of evidence supports a benign interpretation, and there is no conflict with ClinVar status, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.595080 | Disordered | 0.489838 | Uncertain | 0.306 | 0.917 | 0.375 | -4.035 | Likely Benign | 0.175 | Likely Benign | Likely Benign | 0.124 | Likely Benign | -0.17 | Neutral | 0.918 | Possibly Damaging | 0.500 | Possibly Damaging | 2.65 | Benign | 0.19 | Tolerated | 0.1930 | 0.5941 | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2701G>T | A901S 2D  AIThe SynGAP1 missense variant A901S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools that assess pathogenicity uniformly predict a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate benign. No tool in the dataset predicts pathogenicity, so the pathogenic group is empty. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are not available. Overall, the collective predictions strongly suggest that the variant is most likely benign, and this conclusion does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.595080 | Disordered | 0.489838 | Uncertain | 0.306 | 0.917 | 0.375 | -3.284 | Likely Benign | 0.097 | Likely Benign | Likely Benign | 0.043 | Likely Benign | -0.62 | Neutral | 0.009 | Benign | 0.015 | Benign | 2.73 | Benign | 0.35 | Tolerated | 0.2695 | 0.6582 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2702C>A | A901E 2D  AIThe SynGAP1 missense variant A901E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.595080 | Disordered | 0.489838 | Uncertain | 0.306 | 0.917 | 0.375 | -4.350 | Likely Benign | 0.802 | Likely Pathogenic | Ambiguous | 0.082 | Likely Benign | -0.53 | Neutral | 0.800 | Possibly Damaging | 0.300 | Benign | 2.64 | Benign | 0.12 | Tolerated | 0.1433 | 0.2424 | 0 | -1 | -5.3 | 58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2702C>G | A901G 2D  AIThe SynGAP1 missense variant A901G is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Overall, the consensus of available predictions points to a benign impact, with no conflict with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.595080 | Disordered | 0.489838 | Uncertain | 0.306 | 0.917 | 0.375 | -3.928 | Likely Benign | 0.127 | Likely Benign | Likely Benign | 0.047 | Likely Benign | -1.52 | Neutral | 0.622 | Possibly Damaging | 0.165 | Benign | 2.61 | Benign | 0.37 | Tolerated | 0.2030 | 0.5188 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2704G>C | A902P 2D  AIThe SynGAP1 missense variant A902P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for A902P, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.517703 | Binding | 0.319 | 0.919 | 0.375 | -4.509 | Likely Benign | 0.154 | Likely Benign | Likely Benign | 0.146 | Likely Benign | 0.09 | Neutral | 0.995 | Probably Damaging | 0.846 | Possibly Damaging | 2.56 | Benign | 0.01 | Affected | 0.1694 | 0.5304 | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2704G>T | A902S 2D  AIThe SynGAP1 missense variant A902S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic outcome, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also reports Likely Benign. No Foldetta stability data are available, so it does not influence the assessment. Overall, the preponderance of evidence points to a benign effect for A902S, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.517703 | Binding | 0.319 | 0.919 | 0.375 | -4.176 | Likely Benign | 0.099 | Likely Benign | Likely Benign | 0.048 | Likely Benign | -0.74 | Neutral | 0.798 | Possibly Damaging | 0.433 | Benign | 2.63 | Benign | 0.27 | Tolerated | 0.2515 | 0.5565 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2705C>A | A902D 2D  AIThe SynGAP1 missense variant A902D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which collectively suggest a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default predict a pathogenic impact. The high‑accuracy AlphaMissense‑Optimized result is uncertain, and Foldetta stability analysis is unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this assessment does not contradict any ClinVar annotation because no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.517703 | Binding | 0.319 | 0.919 | 0.375 | -5.273 | Likely Benign | 0.823 | Likely Pathogenic | Ambiguous | 0.101 | Likely Benign | -1.21 | Neutral | 0.986 | Probably Damaging | 0.787 | Possibly Damaging | 2.59 | Benign | 0.00 | Affected | 0.1653 | 0.1609 | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||||||
| c.2705C>G | A902G 2D  AIThe SynGAP1 missense variant A902G is not listed in ClinVar and has no entry in gnomAD, indicating no reported clinical classification or population frequency data. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only SIFT predicts a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, SGM‑Consensus is benign, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) is unavailable for this variant. Overall, the consensus of available predictions points to a benign impact, with no conflict with ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.517703 | Binding | 0.319 | 0.919 | 0.375 | -4.046 | Likely Benign | 0.104 | Likely Benign | Likely Benign | 0.020 | Likely Benign | -0.65 | Neutral | 0.004 | Benign | 0.008 | Benign | 2.69 | Benign | 0.03 | Affected | 0.2177 | 0.4461 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2705C>T | A902V 2D  AIThe SynGAP1 missense variant A902V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for A902V, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.517703 | Binding | 0.319 | 0.919 | 0.375 | -4.768 | Likely Benign | 0.223 | Likely Benign | Likely Benign | 0.066 | Likely Benign | -1.15 | Neutral | 0.983 | Probably Damaging | 0.704 | Possibly Damaging | 2.66 | Benign | 0.01 | Affected | 0.1124 | 0.6602 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||||||||||||||
| c.2707G>C | G903R 2D  AIThe SynGAP1 missense variant G903R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and ESM1b. Tools that predict a pathogenic effect are SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments are mixed: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 benign vs 2 pathogenic), and Foldetta results are unavailable. Overall, the majority of standard predictors lean toward a benign impact, and no ClinVar annotation contradicts this assessment. Thus, the variant is most likely benign based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.680603 | Disordered | 0.549818 | Binding | 0.291 | 0.917 | 0.375 | -3.503 | Likely Benign | 0.865 | Likely Pathogenic | Ambiguous | 0.119 | Likely Benign | -2.02 | Neutral | 0.241 | Benign | 0.244 | Benign | 2.33 | Pathogenic | 0.02 | Affected | 0.0872 | 0.4064 | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||||||
| c.2707G>T | G903C 2D  AIThe SynGAP1 missense variant G903C is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. AlphaMissense‑Default is uncertain and does not influence the consensus. High‑accuracy assessments show AlphaMissense‑Optimized predicts a benign outcome, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) predicts pathogenic. Foldetta, which would provide a protein‑folding stability estimate, is unavailable for this variant. Overall, the majority of evidence (five pathogenic versus three benign predictions) points to a pathogenic impact. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.680603 | Disordered | 0.549818 | Binding | 0.291 | 0.917 | 0.375 | -6.593 | Likely Benign | 0.471 | Ambiguous | Likely Benign | 0.151 | Likely Benign | -3.28 | Deleterious | 1.000 | Probably Damaging | 0.990 | Probably Damaging | 2.32 | Pathogenic | 0.02 | Affected | 0.1158 | 0.4420 | -3 | -3 | 2.9 | 46.09 | ||||||||||||||||||||||||||||||||||||||||
| c.26A>C | H9P 2D  AIThe SynGAP1 missense variant H9P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The prediction is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -2.838 | Likely Benign | 0.061 | Likely Benign | Likely Benign | 0.224 | Likely Benign | -0.16 | Neutral | 0.107 | Benign | 0.006 | Benign | 4.19 | Benign | 0.00 | Affected | 0.2644 | 0.5169 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.269T>G | V90G 2D  AIThe SynGAP1 missense variant V90G is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a benign verdict. Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | -2.617 | Likely Benign | 0.358 | Ambiguous | Likely Benign | 0.094 | Likely Benign | -0.55 | Neutral | 0.024 | Benign | 0.208 | Benign | 4.00 | Benign | 0.00 | Affected | 0.2047 | 0.2936 | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.2693C>T | S898F 2D  AIThe SynGAP1 missense variant S898F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as Likely Pathogenic, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) results are unavailable. Based on the overall distribution of predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.690604 | Disordered | 0.426070 | Uncertain | 0.305 | 0.922 | 0.500 | -5.242 | Likely Benign | 0.673 | Likely Pathogenic | Likely Benign | 0.181 | Likely Benign | -2.87 | Deleterious | 0.998 | Probably Damaging | 0.959 | Probably Damaging | 2.44 | Pathogenic | 0.00 | Affected | 0.1183 | 0.6643 | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||||||||||||
| c.2695A>C | I899L 2D  AIThe SynGAP1 missense variant I899L is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates a benign outcome. Foldetta results are not available, so they do not influence the assessment. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.443727 | Uncertain | 0.292 | 0.928 | 0.375 | -1.137 | Likely Benign | 0.093 | Likely Benign | Likely Benign | 0.055 | Likely Benign | -0.36 | Neutral | 0.220 | Benign | 0.078 | Benign | 2.74 | Benign | 0.04 | Affected | 0.0863 | 0.2840 | 2 | 2 | -0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.2695A>T | I899F 2D  AIThe SynGAP1 missense variant I899F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.443727 | Uncertain | 0.292 | 0.928 | 0.375 | -4.049 | Likely Benign | 0.161 | Likely Benign | Likely Benign | 0.060 | Likely Benign | -1.05 | Neutral | 0.906 | Possibly Damaging | 0.473 | Possibly Damaging | 2.69 | Benign | 0.01 | Affected | 0.0593 | 0.2435 | 1 | 0 | -1.7 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2696T>A | I899N 2D  AIThe SynGAP1 missense variant I899N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect for I899N, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.443727 | Uncertain | 0.292 | 0.928 | 0.375 | -3.328 | Likely Benign | 0.469 | Ambiguous | Likely Benign | 0.058 | Likely Benign | -0.68 | Neutral | 0.611 | Possibly Damaging | 0.140 | Benign | 2.76 | Benign | 0.00 | Affected | 0.0933 | 0.0470 | -2 | -3 | -8.0 | 0.94 | |||||||||||||||||||||||||||||||||||||||
| c.2696T>G | I899S 2D  AIThe SynGAP1 missense variant I899S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic call comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign consensus. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not conflict with the ClinVar record, which contains no assertion for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.443727 | Uncertain | 0.292 | 0.928 | 0.375 | -0.857 | Likely Benign | 0.340 | Likely Benign | Likely Benign | 0.082 | Likely Benign | -0.38 | Neutral | 0.003 | Benign | 0.004 | Benign | 2.88 | Benign | 0.00 | Affected | 0.2838 | 0.0840 | -1 | -2 | -5.3 | -26.08 | |||||||||||||||||||||||||||||||||||||||
| c.2697C>G | I899M 2D  AIThe SynGAP1 missense variant I899M is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of computational predictors and the high‑accuracy tools points to a benign impact for I899M. This conclusion is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.443727 | Uncertain | 0.292 | 0.928 | 0.375 | -3.407 | Likely Benign | 0.090 | Likely Benign | Likely Benign | 0.067 | Likely Benign | -0.15 | Neutral | 0.971 | Probably Damaging | 0.690 | Possibly Damaging | 2.70 | Benign | 0.01 | Affected | 0.0687 | 0.2489 | 2 | 1 | -2.6 | 18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2698A>C | T900P 2D  AIThe SynGAP1 missense variant T900P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.465347 | Uncertain | 0.289 | 0.924 | 0.375 | -2.684 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.067 | Likely Benign | -0.40 | Neutral | 0.812 | Possibly Damaging | 0.473 | Possibly Damaging | 2.67 | Benign | 0.22 | Tolerated | 0.1863 | 0.4874 | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2698A>G | T900A 2D  AIThe SynGAP1 missense variant T900A is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.465347 | Uncertain | 0.289 | 0.924 | 0.375 | -2.289 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.028 | Likely Benign | -0.49 | Neutral | 0.059 | Benign | 0.061 | Benign | 2.73 | Benign | 0.40 | Tolerated | 0.4018 | 0.4059 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2698A>T | T900S 2D  AIThe SynGAP1 missense variant T900S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.465347 | Uncertain | 0.289 | 0.924 | 0.375 | -2.031 | Likely Benign | 0.098 | Likely Benign | Likely Benign | 0.056 | Likely Benign | -0.17 | Neutral | 0.224 | Benign | 0.171 | Benign | 2.70 | Benign | 0.71 | Tolerated | 0.3311 | 0.4301 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2699C>A | T900K 2D  AISynGAP1 missense variant T900K has no ClinVar entry and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign), whereas pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized reports Benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign effect for T900K, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.465347 | Uncertain | 0.289 | 0.924 | 0.375 | -4.566 | Likely Benign | 0.721 | Likely Pathogenic | Likely Benign | 0.080 | Likely Benign | -0.64 | Neutral | 0.782 | Possibly Damaging | 0.447 | Possibly Damaging | 2.69 | Benign | 0.92 | Tolerated | 0.1189 | 0.3038 | 0 | -1 | -3.2 | 27.07 | |||||||||||||||||||||||||||||||||||||||
| c.2699C>G | T900R 2D  AIThe SynGAP1 missense variant T900R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (HumDiv and HumVar) and AlphaMissense‑Default predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates Likely Benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of computational evidence points to a benign effect, and this is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.465347 | Uncertain | 0.289 | 0.924 | 0.375 | -3.340 | Likely Benign | 0.617 | Likely Pathogenic | Likely Benign | 0.109 | Likely Benign | 0.34 | Neutral | 0.782 | Possibly Damaging | 0.530 | Possibly Damaging | 2.68 | Benign | 0.83 | Tolerated | 0.1027 | 0.2851 | -1 | -1 | -3.8 | 55.08 | |||||||||||||||||||||||||||||||||||||||
| c.269T>A | V90E 2D  AIThe SynGAP1 missense variant V90E is listed in ClinVar (ID 971665.0) with an uncertain significance status and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all predict benign. Only two tools—SIFT and AlphaMissense‑Default—suggest a pathogenic outcome. When the high‑accuracy consensus is considered, AlphaMissense‑Optimized remains benign, and the SGM Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign effect. No Foldetta stability assessment is available for this variant. Overall, the preponderance of evidence points to a benign impact, which does not contradict the ClinVar uncertain classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | Uncertain | 1 | -4.079 | Likely Benign | 0.703 | Likely Pathogenic | Likely Benign | 0.108 | Likely Benign | -0.38 | Neutral | 0.001 | Benign | 0.000 | Benign | 4.00 | Benign | 0.00 | Affected | 4.32 | 1 | 0.1323 | 0.2233 | -2 | -2 | -7.7 | 29.98 | |||||||||||||||||||||||||||||||||||
| c.269T>C | V90A 2D  AIThe SynGAP1 missense variant V90A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that V90A is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | -0.984 | Likely Benign | 0.288 | Likely Benign | Likely Benign | 0.055 | Likely Benign | 0.27 | Neutral | 0.053 | Benign | 0.008 | Benign | 4.11 | Benign | 0.00 | Affected | 0.3129 | 0.3154 | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||||||||||
| c.2708G>A | G903D 2D  AIThe SynGAP1 missense variant G903D is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (five pathogenic vs. three benign) lean toward a pathogenic interpretation. Thus, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.680603 | Disordered | 0.549818 | Binding | 0.291 | 0.917 | 0.375 | -4.481 | Likely Benign | 0.849 | Likely Pathogenic | Ambiguous | 0.137 | Likely Benign | -1.85 | Neutral | 0.997 | Probably Damaging | 0.939 | Probably Damaging | 2.33 | Pathogenic | 0.01 | Affected | 0.1535 | 0.1321 | 1 | -1 | -3.1 | 58.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2675C>G | S892C 2D  AIThe SynGAP1 missense variant S892C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for S892C, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.473390 | Uncertain | 0.319 | 0.926 | 0.875 | -6.855 | Likely Benign | 0.280 | Likely Benign | Likely Benign | 0.113 | Likely Benign | -2.42 | Neutral | 0.999 | Probably Damaging | 0.969 | Probably Damaging | 2.54 | Benign | 0.00 | Affected | 0.1189 | 0.5323 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||||||
| c.263T>A | V88E 2D  AIThe SynGAP1 missense variant V88E is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) favors a benign outcome. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of predictions (five benign vs. three pathogenic) and the SGM Consensus support a benign classification, whereas AlphaMissense‑Optimized alone suggests pathogenicity. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | -7.978 | In-Between | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.095 | Likely Benign | -1.95 | Neutral | 0.001 | Benign | 0.000 | Benign | 3.68 | Benign | 0.00 | Affected | 0.1444 | 0.1725 | -2 | -2 | -7.7 | 29.98 | ||||||||||||||||||||||||||||||||||||||||
| c.2647C>G | L883V 2D  AIThe SynGAP1 missense variant L883V is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification—there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.641952 | Binding | 0.334 | 0.886 | 0.250 | -4.075 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.059 | Likely Benign | -0.28 | Neutral | 0.451 | Benign | 0.157 | Benign | 2.67 | Benign | 0.28 | Tolerated | 0.1611 | 0.3744 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2648T>C | L883P 2D  AIThe SynGAP1 missense variant L883P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic effect. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence supports a benign classification for this variant, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.641952 | Binding | 0.334 | 0.886 | 0.250 | -2.572 | Likely Benign | 0.105 | Likely Benign | Likely Benign | 0.074 | Likely Benign | -0.58 | Neutral | 0.934 | Possibly Damaging | 0.516 | Possibly Damaging | 2.62 | Benign | 0.21 | Tolerated | 0.3462 | 0.1658 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2648T>G | L883R 2D  AIThe SynGAP1 missense variant L883R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are not available. Overall, the preponderance of evidence points to a benign impact for the L883R variant, and this conclusion is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.641952 | Binding | 0.334 | 0.886 | 0.250 | -3.026 | Likely Benign | 0.286 | Likely Benign | Likely Benign | 0.110 | Likely Benign | -0.83 | Neutral | 0.934 | Possibly Damaging | 0.435 | Benign | 2.73 | Benign | 0.11 | Tolerated | 0.1237 | 0.1147 | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||||||
| c.2650C>G | R884G 2D  AIThe SynGAP1 missense variant R884G is not reported in ClinVar and is absent from gnomAD, indicating no documented clinical or population evidence. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign status. Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.641526 | Binding | 0.305 | 0.898 | 0.250 | -1.332 | Likely Benign | 0.183 | Likely Benign | Likely Benign | 0.030 | Likely Benign | -0.58 | Neutral | 0.000 | Benign | 0.002 | Benign | 2.64 | Benign | 0.27 | Tolerated | 0.3502 | 0.3655 | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2651G>C | R884P 2D  AIThe SynGAP1 missense variant R884P is not reported in ClinVar and is absent from gnomAD. All evaluated in‑silico predictors classify the change as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign effect. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the computational evidence strongly supports a benign classification, and this is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.641526 | Binding | 0.305 | 0.898 | 0.250 | -1.882 | Likely Benign | 0.185 | Likely Benign | Likely Benign | 0.188 | Likely Benign | -1.28 | Neutral | 0.000 | Benign | 0.002 | Benign | 2.58 | Benign | 0.20 | Tolerated | 0.2186 | 0.4337 | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2651G>T | R884L 2D  AIThe SynGAP1 missense variant R884L is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.641526 | Binding | 0.305 | 0.898 | 0.250 | -3.482 | Likely Benign | 0.280 | Likely Benign | Likely Benign | 0.095 | Likely Benign | -1.08 | Neutral | 0.300 | Benign | 0.191 | Benign | 2.63 | Benign | 0.24 | Tolerated | 0.1809 | 0.4177 | -3 | -2 | 8.3 | -43.03 | |||||||||||||||||||||||||||||||||||||||
| c.2653C>A | P885T 2D  AIThe SynGAP1 missense variant P885T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -5.152 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.068 | Likely Benign | -1.44 | Neutral | 0.369 | Benign | 0.171 | Benign | 2.78 | Benign | 0.00 | Affected | 0.1438 | 0.6484 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2653C>G | P885A 2D  AIThe SynGAP1 missense variant P885A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -3.908 | Likely Benign | 0.062 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -1.29 | Neutral | 0.112 | Benign | 0.084 | Benign | 2.80 | Benign | 0.00 | Affected | 0.3425 | 0.5481 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2653C>T | P885S 2D  AIThe SynGAP1 missense variant P885S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -4.089 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.037 | Likely Benign | -1.10 | Neutral | 0.369 | Benign | 0.222 | Benign | 2.87 | Benign | 0.00 | Affected | 0.3363 | 0.5881 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2654C>A | P885H 2D  AIThe SynGAP1 missense variant P885H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence, including the high‑accuracy tools, points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -5.239 | Likely Benign | 0.171 | Likely Benign | Likely Benign | 0.083 | Likely Benign | -1.68 | Neutral | 0.938 | Possibly Damaging | 0.749 | Possibly Damaging | 2.74 | Benign | 0.00 | Affected | 0.1552 | 0.5280 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2654C>T | P885L 2D  AIThe SynGAP1 missense variant P885L is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -4.352 | Likely Benign | 0.150 | Likely Benign | Likely Benign | 0.089 | Likely Benign | -1.99 | Neutral | 0.000 | Benign | 0.001 | Benign | 2.75 | Benign | 0.00 | Affected | 0.2138 | 0.6951 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2656G>A | A886T 2D  AIThe SynGAP1 missense variant A886T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while only SIFT and FATHMM predict pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -4.319 | Likely Benign | 0.075 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -0.78 | Neutral | 0.369 | Benign | 0.237 | Benign | 2.21 | Pathogenic | 0.00 | Affected | 0.1581 | 0.6737 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2656G>C | A886P 2D  AIThe SynGAP1 missense variant A886P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) support a benign classification. This consensus does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -1.931 | Likely Benign | 0.091 | Likely Benign | Likely Benign | 0.073 | Likely Benign | -1.20 | Neutral | 0.812 | Possibly Damaging | 0.575 | Possibly Damaging | 2.15 | Pathogenic | 0.00 | Affected | 0.1985 | 0.4773 | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2647C>A | L883I 2D  AIThe SynGAP1 missense variant L883I is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.641952 | Binding | 0.334 | 0.886 | 0.250 | -5.046 | Likely Benign | 0.102 | Likely Benign | Likely Benign | 0.049 | Likely Benign | -0.26 | Neutral | 0.802 | Possibly Damaging | 0.355 | Benign | 2.66 | Benign | 0.35 | Tolerated | 0.0970 | 0.4093 | 2 | 2 | 0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.2645G>T | G882V 2D  AIThe SynGAP1 missense variant G882V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of ClinVar annotation—there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.632888 | Binding | 0.306 | 0.878 | 0.250 | -5.893 | Likely Benign | 0.257 | Likely Benign | Likely Benign | 0.115 | Likely Benign | -2.41 | Neutral | 0.224 | Benign | 0.066 | Benign | 2.06 | Pathogenic | 0.02 | Affected | 0.1178 | 0.4717 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.263T>C | V88A 2D  AIThe SynGAP1 missense variant V88A is listed in ClinVar (ID 2656486.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are AlphaMissense‑Default, AlphaMissense‑Optimized, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote) is benign; Foldetta stability analysis is unavailable. Overall, the balance of evidence favors a benign interpretation, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | Uncertain | 1 | -5.860 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.050 | Likely Benign | -1.22 | Neutral | 0.053 | Benign | 0.008 | Benign | 3.75 | Benign | 0.00 | Affected | 4.32 | 1 | 0.3563 | 0.2769 | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||||||
| c.263T>G | V88G 2D  AIThe SynGAP1 missense variant V88G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. Tools that predict a pathogenic effect are SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2) and Foldetta results are unavailable. Overall, the majority of predictions (5 benign vs. 4 pathogenic) and the lack of a ClinVar pathogenic classification suggest that V88G is most likely benign, with no contradiction to existing ClinVar data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | -8.588 | Likely Pathogenic | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.084 | Likely Benign | -2.40 | Neutral | 0.024 | Benign | 0.208 | Benign | 3.68 | Benign | 0.00 | Affected | 0.2608 | 0.2609 | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.2641T>A | L881M 2D  AIThe SynGAP1 missense variant L881M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are not available. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign based on current computational evidence, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.629350 | Binding | 0.299 | 0.874 | 0.250 | -4.419 | Likely Benign | 0.090 | Likely Benign | Likely Benign | 0.045 | Likely Benign | -0.31 | Neutral | 0.990 | Probably Damaging | 0.796 | Possibly Damaging | 2.49 | Pathogenic | 0.03 | Affected | 0.0807 | 0.3997 | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2641T>G | L881V 2D  AIThe SynGAP1 missense variant L881V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only polyPhen‑2 HumDiv predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.629350 | Binding | 0.299 | 0.874 | 0.250 | -3.961 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.061 | Likely Benign | -0.29 | Neutral | 0.790 | Possibly Damaging | 0.266 | Benign | 2.60 | Benign | 0.39 | Tolerated | 0.1599 | 0.3053 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2642T>C | L881S 2D  AIThe SynGAP1 missense variant L881S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for the L881S variant, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.629350 | Binding | 0.299 | 0.874 | 0.250 | -3.063 | Likely Benign | 0.169 | Likely Benign | Likely Benign | 0.051 | Likely Benign | -0.49 | Neutral | 0.068 | Benign | 0.012 | Benign | 2.49 | Pathogenic | 0.00 | Affected | 0.3363 | 0.0850 | -3 | -2 | -4.6 | -26.08 | |||||||||||||||||||||||||||||||||||||||
| c.2642T>G | L881W 2D  AIThe SynGAP1 missense variant L881W is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for the variant, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.629350 | Binding | 0.299 | 0.874 | 0.250 | -6.646 | Likely Benign | 0.231 | Likely Benign | Likely Benign | 0.075 | Likely Benign | -0.96 | Neutral | 0.014 | Benign | 0.008 | Benign | 2.44 | Pathogenic | 0.00 | Affected | 0.0662 | 0.3288 | -2 | -2 | -4.7 | 73.05 | |||||||||||||||||||||||||||||||||||||||
| c.2643G>C | L881F 2D  AIThe SynGAP1 missense variant L881F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for L881F, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.629350 | Binding | 0.299 | 0.874 | 0.250 | -5.759 | Likely Benign | 0.101 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -0.87 | Neutral | 0.818 | Possibly Damaging | 0.360 | Benign | 2.48 | Pathogenic | 0.02 | Affected | 0.0711 | 0.3415 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2643G>T | L881F 2D  AIThe SynGAP1 missense variant L881F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for L881F, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.629350 | Binding | 0.299 | 0.874 | 0.250 | -5.759 | Likely Benign | 0.101 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -0.87 | Neutral | 0.818 | Possibly Damaging | 0.360 | Benign | 2.48 | Pathogenic | 0.02 | Affected | 0.0711 | 0.3415 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2644G>A | G882R 2D  AIThe SynGAP1 missense variant G882R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive because it yields a 2‑to‑2 split. Foldetta results are unavailable. Overall, the majority of predictions lean toward a benign impact, and there is no ClinVar annotation to contradict this assessment. Thus, the variant is most likely benign based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.632888 | Binding | 0.306 | 0.878 | 0.250 | -4.715 | Likely Benign | 0.758 | Likely Pathogenic | Likely Benign | 0.051 | Likely Benign | -1.48 | Neutral | 0.001 | Benign | 0.003 | Benign | 2.04 | Pathogenic | 0.01 | Affected | 0.0905 | 0.4126 | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||||||
| c.2644G>C | G882R 2D  AIThe SynGAP1 missense variant G882R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 benign vs 2 pathogenic). Foldetta results are unavailable. Overall, the majority of predictions (six benign vs three pathogenic) support a benign classification. This consensus does not contradict any ClinVar status, as the variant is not yet catalogued there. Thus, the variant is most likely benign based on current predictive evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.632888 | Binding | 0.306 | 0.878 | 0.250 | -4.715 | Likely Benign | 0.758 | Likely Pathogenic | Likely Benign | 0.051 | Likely Benign | -1.48 | Neutral | 0.001 | Benign | 0.003 | Benign | 2.04 | Pathogenic | 0.01 | Affected | 0.0905 | 0.4126 | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||||||
| c.2644G>T | G882W 2D  AIThe SynGAP1 missense variant G882W has no ClinVar entry and is absent from gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further show AlphaMissense‑Optimized as benign, while the SGM‑Consensus remains pathogenic; Foldetta stability analysis is unavailable. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion does not conflict with the ClinVar status, which currently contains no classification for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.690604 | Disordered | 0.632888 | Binding | 0.306 | 0.878 | 0.250 | -8.637 | Likely Pathogenic | 0.606 | Likely Pathogenic | Likely Benign | 0.166 | Likely Benign | -2.35 | Neutral | 0.983 | Probably Damaging | 0.813 | Possibly Damaging | 2.01 | Pathogenic | 0.00 | Affected | 0.0829 | 0.4616 | -7 | -2 | -0.5 | 129.16 | |||||||||||||||||||||||||||||||||||||||
| c.2645G>A | G882E 2D  AIThe SynGAP1 missense variant G882E is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑vs‑2 split; Foldetta results are unavailable. Overall, more tools (six) predict benign than pathogenic (three), and no ClinVar evidence contradicts this assessment. Thus, the variant is most likely benign based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.632888 | Binding | 0.306 | 0.878 | 0.250 | -4.246 | Likely Benign | 0.613 | Likely Pathogenic | Likely Benign | 0.079 | Likely Benign | -1.16 | Neutral | 0.004 | Benign | 0.005 | Benign | 2.04 | Pathogenic | 0.01 | Affected | 0.1263 | 0.3244 | 0 | -2 | -3.1 | 72.06 | ||||||||||||||||||||||||||||||||||||||||
| c.2645G>C | G882A 2D  AIThe SynGAP1 missense variant G882A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, whereas only FATHMM predicts pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence indicates the variant is most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.632888 | Binding | 0.306 | 0.878 | 0.250 | -3.876 | Likely Benign | 0.131 | Likely Benign | Likely Benign | 0.048 | Likely Benign | -1.05 | Neutral | 0.004 | Benign | 0.002 | Benign | 2.14 | Pathogenic | 0.79 | Tolerated | 0.3345 | 0.5268 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2656G>T | A886S 2D  AIThe SynGAP1 missense variant A886S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -2.366 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.055 | Likely Benign | -0.50 | Neutral | 0.224 | Benign | 0.185 | Benign | 2.24 | Pathogenic | 0.00 | Affected | 0.2755 | 0.5639 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2657C>A | A886E 2D  AIThe SynGAP1 missense variant A886E is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors a benign outcome. Foldetta, which would provide a protein‑folding stability estimate, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact. This conclusion is not contradicted by ClinVar, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -3.747 | Likely Benign | 0.422 | Ambiguous | Likely Benign | 0.096 | Likely Benign | -1.31 | Neutral | 0.423 | Benign | 0.230 | Benign | 2.17 | Pathogenic | 0.00 | Affected | 0.1483 | 0.2008 | 0 | -1 | -5.3 | 58.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2657C>G | A886G 2D  AIThe SynGAP1 missense variant A886G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for A886G, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -1.993 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.074 | Likely Benign | -0.70 | Neutral | 0.597 | Possibly Damaging | 0.366 | Benign | 2.28 | Pathogenic | 0.00 | Affected | 0.2173 | 0.3913 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2668C>G | R890G 2D  AIThe SynGAP1 missense variant R890G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also likely benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence indicates that R890G is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.531156 | Binding | 0.284 | 0.928 | 0.625 | -2.080 | Likely Benign | 0.310 | Likely Benign | Likely Benign | 0.146 | Likely Benign | -1.99 | Neutral | 0.990 | Probably Damaging | 0.894 | Possibly Damaging | 3.97 | Benign | 0.33 | Tolerated | 0.3734 | 0.2711 | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2669G>T | R890L 2D  AIThe SynGAP1 missense variant R890L is not reported in ClinVar (ClinVar status: none) and is absent from gnomAD (gnomAD ID: none). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, and polyPhen‑2 HumVar. AlphaMissense‑Default is uncertain. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a benign prediction (2 benign vs. 1 pathogenic, with one uncertain). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑accuracy and consensus predictions indicate a benign impact. This conclusion is not contradicted by ClinVar status, which has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.720929 | Disordered | 0.531156 | Binding | 0.284 | 0.928 | 0.625 | -2.387 | Likely Benign | 0.389 | Ambiguous | Likely Benign | 0.213 | Likely Benign | -2.74 | Deleterious | 0.990 | Probably Damaging | 0.921 | Probably Damaging | 3.98 | Benign | 0.20 | Tolerated | 0.1876 | 0.3406 | -3 | -2 | 8.3 | -43.03 | ||||||||||||||||||||||||||||||||||||||||
| c.266C>A | P89Q 2D  AIThe SynGAP1 missense variant P89Q is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 pathogenic vs. 2 benign). Foldetta results are unavailable. Overall, the balance of evidence (five pathogenic vs. four benign predictions) indicates the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | -4.779 | Likely Benign | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.107 | Likely Benign | -2.63 | Deleterious | 0.642 | Possibly Damaging | 0.038 | Benign | 3.74 | Benign | 0.00 | Affected | 0.1633 | 0.4310 | 0 | -1 | -1.9 | 31.01 | ||||||||||||||||||||||||||||||||||||||||
| c.266C>G | P89R 2D  AIThe SynGAP1 missense variant P89R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 pathogenic vs. 2 benign). Foldetta results are unavailable. Overall, the balance of evidence (five pathogenic vs. four benign predictions) indicates the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | -3.636 | Likely Benign | 0.983 | Likely Pathogenic | Likely Pathogenic | 0.116 | Likely Benign | -3.00 | Deleterious | 0.642 | Possibly Damaging | 0.097 | Benign | 3.83 | Benign | 0.00 | Affected | 0.1720 | 0.3362 | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||||||||
| c.266C>T | P89L 2D  AISynGAP1 missense variant P89L is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM, while pathogenic calls are made by PROVEAN, polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments indicate that AlphaMissense‑Optimized predicts pathogenicity, whereas the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑to‑2 split, and Foldetta results are unavailable. Overall, the majority of tools favor a pathogenic effect, but the evidence is not unanimous. Therefore, the variant is most likely pathogenic according to the current predictions, and this assessment does not contradict its ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | Uncertain | 2 | -6.775 | Likely Benign | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.119 | Likely Benign | -3.29 | Deleterious | 0.889 | Possibly Damaging | 0.058 | Benign | 3.73 | Benign | 0.00 | Affected | 4.32 | 1 | 0.2399 | 0.5638 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||
| c.2671C>A | L891I 2D  AIThe SynGAP1 missense variant L891I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only polyPhen‑2 HumDiv predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta results are unavailable, so they do not influence the assessment. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.505861 | Binding | 0.305 | 0.923 | 0.750 | -5.803 | Likely Benign | 0.099 | Likely Benign | Likely Benign | 0.048 | Likely Benign | -0.71 | Neutral | 0.481 | Possibly Damaging | 0.202 | Benign | 2.74 | Benign | 0.14 | Tolerated | 0.0957 | 0.3494 | 2 | 2 | 0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.2671C>G | L891V 2D  AIThe SynGAP1 missense variant L891V is not reported in ClinVar and is absent from gnomAD, indicating no documented clinical or population evidence. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification. Foldetta results are unavailable, so they do not influence the assessment. Overall, the variant is most likely benign, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.505861 | Binding | 0.305 | 0.923 | 0.750 | -4.992 | Likely Benign | 0.094 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -0.65 | Neutral | 0.057 | Benign | 0.032 | Benign | 2.72 | Benign | 0.70 | Tolerated | 0.1614 | 0.2945 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2671C>T | L891F 2D  AIThe SynGAP1 missense variant L891F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available. Overall, the majority of evidence points to a benign effect for L891F, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.505861 | Binding | 0.305 | 0.923 | 0.750 | -5.650 | Likely Benign | 0.126 | Likely Benign | Likely Benign | 0.102 | Likely Benign | -1.80 | Neutral | 0.970 | Probably Damaging | 0.744 | Possibly Damaging | 2.66 | Benign | 0.04 | Affected | 0.0652 | 0.2907 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2672T>A | L891H 2D  AIThe SynGAP1 missense variant L891H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.505861 | Binding | 0.305 | 0.923 | 0.750 | -4.604 | Likely Benign | 0.320 | Likely Benign | Likely Benign | 0.111 | Likely Benign | -1.79 | Neutral | 0.122 | Benign | 0.134 | Benign | 2.69 | Benign | 0.00 | Affected | 0.1099 | 0.1189 | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||||||
| c.2672T>C | L891P 2D  AIThe SynGAP1 missense variant L891P is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for L891P, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.505861 | Binding | 0.305 | 0.923 | 0.750 | -3.835 | Likely Benign | 0.169 | Likely Benign | Likely Benign | 0.126 | Likely Benign | -1.61 | Neutral | 0.990 | Probably Damaging | 0.900 | Possibly Damaging | 2.67 | Benign | 0.01 | Affected | 0.3594 | 0.1138 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2672T>G | L891R 2D  AIThe SynGAP1 missense variant L891R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar) and SIFT. AlphaMissense‑Default remains uncertain. The high‑accuracy consensus methods reinforce the benign assessment: AlphaMissense‑Optimized predicts benign, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign,” and the protein‑folding stability tool Foldetta has no available result for this variant. Based on the aggregate evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.505861 | Binding | 0.305 | 0.923 | 0.750 | -4.120 | Likely Benign | 0.419 | Ambiguous | Likely Benign | 0.122 | Likely Benign | -1.83 | Neutral | 0.970 | Probably Damaging | 0.801 | Possibly Damaging | 2.65 | Benign | 0.01 | Affected | 0.1233 | 0.0863 | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||||||
| c.2674T>A | S892T 2D  AIThe SynGAP1 missense variant S892T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.473390 | Uncertain | 0.319 | 0.926 | 0.875 | -4.806 | Likely Benign | 0.210 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -1.09 | Neutral | 0.975 | Probably Damaging | 0.748 | Possibly Damaging | 2.61 | Benign | 0.03 | Affected | 0.1606 | 0.5857 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2674T>G | S892A 2D  AIThe SynGAP1 missense variant S892A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence—including the consensus and high‑accuracy predictions—supports a benign classification, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.473390 | Uncertain | 0.319 | 0.926 | 0.875 | -3.867 | Likely Benign | 0.188 | Likely Benign | Likely Benign | 0.037 | Likely Benign | -1.45 | Neutral | 0.889 | Possibly Damaging | 0.682 | Possibly Damaging | 2.63 | Benign | 0.02 | Affected | 0.5131 | 0.4481 | Weaken | 1 | 1 | 2.6 | -16.00 | ||||||||||||||||||||||||||||||||||||||
| c.2668C>A | R890S 2D  AIThe SynGAP1 missense variant R890S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this assessment, so the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.531156 | Binding | 0.284 | 0.928 | 0.625 | -2.481 | Likely Benign | 0.600 | Likely Pathogenic | Likely Benign | 0.176 | Likely Benign | -1.73 | Neutral | 0.990 | Probably Damaging | 0.894 | Possibly Damaging | 4.02 | Benign | 0.27 | Tolerated | 0.3477 | 0.2875 | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||||||
| c.2666G>T | G889V 2D  AIThe SynGAP1 missense variant G889V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G889V, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.552581 | Binding | 0.331 | 0.928 | 0.625 | -5.781 | Likely Benign | 0.148 | Likely Benign | Likely Benign | 0.101 | Likely Benign | -2.11 | Neutral | 0.611 | Possibly Damaging | 0.243 | Benign | 2.39 | Pathogenic | 0.03 | Affected | 0.1198 | 0.4523 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.2659C>A | P887T 2D  AIThe SynGAP1 missense variant P887T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign,” while Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.602269 | Binding | 0.348 | 0.925 | 0.500 | -4.780 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.076 | Likely Benign | -1.47 | Neutral | 0.292 | Benign | 0.110 | Benign | 2.79 | Benign | 0.21 | Tolerated | 0.1228 | 0.3822 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2659C>G | P887A 2D  AIThe SynGAP1 missense variant P887A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign,” while Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.602269 | Binding | 0.348 | 0.925 | 0.500 | -2.986 | Likely Benign | 0.047 | Likely Benign | Likely Benign | 0.056 | Likely Benign | -1.64 | Neutral | 0.011 | Benign | 0.004 | Benign | 2.81 | Benign | 0.36 | Tolerated | 0.2744 | 0.3597 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2659C>T | P887S 2D  AIThe SynGAP1 missense variant P887S is not reported in ClinVar and is absent from gnomAD, indicating no documented clinical or population evidence. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this view: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the predictions strongly suggest that P887S is most likely benign, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.602269 | Binding | 0.348 | 0.925 | 0.500 | -3.462 | Likely Benign | 0.065 | Likely Benign | Likely Benign | 0.066 | Likely Benign | -1.26 | Neutral | 0.032 | Benign | 0.017 | Benign | 2.85 | Benign | 0.25 | Tolerated | 0.2770 | 0.3946 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||||||
| c.265C>A | P89T 2D  AIThe SynGAP1 missense variant P89T has no ClinVar entry and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while pathogenic calls come from polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments further highlight this discordance: AlphaMissense‑Optimized predicts pathogenic, whereas the SGM‑Consensus (majority vote) predicts benign. Foldetta stability analysis is unavailable. Overall, the preponderance of evidence leans toward a benign effect, but the presence of a pathogenic prediction from AlphaMissense‑Optimized introduces uncertainty. This assessment does not contradict ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | -5.429 | Likely Benign | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.105 | Likely Benign | -2.49 | Neutral | 0.588 | Possibly Damaging | 0.036 | Benign | 3.74 | Benign | 0.00 | Affected | 0.1786 | 0.4702 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.265C>G | P89A 2D  AIThe SynGAP1 missense variant P89A is listed in ClinVar (ID 1031674.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a “Likely Benign” outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑confidence predictions indicate a benign impact, and this is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Thus, the variant is most likely benign based on current predictive evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | Uncertain | 2 | -5.778 | Likely Benign | 0.920 | Likely Pathogenic | Ambiguous | 0.095 | Likely Benign | -2.47 | Neutral | 0.225 | Benign | 0.020 | Benign | 3.77 | Benign | 0.00 | Affected | 4.32 | 1 | 0.3407 | 0.3768 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.265C>T | P89S 2D  AIThe SynGAP1 missense variant P89S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as likely benign. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM‑Consensus (majority vote) indicates likely benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of predictions lean toward a benign impact, and this assessment does not contradict the absence of a ClinVar classification. Thus, based on the available computational evidence, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | -5.198 | Likely Benign | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.099 | Likely Benign | -2.40 | Neutral | 0.225 | Benign | 0.020 | Benign | 3.76 | Benign | 0.00 | Affected | 0.3459 | 0.4191 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2660C>A | P887H 2D  AIThe SynGAP1 missense variant P887H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for P887H, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.602269 | Binding | 0.348 | 0.925 | 0.500 | -4.900 | Likely Benign | 0.136 | Likely Benign | Likely Benign | 0.072 | Likely Benign | -1.71 | Neutral | 0.977 | Probably Damaging | 0.777 | Possibly Damaging | 2.73 | Benign | 0.13 | Tolerated | 0.1236 | 0.3275 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2660C>G | P887R 2D  AIThe SynGAP1 missense variant P887R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also reports Likely Benign. No Foldetta stability data are available, so it does not influence the assessment. Overall, the preponderance of evidence supports a benign classification for P887R, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.602269 | Binding | 0.348 | 0.925 | 0.500 | -4.759 | Likely Benign | 0.201 | Likely Benign | Likely Benign | 0.091 | Likely Benign | -1.06 | Neutral | 0.802 | Possibly Damaging | 0.413 | Benign | 2.76 | Benign | 1.00 | Tolerated | 0.1306 | 0.2238 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2660C>T | P887L 2D  AIThe SynGAP1 missense variant P887L is not reported in ClinVar and is absent from gnomAD, indicating no documented allele frequency data. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification. Foldetta results are not available, so they do not influence the assessment. Based on the collective predictions, the variant is most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.602269 | Binding | 0.348 | 0.925 | 0.500 | -4.008 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.067 | Likely Benign | -1.72 | Neutral | 0.152 | Benign | 0.070 | Benign | 2.81 | Benign | 0.18 | Tolerated | 0.1800 | 0.5164 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2662G>T | A888S 2D  AIThe SynGAP1 missense variant A888S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.575860 | Binding | 0.352 | 0.928 | 0.625 | -3.580 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.034 | Likely Benign | -0.66 | Neutral | 0.017 | Benign | 0.025 | Benign | 2.64 | Benign | 0.00 | Affected | 0.2661 | 0.5626 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2663C>A | A888D 2D  AIThe SynGAP1 missense variant A888D is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all classify the change as benign, while SIFT uniquely predicts it as pathogenic. The consensus prediction from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized reports benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not conflict with the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.575860 | Binding | 0.352 | 0.928 | 0.625 | -3.870 | Likely Benign | 0.526 | Ambiguous | Likely Benign | 0.078 | Likely Benign | -1.52 | Neutral | 0.077 | Benign | 0.097 | Benign | 2.56 | Benign | 0.00 | Affected | 0.1646 | 0.1386 | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||||||
| c.2665G>C | G889R 2D  AIThe SynGAP1 missense variant G889R is not reported in ClinVar (ClinVar status: not present) and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 pathogenic vs. 2 benign), and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.552581 | Binding | 0.331 | 0.928 | 0.625 | -3.357 | Likely Benign | 0.685 | Likely Pathogenic | Likely Benign | 0.072 | Likely Benign | -1.96 | Neutral | 0.027 | Benign | 0.009 | Benign | 2.38 | Pathogenic | 0.02 | Affected | 4.32 | 4 | 0.0925 | 0.4165 | -2 | -3 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||||
| c.2666G>A | G889E 2D  AIThe SynGAP1 missense variant G889E is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions lean toward a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.552581 | Binding | 0.331 | 0.928 | 0.625 | -5.352 | Likely Benign | 0.572 | Likely Pathogenic | Likely Benign | 0.083 | Likely Benign | -1.96 | Neutral | 0.611 | Possibly Damaging | 0.187 | Benign | 2.41 | Pathogenic | 0.02 | Affected | 0.1442 | 0.3719 | 0 | -2 | -3.1 | 72.06 | ||||||||||||||||||||||||||||||||||||||||
| c.2675C>A | S892Y 2D  AIThe SynGAP1 missense variant S892Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, the SGM‑Consensus (majority vote) also predicts benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.473390 | Uncertain | 0.319 | 0.926 | 0.875 | -4.696 | Likely Benign | 0.645 | Likely Pathogenic | Likely Benign | 0.113 | Likely Benign | -2.29 | Neutral | 0.998 | Probably Damaging | 0.959 | Probably Damaging | 2.55 | Benign | 0.00 | Affected | 0.0814 | 0.5051 | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||||||
| c.2708G>C | G903A 2D  AIThe SynGAP1 missense variant G903A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact for G903A. This conclusion is not contradicted by ClinVar, which has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.680603 | Disordered | 0.549818 | Binding | 0.291 | 0.917 | 0.375 | -2.843 | Likely Benign | 0.227 | Likely Benign | Likely Benign | 0.141 | Likely Benign | -1.54 | Neutral | 0.989 | Probably Damaging | 0.829 | Possibly Damaging | 2.43 | Pathogenic | 0.08 | Tolerated | 0.3490 | 0.5025 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2738C>A | T913K 2D  AIThe SynGAP1 missense variant T913K is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact for T913K, and this conclusion does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.763517 | Binding | 0.339 | 0.899 | 0.375 | -4.145 | Likely Benign | 0.619 | Likely Pathogenic | Likely Benign | 0.152 | Likely Benign | -1.46 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.70 | Benign | 0.05 | Affected | 0.1204 | 0.3491 | 0 | -1 | -3.2 | 27.07 | |||||||||||||||||||||||||||||||||||||||
| c.2747T>A | V916D 2D  AIThe SynGAP1 missense variant V916D is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (HumDiv and HumVar) and SIFT uniformly predict a pathogenic outcome. AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus indicates Likely Benign, and Foldetta (which integrates FoldX‑MD and Rosetta outputs) is not available for this variant. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.835395 | Binding | 0.308 | 0.879 | 0.250 | -2.653 | Likely Benign | 0.392 | Ambiguous | Likely Benign | 0.139 | Likely Benign | -1.48 | Neutral | 0.975 | Probably Damaging | 0.767 | Possibly Damaging | 2.69 | Benign | 0.01 | Affected | 0.1580 | 0.1113 | -2 | -3 | -7.7 | 15.96 | |||||||||||||||||||||||||||||||||||||||
| c.2747T>C | V916A 2D  AIThe SynGAP1 missense variant V916A is not reported in ClinVar and is absent from gnomAD. Prediction tools that assess pathogenicity all converge on a benign outcome: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. No tool in the dataset indicates a pathogenic effect, so the pathogenic‑prediction group is empty. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign,” and the Foldetta stability analysis is not available. Based on the collective predictions, the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.835395 | Binding | 0.308 | 0.879 | 0.250 | -2.524 | Likely Benign | 0.108 | Likely Benign | Likely Benign | 0.116 | Likely Benign | -0.18 | Neutral | 0.244 | Benign | 0.171 | Benign | 2.91 | Benign | 1.00 | Tolerated | 0.3455 | 0.2841 | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||||||||||
| c.2747T>G | V916G 2D  AIThe SynGAP1 missense variant V916G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.642678 | Disordered | 0.835395 | Binding | 0.308 | 0.879 | 0.250 | -1.950 | Likely Benign | 0.091 | Likely Benign | Likely Benign | 0.093 | Likely Benign | -0.63 | Neutral | 0.666 | Possibly Damaging | 0.917 | Probably Damaging | 2.69 | Benign | 0.05 | Affected | 0.2420 | 0.2681 | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.2749C>A | P917T 2D  AIThe SynGAP1 missense variant P917T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | -4.012 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -1.68 | Neutral | 0.139 | Benign | 0.088 | Benign | 2.69 | Benign | 0.00 | Affected | 0.1445 | 0.5711 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.274G>C | G92R 2D  AIThe SynGAP1 missense variant G92R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the majority of high‑confidence tools and the consensus prediction lean toward a benign interpretation, with no conflict with ClinVar status. Thus, the variant is most likely benign based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -2.909 | Likely Benign | 0.876 | Likely Pathogenic | Ambiguous | 0.139 | Likely Benign | -2.38 | Neutral | 0.999 | Probably Damaging | 0.979 | Probably Damaging | 4.01 | Benign | 0.00 | Affected | 4.32 | 1 | 0.1041 | 0.4605 | -2 | -3 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||
| c.274G>T | G92W 2D  AIThe SynGAP1 missense variant G92W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Tools that agree on a pathogenic effect include PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments are mixed: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of available predictions (five pathogenic vs. three benign) suggest a pathogenic impact. This conclusion is not contradicted by ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -6.502 | Likely Benign | 0.789 | Likely Pathogenic | Ambiguous | 0.224 | Likely Benign | -2.61 | Deleterious | 1.000 | Probably Damaging | 0.979 | Probably Damaging | 3.95 | Benign | 0.00 | Affected | 0.0640 | 0.4546 | -7 | -2 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||||||||||
| c.2750C>A | P917H 2D  AIThe SynGAP1 missense variant P917H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence—including the high‑accuracy tools—points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | -5.395 | Likely Benign | 0.182 | Likely Benign | Likely Benign | 0.122 | Likely Benign | -2.00 | Neutral | 0.975 | Probably Damaging | 0.766 | Possibly Damaging | 2.65 | Benign | 0.00 | Affected | 0.1584 | 0.4410 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2750C>T | P917L 2D  AIThe SynGAP1 missense variant P917L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | -3.369 | Likely Benign | 0.172 | Likely Benign | Likely Benign | 0.075 | Likely Benign | -2.35 | Neutral | 0.425 | Benign | 0.233 | Benign | 2.75 | Benign | 0.00 | Affected | 0.2001 | 0.6351 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||||
| c.2752G>C | A918P 2D  AIThe SynGAP1 missense variant A918P is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.891459 | Binding | 0.317 | 0.860 | 0.250 | -2.087 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.136 | Likely Benign | -0.60 | Neutral | 0.998 | Probably Damaging | 0.924 | Probably Damaging | 2.59 | Benign | 0.04 | Affected | 0.2052 | 0.4985 | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2752G>T | A918S 2D  AIThe SynGAP1 missense variant A918S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also likely benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence indicates that A918S is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.891459 | Binding | 0.317 | 0.860 | 0.250 | -3.279 | Likely Benign | 0.070 | Likely Benign | Likely Benign | 0.058 | Likely Benign | -0.04 | Neutral | 0.910 | Possibly Damaging | 0.554 | Possibly Damaging | 2.66 | Benign | 0.07 | Tolerated | 0.2824 | 0.5442 | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||||||
| c.2753C>A | A918D 2D  AIThe SynGAP1 missense variant A918D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic outcome. AlphaMissense‑Default remains uncertain. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized independently predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence—including the high‑accuracy tools—points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation; there is no contradictory ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.891459 | Binding | 0.317 | 0.860 | 0.250 | -4.281 | Likely Benign | 0.445 | Ambiguous | Likely Benign | 0.102 | Likely Benign | -0.99 | Neutral | 0.961 | Probably Damaging | 0.721 | Possibly Damaging | 2.64 | Benign | 0.01 | Affected | 0.2037 | 0.2412 | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||||||
| c.2753C>G | A918G 2D  AIThe SynGAP1 missense variant A918G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic outcome. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta results are not available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.891459 | Binding | 0.317 | 0.860 | 0.250 | -2.906 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.099 | Likely Benign | 0.14 | Neutral | 0.954 | Possibly Damaging | 0.630 | Possibly Damaging | 2.72 | Benign | 0.70 | Tolerated | 0.2125 | 0.4376 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2755C>A | Q919K 2D  AIThe SynGAP1 missense variant Q919K is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign. Only polyPhen‑2 HumDiv flags it as pathogenic, while AlphaMissense‑Default remains uncertain. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Taken together, the majority of evidence supports a benign interpretation, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.911223 | Binding | 0.299 | 0.841 | 0.250 | -4.357 | Likely Benign | 0.347 | Ambiguous | Likely Benign | 0.125 | Likely Benign | -1.43 | Neutral | 0.771 | Possibly Damaging | 0.412 | Benign | 2.54 | Benign | 0.21 | Tolerated | 0.1950 | 0.4000 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.2746G>T | V916F 2D  AIThe SynGAP1 missense variant V916F is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and SIFT—suggest a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.835395 | Binding | 0.308 | 0.879 | 0.250 | -4.011 | Likely Benign | 0.123 | Likely Benign | Likely Benign | 0.121 | Likely Benign | -1.18 | Neutral | 0.642 | Possibly Damaging | 0.265 | Benign | 2.67 | Benign | 0.02 | Affected | 0.0729 | 0.4593 | -1 | -1 | -1.4 | 48.04 | |||||||||||||||||||||||||||||||||||||||
| c.2746G>C | V916L 2D  AIThe SynGAP1 missense variant V916L is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the variant is most likely benign, and this conclusion does not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.835395 | Binding | 0.308 | 0.879 | 0.250 | -2.645 | Likely Benign | 0.086 | Likely Benign | Likely Benign | 0.064 | Likely Benign | 0.31 | Neutral | 0.001 | Benign | 0.002 | Benign | 2.73 | Benign | 0.09 | Tolerated | 0.1190 | 0.5323 | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2738C>G | T913R 2D  AIThe SynGAP1 missense variant T913R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.763517 | Binding | 0.339 | 0.899 | 0.375 | -3.218 | Likely Benign | 0.494 | Ambiguous | Likely Benign | 0.152 | Likely Benign | -0.97 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.68 | Benign | 0.03 | Affected | 0.1025 | 0.3373 | -1 | -1 | -3.8 | 55.08 | |||||||||||||||||||||||||||||||||||||||
| c.2738C>T | T913I 2D  AIThe SynGAP1 missense variant T913I is not reported in ClinVar and has no entries in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for T913I, and this conclusion does not contradict the ClinVar status, which currently has no classification for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.763517 | Binding | 0.339 | 0.899 | 0.375 | -4.030 | Likely Benign | 0.600 | Likely Pathogenic | Likely Benign | 0.144 | Likely Benign | -2.01 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.70 | Benign | 0.04 | Affected | 0.0903 | 0.6119 | 0 | -1 | 5.2 | 12.05 | |||||||||||||||||||||||||||||||||||||||
| c.273G>T | E91D 2D  AIThe SynGAP1 missense variant E91D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of any ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -3.160 | Likely Benign | 0.293 | Likely Benign | Likely Benign | 0.095 | Likely Benign | -0.84 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 3.98 | Benign | 0.00 | Affected | 4.32 | 1 | 0.2000 | 0.5219 | 2 | 3 | 0.0 | -14.03 | |||||||||||||||||||||||||||||||||||||
| c.2740G>A | D914N 2D  AIThe SynGAP1 missense variant D914N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) all classify the change as benign or likely benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns a benign prediction, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.785987 | Binding | 0.320 | 0.892 | 0.250 | -3.859 | Likely Benign | 0.291 | Likely Benign | Likely Benign | 0.126 | Likely Benign | -1.22 | Neutral | 0.998 | Probably Damaging | 0.966 | Probably Damaging | 2.69 | Benign | 0.02 | Affected | 0.1770 | 0.7848 | 2 | 1 | 0.0 | -0.98 | |||||||||||||||||||||||||||||||||||||||
| c.2740G>C | D914H 2D  AIThe SynGAP1 missense variant D914H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) support a benign classification. This consensus does not contradict ClinVar status, as no ClinVar entry exists for this variant. Thus, based on current computational evidence, the D914H variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.785987 | Binding | 0.320 | 0.892 | 0.250 | -3.755 | Likely Benign | 0.651 | Likely Pathogenic | Likely Benign | 0.184 | Likely Benign | -1.26 | Neutral | 1.000 | Probably Damaging | 0.993 | Probably Damaging | 2.74 | Benign | 0.01 | Affected | 0.1963 | 0.8451 | 1 | -1 | 0.3 | 22.05 | |||||||||||||||||||||||||||||||||||||||
| c.2740G>T | D914Y 2D  AIThe SynGAP1 missense variant D914Y is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split, and Foldetta results are unavailable. Overall, the majority of tools (five pathogenic vs. four benign) and the lack of a benign consensus from the high‑accuracy methods suggest that D914Y is most likely pathogenic. This prediction does not contradict ClinVar status, as the variant has not yet been classified in that database. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.699094 | Disordered | 0.785987 | Binding | 0.320 | 0.892 | 0.250 | -4.768 | Likely Benign | 0.740 | Likely Pathogenic | Likely Benign | 0.239 | Likely Benign | -2.65 | Deleterious | 1.000 | Probably Damaging | 0.993 | Probably Damaging | 2.62 | Benign | 0.01 | Affected | 0.0907 | 0.7583 | -4 | -3 | 2.2 | 48.09 | ||||||||||||||||||||||||||||||||||||||||
| c.2741A>C | D914A 2D  AIThe SynGAP1 missense variant D914A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default remains uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for D914A, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.785987 | Binding | 0.320 | 0.892 | 0.250 | -1.690 | Likely Benign | 0.387 | Ambiguous | Likely Benign | 0.128 | Likely Benign | -1.48 | Neutral | 0.996 | Probably Damaging | 0.953 | Probably Damaging | 2.69 | Benign | 0.05 | Affected | 0.4413 | 0.7086 | 0 | -2 | 5.3 | -44.01 | |||||||||||||||||||||||||||||||||||||||
| c.2741A>G | D914G 2D  AIThe SynGAP1 missense variant D914G is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized reports Benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for D914G, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.785987 | Binding | 0.320 | 0.892 | 0.250 | -1.486 | Likely Benign | 0.314 | Likely Benign | Likely Benign | 0.123 | Likely Benign | -1.46 | Neutral | 0.998 | Probably Damaging | 0.966 | Probably Damaging | 2.65 | Benign | 0.02 | Affected | 0.4421 | 0.7257 | 1 | -1 | 3.1 | -58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2741A>T | D914V 2D  AIThe SynGAP1 missense variant D914V is listed in ClinVar (ID 2582846.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) as benign; Foldetta results are unavailable. Overall, the balance of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.785987 | Binding | 0.320 | 0.892 | 0.250 | Uncertain | 1 | -4.260 | Likely Benign | 0.723 | Likely Pathogenic | Likely Benign | 0.187 | Likely Benign | -2.24 | Neutral | 0.999 | Probably Damaging | 0.986 | Probably Damaging | 2.64 | Benign | 0.01 | Affected | 3.77 | 5 | 0.1316 | 0.7249 | -3 | -2 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||
| c.2742C>G | D914E 2D  AIThe SynGAP1 missense variant D914E is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also reports Likely Benign. No Foldetta stability data are available, so it does not influence the assessment. Overall, the preponderance of evidence supports a benign classification for D914E, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.785987 | Binding | 0.320 | 0.892 | 0.250 | -3.231 | Likely Benign | 0.170 | Likely Benign | Likely Benign | 0.133 | Likely Benign | -0.29 | Neutral | 0.893 | Possibly Damaging | 0.418 | Benign | 2.87 | Benign | 1.00 | Tolerated | 3.77 | 5 | 0.1909 | 0.7088 | 2 | 3 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||||
| c.2743G>C | G915R 2D  AIThe SynGAP1 missense variant G915R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G915R, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.808641 | Binding | 0.302 | 0.880 | 0.375 | -4.528 | Likely Benign | 0.656 | Likely Pathogenic | Likely Benign | 0.104 | Likely Benign | -2.31 | Neutral | 0.999 | Probably Damaging | 0.969 | Probably Damaging | 2.69 | Benign | 0.00 | Affected | 0.0805 | 0.4204 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2744G>A | G915D 2D  AIThe SynGAP1 missense variant G915D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G915D, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.808641 | Binding | 0.302 | 0.880 | 0.375 | -3.409 | Likely Benign | 0.502 | Ambiguous | Likely Benign | 0.134 | Likely Benign | -1.75 | Neutral | 0.978 | Probably Damaging | 0.871 | Possibly Damaging | 2.70 | Benign | 0.01 | Affected | 0.1574 | 0.1559 | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2744G>C | G915A 2D  AIThe SynGAP1 missense variant G915A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available. Overall, the majority of evidence points to a benign effect for G915A, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.808641 | Binding | 0.302 | 0.880 | 0.375 | -4.007 | Likely Benign | 0.103 | Likely Benign | Likely Benign | 0.053 | Likely Benign | -1.22 | Neutral | 0.900 | Possibly Damaging | 0.622 | Possibly Damaging | 2.87 | Benign | 0.05 | Affected | 0.3454 | 0.4930 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2755C>G | Q919E 2D  AIThe SynGAP1 missense variant Q919E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of high‑confidence predictions and the consensus score favor a benign impact. Thus, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.911223 | Binding | 0.299 | 0.841 | 0.250 | -3.352 | Likely Benign | 0.157 | Likely Benign | Likely Benign | 0.143 | Likely Benign | -1.13 | Neutral | 0.771 | Possibly Damaging | 0.492 | Possibly Damaging | 2.44 | Pathogenic | 0.05 | Affected | 0.1366 | 0.2692 | 2 | 2 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.2756A>C | Q919P 2D  AIThe SynGAP1 missense variant Q919P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign based on current computational evidence, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.911223 | Binding | 0.299 | 0.841 | 0.250 | -2.358 | Likely Benign | 0.058 | Likely Benign | Likely Benign | 0.140 | Likely Benign | -1.15 | Neutral | 0.989 | Probably Damaging | 0.834 | Possibly Damaging | 2.38 | Pathogenic | 0.03 | Affected | 0.2364 | 0.6108 | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||||||||||||
| c.2756A>G | Q919R 2D  AIThe SynGAP1 missense variant Q919R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic effect. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also likely benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence indicates that the variant is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.911223 | Binding | 0.299 | 0.841 | 0.250 | -3.636 | Likely Benign | 0.272 | Likely Benign | Likely Benign | 0.105 | Likely Benign | -0.96 | Neutral | 0.961 | Probably Damaging | 0.596 | Possibly Damaging | 2.65 | Benign | 0.85 | Tolerated | 0.1558 | 0.2305 | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||||||||||
| c.2762T>G | L921R 2D  AIThe SynGAP1 missense variant L921R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also predicts benign, and Foldetta results are unavailable. Overall, the majority of high‑confidence tools predict a benign impact, and there is no conflict with ClinVar status. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.608892 | Disordered | 0.943282 | Binding | 0.311 | 0.845 | 0.375 | -2.205 | Likely Benign | 0.563 | Ambiguous | Likely Benign | 0.217 | Likely Benign | -1.19 | Neutral | 0.994 | Probably Damaging | 0.912 | Probably Damaging | 2.41 | Pathogenic | 0.00 | Affected | 0.1306 | 0.0761 | -3 | -2 | -8.3 | 43.03 | ||||||||||||||||||||||||||||||||||||||||
| c.2764C>G | R922G 2D  AIThe SynGAP1 missense variant R922G is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree that the change is benign: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all predict a benign effect, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic effect. AlphaMissense‑Default remains uncertain, and no Foldetta stability assessment is available. High‑accuracy tools reinforce the benign prediction: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta data are missing. Overall, the preponderance of evidence points to a benign impact for R922G, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.549308 | Disordered | 0.955308 | Binding | 0.277 | 0.845 | 0.375 | -2.490 | Likely Benign | 0.472 | Ambiguous | Likely Benign | 0.104 | Likely Benign | -1.48 | Neutral | 0.967 | Probably Damaging | 0.626 | Possibly Damaging | 2.55 | Benign | 0.10 | Tolerated | 0.3473 | 0.3768 | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2765G>C | R922P 2D  AIThe SynGAP1 missense variant R922P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for R922P, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.549308 | Disordered | 0.955308 | Binding | 0.277 | 0.845 | 0.375 | -2.502 | Likely Benign | 0.578 | Likely Pathogenic | Likely Benign | 0.124 | Likely Benign | -1.36 | Neutral | 0.998 | Probably Damaging | 0.942 | Probably Damaging | 2.54 | Benign | 0.10 | Tolerated | 0.2391 | 0.4966 | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2765G>T | R922L 2D  AIThe SynGAP1 missense variant R922L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all classify the substitution as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports it as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic impact. The AlphaMissense‑Default score is uncertain, and no Foldetta stability assessment is available. High‑accuracy methods that are available—AlphaMissense‑Optimized and the SGM‑Consensus—both support a benign interpretation. Therefore, the variant is most likely benign according to the consensus of predictive tools, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.549308 | Disordered | 0.955308 | Binding | 0.277 | 0.845 | 0.375 | -3.714 | Likely Benign | 0.546 | Ambiguous | Likely Benign | 0.150 | Likely Benign | 0.84 | Neutral | 0.983 | Probably Damaging | 0.828 | Possibly Damaging | 3.04 | Benign | 1.00 | Tolerated | 0.2033 | 0.5022 | -3 | -2 | 8.3 | -43.03 | |||||||||||||||||||||||||||||||||||||||
| c.2767A>C | I923L 2D  AIThe SynGAP1 missense variant I923L is not reported in ClinVar and is absent from gnomAD. All evaluated in‑silico predictors—including REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods likewise support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” Foldetta results are unavailable. Consequently, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.562014 | Disordered | 0.964857 | Binding | 0.292 | 0.852 | 0.250 | -1.637 | Likely Benign | 0.157 | Likely Benign | Likely Benign | 0.069 | Likely Benign | -0.03 | Neutral | 0.166 | Benign | 0.101 | Benign | 2.82 | Benign | 0.56 | Tolerated | 0.1133 | 0.4528 | 2 | 2 | -0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.2767A>T | I923F 2D  AIThe SynGAP1 missense variant I923F is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.562014 | Disordered | 0.964857 | Binding | 0.292 | 0.852 | 0.250 | -2.967 | Likely Benign | 0.169 | Likely Benign | Likely Benign | 0.112 | Likely Benign | -0.85 | Neutral | 0.007 | Benign | 0.005 | Benign | 2.70 | Benign | 0.11 | Tolerated | 0.0726 | 0.3887 | 1 | 0 | -1.7 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2768T>A | I923N 2D  AIThe SynGAP1 missense variant I923N (ClinVar ID 647043.0) is listed as “Uncertain” and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign” because the majority of its constituent tools (three benign, one pathogenic) favor a benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as benign, and the Foldetta stability analysis is unavailable. Overall, the collective predictions point to a benign impact, which does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.562014 | Disordered | 0.964857 | Binding | 0.292 | 0.852 | 0.250 | Uncertain | 1 | -0.733 | Likely Benign | 0.712 | Likely Pathogenic | Likely Benign | 0.108 | Likely Benign | -1.16 | Neutral | 0.991 | Probably Damaging | 0.793 | Possibly Damaging | 2.70 | Benign | 0.13 | Tolerated | 3.77 | 5 | 0.1164 | 0.1073 | -2 | -3 | -8.0 | 0.94 | |||||||||||||||||||||||||||||||||||
| c.2768T>C | I923T 2D  AIThe SynGAP1 missense variant I923T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain” and the SGM‑Consensus as “Likely Benign”; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.562014 | Disordered | 0.964857 | Binding | 0.292 | 0.852 | 0.250 | -1.180 | Likely Benign | 0.842 | Likely Pathogenic | Ambiguous | 0.097 | Likely Benign | -0.53 | Neutral | 0.837 | Possibly Damaging | 0.348 | Benign | 2.73 | Benign | 0.41 | Tolerated | 0.1327 | 0.1786 | 0 | -1 | -5.2 | -12.05 | |||||||||||||||||||||||||||||||||||||||
| c.2768T>G | I923S 2D  AIThe SynGAP1 missense variant I923S is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for I923S. This conclusion is not contradicted by ClinVar status, which has no classification for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.562014 | Disordered | 0.964857 | Binding | 0.292 | 0.852 | 0.250 | -0.002 | Likely Benign | 0.760 | Likely Pathogenic | Likely Benign | 0.094 | Likely Benign | -0.61 | Neutral | 0.912 | Possibly Damaging | 0.529 | Possibly Damaging | 2.73 | Benign | 0.33 | Tolerated | 0.3300 | 0.1455 | -1 | -2 | -5.3 | -26.08 | |||||||||||||||||||||||||||||||||||||||
| c.2769C>G | I923M 2D  AIThe SynGAP1 missense variant I923M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic effect. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also likely benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence indicates that I923M is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.562014 | Disordered | 0.964857 | Binding | 0.292 | 0.852 | 0.250 | -2.711 | Likely Benign | 0.195 | Likely Benign | Likely Benign | 0.106 | Likely Benign | -0.14 | Neutral | 0.973 | Probably Damaging | 0.721 | Possibly Damaging | 2.70 | Benign | 0.16 | Tolerated | 0.0949 | 0.3874 | 2 | 1 | -2.6 | 18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2770C>A | P924T 2D  AIThe SynGAP1 missense variant P924T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence indicates that P924T is most likely pathogenic, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -6.360 | Likely Benign | 0.961 | Likely Pathogenic | Likely Pathogenic | 0.400 | Likely Benign | -6.03 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.67 | Pathogenic | 0.00 | Affected | 0.1190 | 0.4009 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2770C>G | P924A 2D  AIThe SynGAP1 missense variant P924A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all classify the variant as damaging. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments are mixed: AlphaMissense‑Optimized yields an Uncertain result, SGM‑Consensus indicates Likely Pathogenic, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the preponderance of evidence from multiple in silico predictors points to a pathogenic effect, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -5.995 | Likely Benign | 0.854 | Likely Pathogenic | Ambiguous | 0.383 | Likely Benign | -5.90 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 0.68 | Pathogenic | 0.00 | Affected | 0.2904 | 0.3423 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.2770C>T | P924S 2D  AIThe SynGAP1 missense variant P924S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessment shows AlphaMissense‑Optimized as uncertain, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—labels the variant as Likely Pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this residue. Overall, the preponderance of evidence points to a pathogenic effect for P924S, and this conclusion does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -4.686 | Likely Benign | 0.920 | Likely Pathogenic | Ambiguous | 0.388 | Likely Benign | -5.86 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.68 | Pathogenic | 0.00 | Affected | 0.2952 | 0.3772 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||||||
| c.2762T>A | L921Q 2D  AIThe SynGAP1 missense variant L921Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.943282 | Binding | 0.311 | 0.845 | 0.375 | -2.389 | Likely Benign | 0.311 | Likely Benign | Likely Benign | 0.132 | Likely Benign | -0.62 | Neutral | 0.994 | Probably Damaging | 0.940 | Probably Damaging | 2.41 | Pathogenic | 0.00 | Affected | 0.1097 | 0.1119 | -2 | -2 | -7.3 | 14.97 | |||||||||||||||||||||||||||||||||||||||
| c.2761C>G | L921V 2D  AIThe SynGAP1 missense variant L921V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this conclusion, so the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.943282 | Binding | 0.311 | 0.845 | 0.375 | -4.131 | Likely Benign | 0.221 | Likely Benign | Likely Benign | 0.045 | Likely Benign | -1.03 | Neutral | 0.835 | Possibly Damaging | 0.524 | Possibly Damaging | 2.45 | Pathogenic | 0.27 | Tolerated | 0.1483 | 0.3178 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2757G>C | Q919H 2D  AIThe SynGAP1 missense variant Q919H has no ClinVar record and is not listed in gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status, as none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.911223 | Binding | 0.299 | 0.841 | 0.250 | -3.867 | Likely Benign | 0.237 | Likely Benign | Likely Benign | 0.153 | Likely Benign | -1.83 | Neutral | 0.989 | Probably Damaging | 0.883 | Possibly Damaging | 2.39 | Pathogenic | 0.03 | Affected | 0.1463 | 0.4203 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2757G>T | Q919H 2D  AIThe SynGAP1 missense variant Q919H has no ClinVar record and is not listed in gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status, as none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.911223 | Binding | 0.299 | 0.841 | 0.250 | -3.867 | Likely Benign | 0.237 | Likely Benign | Likely Benign | 0.152 | Likely Benign | -1.83 | Neutral | 0.989 | Probably Damaging | 0.883 | Possibly Damaging | 2.39 | Pathogenic | 0.03 | Affected | 0.1463 | 0.4203 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2758C>A | Q920K 2D  AIThe SynGAP1 missense variant Q920K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for Q920K, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -4.234 | Likely Benign | 0.380 | Ambiguous | Likely Benign | 0.145 | Likely Benign | -1.72 | Neutral | 0.771 | Possibly Damaging | 0.412 | Benign | 2.67 | Benign | 0.00 | Affected | 0.2041 | 0.4331 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.2758C>G | Q920E 2D  AIThe SynGAP1 missense variant Q920E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to the variant being most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -3.863 | Likely Benign | 0.174 | Likely Benign | Likely Benign | 0.125 | Likely Benign | -1.23 | Neutral | 0.771 | Possibly Damaging | 0.492 | Possibly Damaging | 2.67 | Benign | 0.00 | Affected | 0.1443 | 0.2622 | 2 | 2 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.2759A>C | Q920P 2D  AIThe SynGAP1 missense variant Q920P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of ClinVar annotation. Thus, the variant is most likely benign, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -2.127 | Likely Benign | 0.065 | Likely Benign | Likely Benign | 0.235 | Likely Benign | -1.68 | Neutral | 0.989 | Probably Damaging | 0.834 | Possibly Damaging | 2.58 | Benign | 0.00 | Affected | 0.2438 | 0.5318 | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||||||||||||
| c.2759A>G | Q920R 2D  AIThe SynGAP1 missense variant Q920R is not reported in ClinVar and is absent from gnomAD. Consensus from multiple in‑silico predictors shows a split: benign calls come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic calls arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy tools further support this view: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus confirms Likely Benign, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -3.170 | Likely Benign | 0.352 | Ambiguous | Likely Benign | 0.168 | Likely Benign | -1.41 | Neutral | 0.961 | Probably Damaging | 0.596 | Possibly Damaging | 2.63 | Benign | 0.00 | Affected | 0.1626 | 0.2369 | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||||||||||
| c.2759A>T | Q920L 2D  AIThe SynGAP1 missense variant Q920L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -4.048 | Likely Benign | 0.280 | Likely Benign | Likely Benign | 0.181 | Likely Benign | -2.36 | Neutral | 0.891 | Possibly Damaging | 0.596 | Possibly Damaging | 2.60 | Benign | 0.00 | Affected | 0.0774 | 0.5995 | -2 | -2 | 7.3 | -14.97 | |||||||||||||||||||||||||||||||||||||||
| c.275G>A | G92E 2D  AIThe SynGAP1 missense variant G92E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -3.240 | Likely Benign | 0.651 | Likely Pathogenic | Likely Benign | 0.156 | Likely Benign | -2.27 | Neutral | 0.999 | Probably Damaging | 0.972 | Probably Damaging | 4.07 | Benign | 0.00 | Affected | 0.1521 | 0.4426 | 0 | -2 | -3.1 | 72.06 | |||||||||||||||||||||||||||||||||||||||
| c.275G>C | G92A 2D  AIThe SynGAP1 missense variant G92A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized returns “Benign” and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates “Likely Benign.” No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for G92A, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -4.044 | Likely Benign | 0.219 | Likely Benign | Likely Benign | 0.080 | Likely Benign | -1.53 | Neutral | 0.996 | Probably Damaging | 0.910 | Probably Damaging | 4.08 | Benign | 0.00 | Affected | 0.3922 | 0.4930 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.275G>T | G92V 2D  AIThe SynGAP1 missense variant G92V is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic predictions come from PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority vote) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of computational evidence points to a benign impact for G92V, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -4.627 | Likely Benign | 0.338 | Likely Benign | Likely Benign | 0.164 | Likely Benign | -2.62 | Deleterious | 0.999 | Probably Damaging | 0.972 | Probably Damaging | 4.00 | Benign | 0.00 | Affected | 0.1171 | 0.4363 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.2760A>C | Q920H 2D  AIThe SynGAP1 missense variant Q920H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -3.771 | Likely Benign | 0.254 | Likely Benign | Likely Benign | 0.079 | Likely Benign | -1.79 | Neutral | 0.989 | Probably Damaging | 0.883 | Possibly Damaging | 2.63 | Benign | 0.00 | Affected | 0.1419 | 0.4334 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2760A>T | Q920H 2D  AIThe SynGAP1 missense variant Q920H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -3.771 | Likely Benign | 0.254 | Likely Benign | Likely Benign | 0.079 | Likely Benign | -1.79 | Neutral | 0.989 | Probably Damaging | 0.883 | Possibly Damaging | 2.63 | Benign | 0.00 | Affected | 0.1419 | 0.4334 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.2761C>A | L921M 2D  AIThe SynGAP1 missense variant L921M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign impact for L921M, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.943282 | Binding | 0.311 | 0.845 | 0.375 | -4.485 | Likely Benign | 0.142 | Likely Benign | Likely Benign | 0.068 | Likely Benign | -0.38 | Neutral | 0.489 | Possibly Damaging | 0.137 | Benign | 2.40 | Pathogenic | 0.02 | Affected | 0.0762 | 0.3732 | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2771C>A | P924H 2D  AIThe SynGAP1 missense variant P924H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. The Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the preponderance of computational evidence indicates that P924H is most likely pathogenic, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -6.236 | Likely Benign | 0.957 | Likely Pathogenic | Likely Pathogenic | 0.457 | Likely Benign | -5.87 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 0.65 | Pathogenic | 0.00 | Affected | 0.1317 | 0.3281 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.2737A>T | T913S 2D  AIThe SynGAP1 missense variant T913S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta results are not available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.763517 | Binding | 0.339 | 0.899 | 0.375 | -2.636 | Likely Benign | 0.106 | Likely Benign | Likely Benign | 0.129 | Likely Benign | -0.74 | Neutral | 0.999 | Probably Damaging | 0.992 | Probably Damaging | 2.80 | Benign | 0.74 | Tolerated | 0.3541 | 0.5147 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2708G>T | G903V 2D  AIThe SynGAP1 missense variant G903V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also predicts benign, while Foldetta results are unavailable. Overall, the balance of evidence (five benign predictions versus three pathogenic predictions, with no conflicting ClinVar annotation) indicates that the variant is most likely benign. This conclusion does not contradict any ClinVar status, as the variant is not currently catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.680603 | Disordered | 0.549818 | Binding | 0.291 | 0.917 | 0.375 | -4.750 | Likely Benign | 0.491 | Ambiguous | Likely Benign | 0.168 | Likely Benign | -1.93 | Neutral | 0.997 | Probably Damaging | 0.959 | Probably Damaging | 2.44 | Pathogenic | 0.95 | Tolerated | 0.1269 | 0.4266 | -1 | -3 | 4.6 | 42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.2717T>G | L906R 2D  AIThe SynGAP1 missense variant L906R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of standard predictors (5 vs 4) lean toward pathogenicity, while the high‑accuracy AlphaMissense‑Optimized predicts benign and the consensus remains unresolved. Thus, the variant is most likely pathogenic based on the current predictions, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.604312 | Disordered | 0.644316 | Binding | 0.315 | 0.920 | 0.250 | -3.440 | Likely Benign | 0.769 | Likely Pathogenic | Likely Benign | 0.160 | Likely Benign | -0.26 | Neutral | 1.000 | Probably Damaging | 0.990 | Probably Damaging | 2.18 | Pathogenic | 0.04 | Affected | 0.1228 | 0.0947 | -3 | -2 | -8.3 | 43.03 | ||||||||||||||||||||||||||||||||||||||||
| c.2719A>C | S907R 2D  AIThe SynGAP1 missense variant S907R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM‑Consensus (a majority vote of the same four high‑accuracy tools) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this assessment does not contradict the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.661854 | Binding | 0.336 | 0.920 | 0.250 | -3.852 | Likely Benign | 0.963 | Likely Pathogenic | Likely Pathogenic | 0.082 | Likely Benign | -0.71 | Neutral | 0.998 | Probably Damaging | 0.951 | Probably Damaging | 2.73 | Benign | 0.34 | Tolerated | 0.0964 | 0.3441 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||||
| c.2719A>G | S907G 2D  AIThe SynGAP1 missense variant S907G is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.661854 | Binding | 0.336 | 0.920 | 0.250 | -3.018 | Likely Benign | 0.151 | Likely Benign | Likely Benign | 0.078 | Likely Benign | -1.23 | Neutral | 0.942 | Possibly Damaging | 0.788 | Possibly Damaging | 2.63 | Benign | 0.04 | Affected | 0.2625 | 0.5134 | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2719A>T | S907C 2D  AIThe SynGAP1 missense variant S907C is listed in ClinVar as Benign (ClinVar ID 1502069.0) and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence points to a benign effect, aligning with the ClinVar classification and indicating no contradiction with the reported status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.661854 | Binding | 0.336 | 0.920 | 0.250 | Likely Benign | 1 | -6.685 | Likely Benign | 0.298 | Likely Benign | Likely Benign | 0.113 | Likely Benign | -2.34 | Neutral | 0.999 | Probably Damaging | 0.988 | Probably Damaging | 2.60 | Benign | 0.02 | Affected | 3.77 | 5 | 0.1023 | 0.5951 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.271G>A | E91K 2D  AISynGAP1 E91K is not reported in ClinVar and is absent from gnomAD. Computational predictors fall into two groups: benign calls (REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus “Likely Benign”) and pathogenic calls (polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, AlphaMissense‑Optimized). High‑accuracy tools give conflicting results: AlphaMissense‑Optimized predicts pathogenic, whereas the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) predicts benign; Foldetta data are unavailable. Consequently, the evidence is split, but the consensus of the most reliable predictors leans toward a benign effect. Thus, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -4.964 | Likely Benign | 0.962 | Likely Pathogenic | Likely Pathogenic | 0.146 | Likely Benign | -1.07 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 3.92 | Benign | 0.00 | Affected | 0.2693 | 0.7444 | 0 | 1 | -0.4 | -0.94 | |||||||||||||||||||||||||||||||||||||||
| c.271G>C | E91Q 2D  AIThe SynGAP1 missense variant E91Q has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic impact are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. AlphaMissense‑Optimized is uncertain, and no Foldetta stability assessment is available. Overall, the predictions are mixed, with an equal number of benign and pathogenic calls, but the consensus‑based SGM‑Consensus and the majority of individual benign predictions lean toward a benign interpretation. Thus, the variant is most likely benign based on current computational evidence, and this assessment does not contradict ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -4.053 | Likely Benign | 0.805 | Likely Pathogenic | Ambiguous | 0.103 | Likely Benign | -0.89 | Neutral | 0.947 | Possibly Damaging | 0.727 | Possibly Damaging | 3.89 | Benign | 0.00 | Affected | 0.1487 | 0.7442 | 2 | 2 | 0.0 | -0.98 | |||||||||||||||||||||||||||||||||||||||
| c.2720G>A | S907N 2D  AIThe SynGAP1 missense variant S907N is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign, while the majority‑vote SGM‑Consensus also reports a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic impact. The AlphaMissense‑Default score is uncertain, and no Foldetta stability assessment is available. High‑accuracy evaluations further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is likely benign, with no Foldetta data to contradict this. Overall, the preponderance of evidence points to a benign effect, and this assessment does not conflict with any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.661854 | Binding | 0.336 | 0.920 | 0.250 | -4.520 | Likely Benign | 0.430 | Ambiguous | Likely Benign | 0.140 | Likely Benign | -0.26 | Neutral | 0.951 | Possibly Damaging | 0.803 | Possibly Damaging | 2.66 | Benign | 0.08 | Tolerated | 0.1312 | 0.4585 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||||||
| c.2720G>C | S907T 2D  AIThe SynGAP1 missense variant S907T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The only tools that predict a pathogenic outcome are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.661854 | Binding | 0.336 | 0.920 | 0.250 | -4.794 | Likely Benign | 0.220 | Likely Benign | Likely Benign | 0.178 | Likely Benign | -0.53 | Neutral | 0.992 | Probably Damaging | 0.846 | Possibly Damaging | 2.66 | Benign | 0.93 | Tolerated | 0.1324 | 0.6352 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2720G>T | S907I 2D  AIThe SynGAP1 missense variant S907I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus (majority vote) as Likely Benign, and Foldetta results are unavailable. Based on the overall balance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.661854 | Binding | 0.336 | 0.920 | 0.250 | -6.082 | Likely Benign | 0.795 | Likely Pathogenic | Ambiguous | 0.229 | Likely Benign | -2.20 | Neutral | 0.998 | Probably Damaging | 0.967 | Probably Damaging | 2.62 | Benign | 0.03 | Affected | 0.1006 | 0.5853 | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||||||
| c.2721C>A | S907R 2D  AIThe SynGAP1 missense variant S907R has no ClinVar record and is not listed in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while polyPhen‑2 (HumDiv and HumVar) and AlphaMissense‑Default predict a pathogenic outcome. AlphaMissense‑Optimized also classifies the variant as pathogenic, whereas the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as likely benign. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic and the SGM‑Consensus as benign; Foldetta results are unavailable. Overall, the majority of tools (five benign vs. four pathogenic) lean toward a benign interpretation, and there is no ClinVar evidence contradicting this assessment. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.661854 | Binding | 0.336 | 0.920 | 0.250 | -3.852 | Likely Benign | 0.963 | Likely Pathogenic | Likely Pathogenic | 0.089 | Likely Benign | -0.71 | Neutral | 0.998 | Probably Damaging | 0.951 | Probably Damaging | 2.73 | Benign | 0.34 | Tolerated | 0.0964 | 0.3441 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||||
| c.2721C>G | S907R 2D  AIThe SynGAP1 missense variant S907R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, whereas the SGM‑Consensus remains benign; Foldetta results are unavailable. Overall, the predictions are mixed but lean toward a benign interpretation, with no conflict with the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.661854 | Binding | 0.336 | 0.920 | 0.250 | -3.852 | Likely Benign | 0.963 | Likely Pathogenic | Likely Pathogenic | 0.089 | Likely Benign | -0.71 | Neutral | 0.998 | Probably Damaging | 0.951 | Probably Damaging | 2.73 | Benign | 0.34 | Tolerated | 0.0964 | 0.3441 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||||
| c.2722C>A | Q908K 2D  AIThe SynGAP1 missense variant Q908K is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign interpretation: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized all predict benign, while the majority of other predictors (polyPhen‑2 HumDiv and HumVar) indicate pathogenic. When predictions are grouped by agreement, the benign‑predicating tools outnumber the pathogenic ones, and the single uncertain call from AlphaMissense‑Default does not alter the overall trend. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized classifies the variant as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports it as Likely Benign. No Foldetta stability analysis is available. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.678728 | Binding | 0.275 | 0.917 | 0.250 | -5.641 | Likely Benign | 0.549 | Ambiguous | Likely Benign | 0.139 | Likely Benign | -1.15 | Neutral | 0.963 | Probably Damaging | 0.973 | Probably Damaging | 2.67 | Benign | 0.22 | Tolerated | 0.1738 | 0.3711 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.2722C>G | Q908E 2D  AIThe SynGAP1 missense variant Q908E is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign status: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect for Q908E, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.678728 | Binding | 0.275 | 0.917 | 0.250 | -4.178 | Likely Benign | 0.271 | Likely Benign | Likely Benign | 0.139 | Likely Benign | -1.14 | Neutral | 0.963 | Probably Damaging | 0.973 | Probably Damaging | 2.58 | Benign | 1.00 | Tolerated | 0.1255 | 0.1886 | 2 | 2 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.2717T>C | L906P 2D  AIThe SynGAP1 missense variant L906P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also favors a benign outcome. Foldetta, which would provide a protein‑folding stability estimate, is unavailable for this variant. Overall, the preponderance of evidence points to a benign impact. This conclusion is not contradicted by ClinVar, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.604312 | Disordered | 0.644316 | Binding | 0.315 | 0.920 | 0.250 | -3.662 | Likely Benign | 0.478 | Ambiguous | Likely Benign | 0.150 | Likely Benign | 0.08 | Neutral | 1.000 | Probably Damaging | 0.993 | Probably Damaging | 2.18 | Pathogenic | 0.10 | Tolerated | 0.3430 | 0.1458 | -3 | -3 | -5.4 | -16.04 | ||||||||||||||||||||||||||||||||||||||||
| c.2717T>A | L906H 2D  AIThe SynGAP1 missense variant L906H is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (two benign vs. two pathogenic votes) and Foldetta results are unavailable. Overall, the majority of standard predictors lean toward pathogenicity, but the most reliable high‑accuracy tool suggests a benign outcome and the consensus is unresolved. Thus, the variant is most likely pathogenic based on the prevailing predictions, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.604312 | Disordered | 0.644316 | Binding | 0.315 | 0.920 | 0.250 | -4.367 | Likely Benign | 0.732 | Likely Pathogenic | Likely Benign | 0.124 | Likely Benign | -1.01 | Neutral | 1.000 | Probably Damaging | 0.997 | Probably Damaging | 2.18 | Pathogenic | 0.02 | Affected | 0.1125 | 0.1073 | -2 | -3 | -7.0 | 23.98 | ||||||||||||||||||||||||||||||||||||||||
| c.2710A>C | M904L 2D  AIThe SynGAP1 missense variant M904L is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.589073 | Binding | 0.350 | 0.920 | 0.250 | -1.839 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.050 | Likely Benign | -0.24 | Neutral | 0.000 | Benign | 0.001 | Benign | 2.86 | Benign | 0.17 | Tolerated | 0.1915 | 0.4584 | 4 | 2 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2710A>T | M904L 2D  AIThe SynGAP1 missense variant M904L is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.589073 | Binding | 0.350 | 0.920 | 0.250 | -1.839 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.050 | Likely Benign | -0.24 | Neutral | 0.000 | Benign | 0.001 | Benign | 2.86 | Benign | 0.17 | Tolerated | 0.1915 | 0.4584 | 4 | 2 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2711T>A | M904K 2D  AIThe SynGAP1 missense variant M904K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Only AlphaMissense‑Default predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.589073 | Binding | 0.350 | 0.920 | 0.250 | -2.333 | Likely Benign | 0.744 | Likely Pathogenic | Likely Benign | 0.033 | Likely Benign | -1.08 | Neutral | 0.277 | Benign | 0.137 | Benign | 2.80 | Benign | 1.00 | Tolerated | 0.1696 | 0.0871 | 0 | -1 | -5.8 | -3.02 | |||||||||||||||||||||||||||||||||||||||
| c.2711T>C | M904T 2D  AIThe SynGAP1 missense variant M904T is listed in ClinVar (ID 1311496.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only AlphaMissense‑Default predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; a Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.589073 | Binding | 0.350 | 0.920 | 0.250 | Uncertain | 1 | -2.721 | Likely Benign | 0.668 | Likely Pathogenic | Likely Benign | 0.042 | Likely Benign | -1.15 | Neutral | 0.277 | Benign | 0.103 | Benign | 2.78 | Benign | 0.18 | Tolerated | 3.77 | 5 | 0.2394 | 0.2568 | -1 | -1 | -2.6 | -30.09 | |||||||||||||||||||||||||||||||||||
| c.2711T>G | M904R 2D  AIThe SynGAP1 missense variant M904R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for M904R, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.589073 | Binding | 0.350 | 0.920 | 0.250 | -1.238 | Likely Benign | 0.693 | Likely Pathogenic | Likely Benign | 0.078 | Likely Benign | -0.81 | Neutral | 0.468 | Possibly Damaging | 0.206 | Benign | 2.76 | Benign | 0.82 | Tolerated | 0.1742 | 0.1318 | 0 | -1 | -6.4 | 24.99 | |||||||||||||||||||||||||||||||||||||||
| c.2712G>A | M904I 2D  AIThe SynGAP1 missense variant M904I is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools uniformly classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM all return benign scores, and AlphaMissense‑Optimized also predicts a benign effect. No tool predicts pathogenicity, and the only uncertain result comes from AlphaMissense‑Default, which is inconclusive. The high‑accuracy consensus methods corroborate this benign assessment: the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign,” and AlphaMissense‑Optimized explicitly labels the variant as benign. Foldetta, a protein‑folding stability predictor, has no available result for this residue. Taken together, the evidence overwhelmingly supports a benign impact for M904I, and this conclusion is consistent with the absence of a ClinVar pathogenic annotation. Thus, the variant is most likely benign, and this conclusion does not contradict the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.589073 | Binding | 0.350 | 0.920 | 0.250 | -3.845 | Likely Benign | 0.385 | Ambiguous | Likely Benign | 0.023 | Likely Benign | -0.48 | Neutral | 0.039 | Benign | 0.023 | Benign | 2.78 | Benign | 0.07 | Tolerated | 0.1694 | 0.3633 | 2 | 1 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2712G>C | M904I 2D  AIThe SynGAP1 missense variant M904I is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools uniformly classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM all return benign scores, and AlphaMissense‑Optimized also predicts a benign effect. No tool predicts pathogenicity, and the only uncertain result comes from AlphaMissense‑Default, which is inconclusive. The high‑accuracy consensus methods corroborate this benign assessment: the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign,” and AlphaMissense‑Optimized also indicates a benign outcome. Foldetta, a protein‑folding stability predictor, has no available result for this variant. Taken together, the evidence overwhelmingly supports a benign impact, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.589073 | Binding | 0.350 | 0.920 | 0.250 | -3.845 | Likely Benign | 0.385 | Ambiguous | Likely Benign | 0.023 | Likely Benign | -0.48 | Neutral | 0.039 | Benign | 0.023 | Benign | 2.78 | Benign | 0.07 | Tolerated | 0.1694 | 0.3633 | 2 | 1 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2712G>T | M904I 2D  AIThe SynGAP1 missense variant M904I is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools uniformly classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM all return benign scores, and AlphaMissense‑Optimized also predicts a benign effect. No tool predicts pathogenicity, and the only uncertain result comes from AlphaMissense‑Default, which is inconclusive. The high‑accuracy consensus methods corroborate this benign assessment: the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign,” and AlphaMissense‑Optimized also indicates a benign outcome. Foldetta, a protein‑folding stability predictor, has no available result for this variant. Taken together, the evidence overwhelmingly supports a benign impact, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.589073 | Binding | 0.350 | 0.920 | 0.250 | -3.845 | Likely Benign | 0.385 | Ambiguous | Likely Benign | 0.023 | Likely Benign | -0.48 | Neutral | 0.039 | Benign | 0.023 | Benign | 2.78 | Benign | 0.07 | Tolerated | 0.1694 | 0.3633 | 2 | 1 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2713C>G | R905G 2D  AIThe SynGAP1 missense variant R905G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this conclusion, so the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.618085 | Binding | 0.291 | 0.920 | 0.250 | -2.612 | Likely Benign | 0.707 | Likely Pathogenic | Likely Benign | 0.135 | Likely Benign | -2.47 | Neutral | 0.999 | Probably Damaging | 0.948 | Probably Damaging | 2.61 | Benign | 0.06 | Tolerated | 0.3346 | 0.3776 | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2714G>C | R905P 2D  AIThe SynGAP1 missense variant R905P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.618085 | Binding | 0.291 | 0.920 | 0.250 | -3.713 | Likely Benign | 0.701 | Likely Pathogenic | Likely Benign | 0.265 | Likely Benign | -0.86 | Neutral | 1.000 | Probably Damaging | 0.977 | Probably Damaging | 2.64 | Benign | 0.07 | Tolerated | 0.2032 | 0.4616 | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||||||||
| c.2716C>A | L906I 2D  AIThe SynGAP1 missense variant L906I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; no Foldetta stability result is available. Overall, the majority of evidence points to a benign impact for this variant. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.604312 | Disordered | 0.644316 | Binding | 0.315 | 0.920 | 0.250 | -5.882 | Likely Benign | 0.174 | Likely Benign | Likely Benign | 0.036 | Likely Benign | -0.50 | Neutral | 0.905 | Possibly Damaging | 0.545 | Possibly Damaging | 2.45 | Pathogenic | 0.32 | Tolerated | 0.1060 | 0.4093 | 2 | 2 | 0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.2716C>G | L906V 2D  AIThe SynGAP1 missense variant L906V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as tolerated or benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and FATHMM predict a damaging or pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a Likely Benign consensus. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not conflict with ClinVar, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.604312 | Disordered | 0.644316 | Binding | 0.315 | 0.920 | 0.250 | -5.105 | Likely Benign | 0.206 | Likely Benign | Likely Benign | 0.105 | Likely Benign | -0.64 | Neutral | 0.981 | Probably Damaging | 0.832 | Possibly Damaging | 2.46 | Pathogenic | 0.21 | Tolerated | 0.1632 | 0.3744 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2716C>T | L906F 2D  AIThe SynGAP1 missense variant L906F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for the variant. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.604312 | Disordered | 0.644316 | Binding | 0.315 | 0.920 | 0.250 | -4.700 | Likely Benign | 0.304 | Likely Benign | Likely Benign | 0.113 | Likely Benign | -1.79 | Neutral | 0.999 | Probably Damaging | 0.979 | Probably Damaging | 2.22 | Pathogenic | 0.18 | Tolerated | 0.0753 | 0.3342 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.2723A>C | Q908P 2D  AIThe SynGAP1 missense variant Q908P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools, polyPhen‑2 HumDiv and HumVar, predict a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for Q908P, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.678728 | Binding | 0.275 | 0.917 | 0.250 | -4.446 | Likely Benign | 0.151 | Likely Benign | Likely Benign | 0.196 | Likely Benign | -0.59 | Neutral | 0.996 | Probably Damaging | 0.992 | Probably Damaging | 2.51 | Benign | 0.11 | Tolerated | 0.2379 | 0.5121 | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||||||||||||
| c.2723A>G | Q908R 2D  AIThe SynGAP1 missense variant Q908R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (both HumDiv and HumVar) predict a pathogenic outcome. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status because none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.678728 | Binding | 0.275 | 0.917 | 0.250 | -4.284 | Likely Benign | 0.485 | Ambiguous | Likely Benign | 0.170 | Likely Benign | -1.02 | Neutral | 0.985 | Probably Damaging | 0.982 | Probably Damaging | 2.75 | Benign | 0.10 | Tolerated | 0.1391 | 0.1743 | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||||||||||
| c.2723A>T | Q908L 2D  AIThe SynGAP1 missense variant Q908L is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (HumDiv and HumVar) and SIFT uniformly predict a pathogenic impact. AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus is Likely Benign, and Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.678728 | Binding | 0.275 | 0.917 | 0.250 | -3.589 | Likely Benign | 0.420 | Ambiguous | Likely Benign | 0.193 | Likely Benign | -1.40 | Neutral | 0.985 | Probably Damaging | 0.982 | Probably Damaging | 2.53 | Benign | 0.05 | Affected | 0.0706 | 0.5655 | -2 | -2 | 7.3 | -14.97 | |||||||||||||||||||||||||||||||||||||||
| c.272A>T | E91V 2D  AIThe SynGAP1 E91V missense variant has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic impact are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” AlphaMissense‑Optimized yields an uncertain result, and no Foldetta stability assessment is available. Overall, the evidence is mixed, but the consensus of several independent benign predictors and the SGM‑Consensus lean toward a benign interpretation. Thus, the variant is most likely benign based on current predictions, and this assessment does not contradict any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -3.697 | Likely Benign | 0.934 | Likely Pathogenic | Ambiguous | 0.124 | Likely Benign | -2.16 | Neutral | 0.947 | Possibly Damaging | 0.788 | Possibly Damaging | 3.84 | Benign | 0.00 | Affected | 0.0940 | 0.7457 | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||||||||||||
| c.2731G>A | V911I 2D  AIThe SynGAP1 missense variant V911I is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign,” while Foldetta results are unavailable. Consequently, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.724137 | Binding | 0.327 | 0.914 | 0.375 | -3.983 | Likely Benign | 0.085 | Likely Benign | Likely Benign | 0.024 | Likely Benign | -0.32 | Neutral | 0.451 | Benign | 0.209 | Benign | 2.70 | Benign | 0.22 | Tolerated | 0.0881 | 0.4331 | 4 | 3 | 0.3 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2731G>C | V911L 2D  AIThe SynGAP1 missense variant V911L is not reported in ClinVar and is absent from gnomAD. Prediction tools that assess pathogenicity all converge on a benign outcome: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. No tool in the dataset indicates a pathogenic effect. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized reports a benign effect, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is classified as Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the computational evidence strongly supports a benign classification, and this is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.724137 | Binding | 0.327 | 0.914 | 0.375 | -2.722 | Likely Benign | 0.166 | Likely Benign | Likely Benign | 0.100 | Likely Benign | -0.39 | Neutral | 0.451 | Benign | 0.157 | Benign | 2.73 | Benign | 0.16 | Tolerated | 0.1067 | 0.5310 | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2731G>T | V911F 2D  AIThe SynGAP1 missense variant V911F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for V911F, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.724137 | Binding | 0.327 | 0.914 | 0.375 | -4.454 | Likely Benign | 0.169 | Likely Benign | Likely Benign | 0.079 | Likely Benign | -1.08 | Neutral | 0.977 | Probably Damaging | 0.721 | Possibly Damaging | 2.68 | Benign | 0.04 | Affected | 0.0749 | 0.4380 | -1 | -1 | -1.4 | 48.04 | |||||||||||||||||||||||||||||||||||||||
| c.2732T>A | V911D 2D  AIThe SynGAP1 missense variant V911D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of high‑confidence predictors indicate a benign impact, and this is consistent with the lack of ClinVar evidence. Therefore, the variant is most likely benign and does not contradict any existing ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.724137 | Binding | 0.327 | 0.914 | 0.375 | -4.422 | Likely Benign | 0.624 | Likely Pathogenic | Likely Benign | 0.114 | Likely Benign | -0.41 | Neutral | 0.500 | Possibly Damaging | 0.157 | Benign | 2.71 | Benign | 0.05 | Affected | 0.1416 | 0.1289 | -2 | -3 | -7.7 | 15.96 | |||||||||||||||||||||||||||||||||||||||
| c.2732T>C | V911A 2D  AIThe SynGAP1 missense variant V911A is not reported in ClinVar and is absent from gnomAD. All evaluated in silico predictors classify the change as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign effect. Foldetta results are unavailable. Based on the consensus of all predictions, the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.724137 | Binding | 0.327 | 0.914 | 0.375 | -3.030 | Likely Benign | 0.249 | Likely Benign | Likely Benign | 0.118 | Likely Benign | 0.08 | Neutral | 0.264 | Benign | 0.102 | Benign | 2.77 | Benign | 0.41 | Tolerated | 0.3069 | 0.3336 | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||||||||||
| c.2732T>G | V911G 2D  AIThe SynGAP1 missense variant V911G is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). All available in silico predictors classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity, so the benign group includes every listed predictor, while the pathogenic group is empty. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign; the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also yields a benign verdict. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, is not available for this variant. Overall, the computational evidence overwhelmingly supports a benign effect, and this conclusion does not contradict any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.685117 | Disordered | 0.724137 | Binding | 0.327 | 0.914 | 0.375 | -2.754 | Likely Benign | 0.222 | Likely Benign | Likely Benign | 0.124 | Likely Benign | -0.04 | Neutral | 0.004 | Benign | 0.003 | Benign | 2.74 | Benign | 0.16 | Tolerated | 0.2307 | 0.3360 | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||||||
| c.2734A>C | T912P 2D  AIThe SynGAP1 missense variant T912P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | -2.955 | Likely Benign | 0.111 | Likely Benign | Likely Benign | 0.087 | Likely Benign | -0.78 | Neutral | 0.217 | Benign | 0.121 | Benign | 4.00 | Benign | 0.00 | Affected | 0.1832 | 0.4608 | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||||||
| c.2734A>G | T912A 2D  AIThe SynGAP1 missense variant T912A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | -3.084 | Likely Benign | 0.097 | Likely Benign | Likely Benign | 0.088 | Likely Benign | -1.50 | Neutral | 0.973 | Probably Damaging | 0.856 | Possibly Damaging | 4.03 | Benign | 0.00 | Affected | 0.3754 | 0.3841 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2734A>T | T912S 2D  AIThe SynGAP1 missense variant T912S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for T912S, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | -2.647 | Likely Benign | 0.127 | Likely Benign | Likely Benign | 0.074 | Likely Benign | -1.05 | Neutral | 0.994 | Probably Damaging | 0.856 | Possibly Damaging | 4.18 | Benign | 0.00 | Affected | 0.3078 | 0.3893 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2735C>A | T912N 2D  AIThe SynGAP1 missense variant T912N is listed in ClinVar with an uncertain significance (ClinVar ID 2337624.0) and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as likely benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates likely benign. No Foldetta (FoldX‑MD/Rosetta) stability result is available, so it does not influence the overall assessment. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict the current ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | Uncertain | 1 | -4.260 | Likely Benign | 0.190 | Likely Benign | Likely Benign | 0.116 | Likely Benign | -1.15 | Neutral | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 3.96 | Benign | 0.00 | Affected | 3.77 | 5 | 0.1095 | 0.3750 | 0 | 0 | -2.8 | 13.00 | |||||||||||||||||||||||||||||||||||
| c.2735C>G | T912S 2D  AIThe SynGAP1 missense variant T912S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for T912S, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | -2.647 | Likely Benign | 0.127 | Likely Benign | Likely Benign | 0.128 | Likely Benign | -1.05 | Neutral | 0.994 | Probably Damaging | 0.856 | Possibly Damaging | 4.18 | Benign | 0.00 | Affected | 0.3078 | 0.3893 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.2737A>C | T913P 2D  AIThe SynGAP1 missense variant T913P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic effect. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for T913P, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.763517 | Binding | 0.339 | 0.899 | 0.375 | -2.029 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.176 | Likely Benign | -1.43 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.65 | Benign | 0.26 | Tolerated | 0.2082 | 0.5786 | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||||||
| c.272A>G | E91G 2D  AIThe SynGAP1 missense variant E91G is listed in ClinVar (ID 436922.0) as benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicating a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar designation and not contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | Likely Benign | 1 | -3.226 | Likely Benign | 0.783 | Likely Pathogenic | Likely Benign | 0.110 | Likely Benign | -2.18 | Neutral | 0.947 | Possibly Damaging | 0.727 | Possibly Damaging | 3.86 | Benign | 0.00 | Affected | 4.32 | 1 | 0.3418 | 0.6030 | 0 | -2 | 3.1 | -72.06 | |||||||||||||||||||||||||||||||||||
| c.272A>C | E91A 2D  AIThe SynGAP1 missense variant E91A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. AlphaMissense‑Optimized is uncertain, and no Foldetta stability result is available. Overall, the majority of consensus‑based and individual predictors lean toward a benign classification, with several high‑confidence tools indicating pathogenicity, leaving the assessment inconclusive. Based on the available predictions, the variant is most likely benign, and this does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -3.302 | Likely Benign | 0.815 | Likely Pathogenic | Ambiguous | 0.094 | Likely Benign | -1.58 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 3.89 | Benign | 0.00 | Affected | 0.4511 | 0.7077 | 0 | -1 | 5.3 | -58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2724G>T | Q908H 2D  AIThe SynGAP1 missense variant Q908H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) all classify the change as benign or likely benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized reports benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely benign. Foldetta results are not available. Overall, the majority of evidence points to a benign effect for Q908H, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.678728 | Binding | 0.275 | 0.917 | 0.250 | -4.658 | Likely Benign | 0.311 | Likely Benign | Likely Benign | 0.110 | Likely Benign | -0.74 | Neutral | 0.996 | Probably Damaging | 0.995 | Probably Damaging | 2.58 | Benign | 0.05 | Affected | 3.77 | 5 | 0.1293 | 0.3597 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||
| c.2725A>C | M909L 2D  AIThe SynGAP1 missense variant M909L is not reported in ClinVar and is absent from gnomAD. In silico prediction tools that assess pathogenicity uniformly predict a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate benign. No tool in the dataset predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are not available. Overall, the consensus of all available predictions points to a benign effect, and this is consistent with the lack of a ClinVar classification—there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.696196 | Binding | 0.314 | 0.914 | 0.250 | -1.417 | Likely Benign | 0.152 | Likely Benign | Likely Benign | 0.184 | Likely Benign | -0.85 | Neutral | 0.002 | Benign | 0.006 | Benign | 2.82 | Benign | 0.32 | Tolerated | 3.77 | 5 | 0.1591 | 0.4352 | 2 | 4 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||||
| c.2725A>G | M909V 2D  AIThe SynGAP1 missense variant M909V is not reported in ClinVar and is absent from gnomAD, indicating no documented clinical or population evidence. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification. Foldetta results are not available, so they do not influence the assessment. Overall, the variant is most likely benign, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.696196 | Binding | 0.314 | 0.914 | 0.250 | -2.906 | Likely Benign | 0.230 | Likely Benign | Likely Benign | 0.164 | Likely Benign | -0.88 | Neutral | 0.288 | Benign | 0.147 | Benign | 2.97 | Benign | 0.45 | Tolerated | 0.3295 | 0.3600 | 2 | 1 | 2.3 | -32.06 | |||||||||||||||||||||||||||||||||||||||
| c.2726T>A | M909K 2D  AIThe SynGAP1 missense variant M909K is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster into two groups: benign (SGM‑Consensus, REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized) and pathogenic (polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default). High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates likely benign. Foldetta, a protein‑folding stability method, was not available for this variant. Overall, the preponderance of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.696196 | Binding | 0.314 | 0.914 | 0.250 | -5.024 | Likely Benign | 0.748 | Likely Pathogenic | Likely Benign | 0.126 | Likely Benign | -1.17 | Neutral | 0.965 | Probably Damaging | 0.629 | Possibly Damaging | 2.71 | Benign | 0.18 | Tolerated | 0.1585 | 0.0628 | 0 | -1 | -5.8 | -3.02 | |||||||||||||||||||||||||||||||||||||||
| c.2726T>C | M909T 2D  AIThe SynGAP1 missense variant M909T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates likely benign. Foldetta results are unavailable, so they do not influence the assessment. Overall, the majority of evidence points to a benign impact for M909T, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.696196 | Binding | 0.314 | 0.914 | 0.250 | -3.053 | Likely Benign | 0.647 | Likely Pathogenic | Likely Benign | 0.109 | Likely Benign | -0.74 | Neutral | 0.901 | Possibly Damaging | 0.430 | Benign | 2.86 | Benign | 1.00 | Tolerated | 0.2157 | 0.2085 | -1 | -1 | -2.6 | -30.09 | |||||||||||||||||||||||||||||||||||||||
| c.2726T>G | M909R 2D  AIThe SynGAP1 missense variant M909R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this conclusion, so the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.696196 | Binding | 0.314 | 0.914 | 0.250 | -3.064 | Likely Benign | 0.699 | Likely Pathogenic | Likely Benign | 0.127 | Likely Benign | -1.23 | Neutral | 0.965 | Probably Damaging | 0.703 | Possibly Damaging | 2.70 | Benign | 0.12 | Tolerated | 0.1745 | 0.0809 | 0 | -1 | -6.4 | 24.99 | |||||||||||||||||||||||||||||||||||||||
| c.2727G>A | M909I 2D  AIThe SynGAP1 missense variant M909I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.696196 | Binding | 0.314 | 0.914 | 0.250 | -3.636 | Likely Benign | 0.703 | Likely Pathogenic | Likely Benign | 0.097 | Likely Benign | -1.15 | Neutral | 0.481 | Possibly Damaging | 0.220 | Benign | 2.82 | Benign | 0.25 | Tolerated | 0.1392 | 0.3298 | 2 | 1 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2727G>C | M909I 2D  AIThe SynGAP1 missense variant M909I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.696196 | Binding | 0.314 | 0.914 | 0.250 | -3.636 | Likely Benign | 0.703 | Likely Pathogenic | Likely Benign | 0.097 | Likely Benign | -1.15 | Neutral | 0.481 | Possibly Damaging | 0.220 | Benign | 2.82 | Benign | 0.25 | Tolerated | 0.1392 | 0.3298 | 2 | 1 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2727G>T | M909I 2D  AIThe SynGAP1 missense variant M909I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.642678 | Disordered | 0.696196 | Binding | 0.314 | 0.914 | 0.250 | -3.636 | Likely Benign | 0.703 | Likely Pathogenic | Likely Benign | 0.097 | Likely Benign | -1.15 | Neutral | 0.481 | Possibly Damaging | 0.220 | Benign | 2.82 | Benign | 0.25 | Tolerated | 0.1392 | 0.3298 | 2 | 1 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||||||||
| c.2728G>A | G910S 2D  AIThe SynGAP1 missense variant G910S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. The variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.707319 | Binding | 0.264 | 0.917 | 0.250 | -3.195 | Likely Benign | 0.331 | Likely Benign | Likely Benign | 0.234 | Likely Benign | -1.54 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.79 | Benign | 0.06 | Tolerated | 0.2671 | 0.4492 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2728G>C | G910R 2D  AIThe SynGAP1 missense variant G910R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, with no conflict with ClinVar status because no ClinVar assertion exists. Thus, the variant is most likely benign based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.707319 | Binding | 0.264 | 0.917 | 0.250 | -4.566 | Likely Benign | 0.945 | Likely Pathogenic | Ambiguous | 0.235 | Likely Benign | -1.78 | Neutral | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.86 | Benign | 0.02 | Affected | 0.0875 | 0.4198 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.2728G>T | G910C 2D  AIThe SynGAP1 missense variant G910C is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 benign vs 2 pathogenic) and Foldetta data are unavailable. Overall, the majority of tools (five pathogenic vs four benign) lean toward a pathogenic interpretation, and the high‑accuracy benign prediction from AlphaMissense‑Optimized does not override this trend. Thus, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.762850 | Disordered | 0.707319 | Binding | 0.264 | 0.917 | 0.250 | -6.359 | Likely Benign | 0.723 | Likely Pathogenic | Likely Benign | 0.252 | Likely Benign | -3.11 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.69 | Benign | 0.01 | Affected | 0.1179 | 0.4194 | -3 | -3 | 2.9 | 46.09 | ||||||||||||||||||||||||||||||||||||||||
| c.2729G>A | G910D 2D  AIThe SynGAP1 missense variant G910D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain” and the SGM‑Consensus as “Likely Benign”; Foldetta results are unavailable. Overall, the majority of evidence points toward a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign based on current predictive data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.762850 | Disordered | 0.707319 | Binding | 0.264 | 0.917 | 0.250 | -4.190 | Likely Benign | 0.856 | Likely Pathogenic | Ambiguous | 0.214 | Likely Benign | -1.87 | Neutral | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.84 | Benign | 0.05 | Affected | 0.1642 | 0.1477 | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||||||
| c.2737A>G | T913A 2D  AIThe SynGAP1 missense variant T913A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus likewise indicates Likely Benign. Foldetta results are unavailable, so they do not influence the overall assessment. Based on the collective predictions, the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.763517 | Binding | 0.339 | 0.899 | 0.375 | -2.386 | Likely Benign | 0.083 | Likely Benign | Likely Benign | 0.098 | Likely Benign | -1.41 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.79 | Benign | 0.15 | Tolerated | 0.4240 | 0.4905 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.2890C>A | H964N 2D  AIThe SynGAP1 missense variant H964N is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only ESM1b predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.990547 | Disordered | 0.982486 | Binding | 0.364 | 0.886 | 0.750 | -8.073 | Likely Pathogenic | 0.084 | Likely Benign | Likely Benign | 0.098 | Likely Benign | -0.30 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.18 | Benign | 0.64 | Tolerated | 0.1988 | 0.3495 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.303C>G | H101Q 2D  AIThe SynGAP1 missense variant H101Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for H101Q, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -2.827 | Likely Benign | 0.124 | Likely Benign | Likely Benign | 0.149 | Likely Benign | -0.37 | Neutral | 0.824 | Possibly Damaging | 0.880 | Possibly Damaging | 4.24 | Benign | 0.00 | Affected | 4.32 | 1 | 0.1487 | 0.3689 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||
| c.3047A>T | D1016V 2D  AIThe SynGAP1 D1016V missense variant is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools show a split: benign calls come from REVEL and ESM1b, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments give an uncertain result from AlphaMissense‑Optimized, a Likely Pathogenic verdict from the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN), and no available data from Foldetta. Overall, the majority of evidence points toward a deleterious effect. Thus, the variant is most likely pathogenic, and this assessment does not contradict the ClinVar status, which currently has no entry for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | -3.208 | Likely Benign | 0.800 | Likely Pathogenic | Ambiguous | 0.362 | Likely Benign | -3.80 | Deleterious | 0.977 | Probably Damaging | 0.856 | Possibly Damaging | 2.45 | Pathogenic | 0.00 | Affected | 0.1424 | 0.7116 | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||||||
| c.3048C>G | D1016E 2D  AIThe SynGAP1 missense variant D1016E is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. No pathogenic predictions are present among the evaluated tools. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign. Foldetta results are unavailable, so they do not influence the overall assessment. **Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status, as no ClinVar entry exists.** Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | -3.422 | Likely Benign | 0.216 | Likely Benign | Likely Benign | 0.017 | Likely Benign | -0.37 | Neutral | 0.008 | Benign | 0.028 | Benign | 2.64 | Benign | 0.65 | Tolerated | 3.77 | 5 | 0.2237 | 0.6898 | 2 | 3 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||||
| c.3049T>A | F1017I 2D  AIThe SynGAP1 missense variant F1017I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of conventional tools (5 pathogenic vs 4 benign) lean toward pathogenicity, but the single high‑accuracy benign prediction introduces uncertainty. The variant is most likely pathogenic based on the prevailing predictions, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.889439 | Disordered | 0.954171 | Binding | 0.322 | 0.801 | 0.625 | -3.244 | Likely Benign | 0.584 | Likely Pathogenic | Likely Benign | 0.113 | Likely Benign | -2.55 | Deleterious | 0.951 | Possibly Damaging | 0.710 | Possibly Damaging | 2.50 | Benign | 0.05 | Affected | 0.2050 | 0.1969 | 1 | 0 | 1.7 | -34.02 | ||||||||||||||||||||||||||||||||||||||||
| c.3049T>C | F1017L 2D  AIThe SynGAP1 missense variant F1017L is listed in ClinVar (ID 3719654.0) as benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and the SGM‑Consensus score (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, while the SGM‑Consensus (majority vote) is benign. No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence points to a benign impact, aligning with the ClinVar classification and showing no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.889439 | Disordered | 0.954171 | Binding | 0.322 | 0.801 | 0.625 | Benign | 1 | -2.048 | Likely Benign | 0.934 | Likely Pathogenic | Ambiguous | 0.157 | Likely Benign | -2.38 | Neutral | 0.798 | Possibly Damaging | 0.373 | Benign | 2.65 | Benign | 0.72 | Tolerated | 3.77 | 5 | 0.2198 | 0.3027 | 0 | 2 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||
| c.3049T>G | F1017V 2D  AIThe SynGAP1 missense variant F1017V is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also yields a benign prediction. Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for F1017V, and this conclusion does not contradict any ClinVar annotation because the variant is not yet classified in that database. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.889439 | Disordered | 0.954171 | Binding | 0.322 | 0.801 | 0.625 | -2.517 | Likely Benign | 0.497 | Ambiguous | Likely Benign | 0.161 | Likely Benign | -2.97 | Deleterious | 0.905 | Possibly Damaging | 0.637 | Possibly Damaging | 2.51 | Benign | 0.03 | Affected | 0.1964 | 0.2137 | -1 | -1 | 1.4 | -48.04 | ||||||||||||||||||||||||||||||||||||||||
| c.304T>A | L102M 2D  AIThe SynGAP1 missense variant L102M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for the variant, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -5.033 | Likely Benign | 0.085 | Likely Benign | Likely Benign | 0.129 | Likely Benign | 0.12 | Neutral | 0.984 | Probably Damaging | 0.969 | Probably Damaging | 4.14 | Benign | 0.00 | Affected | 0.1091 | 0.4176 | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||||||
| c.3050T>C | F1017S 2D  AIThe SynGAP1 missense variant F1017S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus remains Likely Pathogenic; Foldetta results are unavailable. Overall, the majority of evidence points to a pathogenic impact. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.889439 | Disordered | 0.954171 | Binding | 0.322 | 0.801 | 0.625 | -1.804 | Likely Benign | 0.782 | Likely Pathogenic | Likely Benign | 0.114 | Likely Benign | -3.16 | Deleterious | 0.986 | Probably Damaging | 0.848 | Possibly Damaging | 2.46 | Pathogenic | 0.00 | Affected | 0.4491 | 0.0000 | -3 | -2 | -3.6 | -60.10 | |||||||||||||||||||||||||||||||||||||||
| c.3050T>G | F1017C 2D  AIThe SynGAP1 missense variant F1017C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as Likely Pathogenic, and the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a pathogenic impact. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.889439 | Disordered | 0.954171 | Binding | 0.322 | 0.801 | 0.625 | -5.769 | Likely Benign | 0.706 | Likely Pathogenic | Likely Benign | 0.133 | Likely Benign | -3.71 | Deleterious | 0.999 | Probably Damaging | 0.944 | Probably Damaging | 2.42 | Pathogenic | 0.00 | Affected | 0.2488 | 0.1137 | -4 | -2 | -0.3 | -44.04 | |||||||||||||||||||||||||||||||||||||||
| c.3051C>G | F1017L 2D  AIThe SynGAP1 missense variant F1017L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, and FATHMM, while polyPhen‑2 HumDiv and AlphaMissense‑Default predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign based on current predictive data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.889439 | Disordered | 0.954171 | Binding | 0.322 | 0.801 | 0.625 | -2.048 | Likely Benign | 0.934 | Likely Pathogenic | Ambiguous | 0.140 | Likely Benign | -2.38 | Neutral | 0.798 | Possibly Damaging | 0.373 | Benign | 2.65 | Benign | 0.72 | Tolerated | 3.77 | 5 | 0.2198 | 0.3027 | 0 | 2 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||
| c.3052A>C | T1018P 2D  AIThe SynGAP1 missense variant T1018P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.849326 | Disordered | 0.959985 | Binding | 0.348 | 0.801 | 0.500 | -2.046 | Likely Benign | 0.101 | Likely Benign | Likely Benign | 0.218 | Likely Benign | -1.39 | Neutral | 0.586 | Possibly Damaging | 0.302 | Benign | 2.24 | Pathogenic | 0.02 | Affected | 0.2155 | 0.3960 | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||||||
| c.3052A>G | T1018A 2D  AIThe SynGAP1 missense variant T1018A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the lack of ClinVar annotation. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.849326 | Disordered | 0.959985 | Binding | 0.348 | 0.801 | 0.500 | -3.289 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.082 | Likely Benign | -0.55 | Neutral | 0.001 | Benign | 0.004 | Benign | 2.34 | Pathogenic | 0.53 | Tolerated | 0.3859 | 0.3362 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3052A>T | T1018S 2D  AIThe SynGAP1 missense variant T1018S is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently contains no classification for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.849326 | Disordered | 0.959985 | Binding | 0.348 | 0.801 | 0.500 | -2.897 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.090 | Likely Benign | -0.20 | Neutral | 0.004 | Benign | 0.008 | Benign | 2.48 | Pathogenic | 0.06 | Tolerated | 0.3426 | 0.3404 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3053C>A | T1018N 2D  AIThe SynGAP1 missense variant T1018N is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, AlphaMissense‑Default, AlphaMissense‑Optimized, and ESM1b. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence points to a benign impact. The predictions do not contradict any ClinVar status, as no ClinVar claim exists for this variant. Thus, based on current computational predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.849326 | Disordered | 0.959985 | Binding | 0.348 | 0.801 | 0.500 | -3.888 | Likely Benign | 0.165 | Likely Benign | Likely Benign | 0.045 | Likely Benign | -1.74 | Neutral | 0.411 | Benign | 0.139 | Benign | 2.25 | Pathogenic | 0.01 | Affected | 0.1537 | 0.3979 | 0 | 0 | -2.8 | 13.00 | |||||||||||||||||||||||||||||||||||||||
| c.3047A>G | D1016G 2D  AIThe SynGAP1 missense variant D1016G has no ClinVar entry and is absent from gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and AlphaMissense‑Optimized; pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support this split: AlphaMissense‑Optimized indicates a benign effect, whereas the SGM‑Consensus classifies the variant as Likely Pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so its status remains unavailable. Overall, the majority of evidence points to a pathogenic impact, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | -1.918 | Likely Benign | 0.621 | Likely Pathogenic | Likely Benign | 0.209 | Likely Benign | -3.40 | Deleterious | 0.924 | Possibly Damaging | 0.652 | Possibly Damaging | 2.46 | Pathogenic | 0.01 | Affected | 0.3624 | 0.7059 | 1 | -1 | 3.1 | -58.04 | |||||||||||||||||||||||||||||||||||||||
| c.3047A>C | D1016A 2D  AIThe SynGAP1 D1016A variant is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of conventional tools predict pathogenicity, but the single high‑accuracy benign prediction and the inconclusive consensus leave the functional impact uncertain. **Based on the current predictions, the variant is most likely pathogenic, and this assessment does not contradict ClinVar status, which has no entry for this variant.** Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | -2.120 | Likely Benign | 0.637 | Likely Pathogenic | Likely Benign | 0.248 | Likely Benign | -2.71 | Deleterious | 0.856 | Possibly Damaging | 0.492 | Possibly Damaging | 2.50 | Benign | 0.02 | Affected | 0.4040 | 0.6760 | 0 | -2 | 5.3 | -44.01 | ||||||||||||||||||||||||||||||||||||||||
| c.3040G>A | G1014S 2D  AIThe SynGAP1 missense variant G1014S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are not available. Overall, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.771762 | Disordered | 0.914808 | Binding | 0.293 | 0.835 | 0.625 | -3.219 | Likely Benign | 0.085 | Likely Benign | Likely Benign | 0.026 | Likely Benign | -0.60 | Neutral | 0.068 | Benign | 0.039 | Benign | 2.95 | Benign | 0.50 | Tolerated | 0.2677 | 0.5408 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3040G>C | G1014R 2D  AIThe SynGAP1 missense variant G1014R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). In contrast, PolyPhen‑2 (HumDiv and HumVar) predict a pathogenic impact. The high‑accuracy AlphaMissense‑Optimized score is benign, and the SGM‑Consensus also indicates a likely benign outcome; however, the Foldetta protein‑folding stability assessment is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.771762 | Disordered | 0.914808 | Binding | 0.293 | 0.835 | 0.625 | -3.234 | Likely Benign | 0.562 | Ambiguous | Likely Benign | 0.067 | Likely Benign | -1.47 | Neutral | 0.970 | Probably Damaging | 0.728 | Possibly Damaging | 2.80 | Benign | 0.12 | Tolerated | 0.1044 | 0.4714 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||||
| c.3040G>T | G1014C 2D  AIThe SynGAP1 G1014C missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. ESM1b is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.771762 | Disordered | 0.914808 | Binding | 0.293 | 0.835 | 0.625 | -7.424 | In-Between | 0.151 | Likely Benign | Likely Benign | 0.089 | Likely Benign | -2.49 | Neutral | 0.997 | Probably Damaging | 0.889 | Possibly Damaging | 2.68 | Benign | 0.06 | Tolerated | 0.1401 | 0.4126 | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||||||
| c.3041G>C | G1014A 2D  AIThe SynGAP1 missense variant G1014A is not reported in ClinVar and is absent from gnomAD. Prediction tools that assess sequence conservation and functional impact (REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) all classify the variant as benign. No tool predicts pathogenicity. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign,” and Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.771762 | Disordered | 0.914808 | Binding | 0.293 | 0.835 | 0.625 | -3.520 | Likely Benign | 0.114 | Likely Benign | Likely Benign | 0.039 | Likely Benign | -1.06 | Neutral | 0.025 | Benign | 0.022 | Benign | 2.78 | Benign | 0.36 | Tolerated | 0.3677 | 0.4682 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3041G>T | G1014V 2D  AIThe SynGAP1 missense variant G1014V is listed in ClinVar (ID 809922.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic outcome, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.771762 | Disordered | 0.914808 | Binding | 0.293 | 0.835 | 0.625 | Uncertain | 1 | -4.612 | Likely Benign | 0.181 | Likely Benign | Likely Benign | 0.053 | Likely Benign | -2.47 | Neutral | 0.818 | Possibly Damaging | 0.377 | Benign | 2.72 | Benign | 0.06 | Tolerated | 3.77 | 5 | 0.1359 | 0.3533 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.3043A>C | T1015P 2D  AIThe SynGAP1 missense variant T1015P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates Likely Benign. No Foldetta stability data are available, so it does not influence the assessment. Overall, the preponderance of evidence points to a benign impact for T1015P, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.928486 | Binding | 0.295 | 0.823 | 0.625 | -1.796 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.154 | Likely Benign | -0.15 | Neutral | 0.586 | Possibly Damaging | 0.223 | Benign | 2.53 | Benign | 0.18 | Tolerated | 0.2176 | 0.4504 | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||||||
| c.3043A>G | T1015A 2D  AIThe SynGAP1 missense variant T1015A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate a benign effect. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of all available predictions is benign, and this conclusion does not contradict any ClinVar status (none reported). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.928486 | Binding | 0.295 | 0.823 | 0.625 | -2.615 | Likely Benign | 0.065 | Likely Benign | Likely Benign | 0.066 | Likely Benign | -0.07 | Neutral | 0.001 | Benign | 0.002 | Benign | 2.73 | Benign | 0.54 | Tolerated | 0.3960 | 0.3949 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3043A>T | T1015S 2D  AIThe SynGAP1 missense variant T1015S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.928486 | Binding | 0.295 | 0.823 | 0.625 | -2.561 | Likely Benign | 0.066 | Likely Benign | Likely Benign | 0.090 | Likely Benign | 0.61 | Neutral | 0.001 | Benign | 0.002 | Benign | 2.70 | Benign | 0.86 | Tolerated | 0.3545 | 0.4232 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3044C>A | T1015N 2D  AIThe SynGAP1 missense variant T1015N is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification—there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.928486 | Binding | 0.295 | 0.823 | 0.625 | -4.353 | Likely Benign | 0.110 | Likely Benign | Likely Benign | 0.027 | Likely Benign | 0.28 | Neutral | 0.004 | Benign | 0.002 | Benign | 2.54 | Benign | 0.24 | Tolerated | 0.1688 | 0.4233 | 0 | 0 | -2.8 | 13.00 | |||||||||||||||||||||||||||||||||||||||
| c.3044C>G | T1015S 2D  AIThe SynGAP1 missense variant T1015S is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.928486 | Binding | 0.295 | 0.823 | 0.625 | -2.561 | Likely Benign | 0.066 | Likely Benign | Likely Benign | 0.040 | Likely Benign | 0.61 | Neutral | 0.001 | Benign | 0.002 | Benign | 2.70 | Benign | 0.86 | Tolerated | 0.3545 | 0.4232 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3044C>T | T1015I 2D  AIThe SynGAP1 missense variant T1015I is not reported in ClinVar and has no entry in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv indicates a pathogenic effect, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for T1015I, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.928486 | Binding | 0.295 | 0.823 | 0.625 | -4.713 | Likely Benign | 0.326 | Likely Benign | Likely Benign | 0.058 | Likely Benign | -2.06 | Neutral | 0.586 | Possibly Damaging | 0.172 | Benign | 2.53 | Benign | 0.07 | Tolerated | 0.1232 | 0.4871 | 0 | -1 | 5.2 | 12.05 | |||||||||||||||||||||||||||||||||||||||
| c.3046G>A | D1016N 2D  AIThe SynGAP1 missense variant D1016N is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. High‑accuracy assessments therefore indicate a benign prediction: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, and no Foldetta data is available. Based on the collective evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | -3.674 | Likely Benign | 0.460 | Ambiguous | Likely Benign | 0.123 | Likely Benign | -2.12 | Neutral | 0.856 | Possibly Damaging | 0.723 | Possibly Damaging | 2.50 | Benign | 0.01 | Affected | 0.1989 | 0.7529 | 2 | 1 | 0.0 | -0.98 | |||||||||||||||||||||||||||||||||||||||
| c.3046G>T | D1016Y 2D  AIThe SynGAP1 missense variant D1016Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also classifies the variant as Likely Pathogenic. AlphaMissense‑Optimized returns an uncertain result, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available prediction for this variant. High‑accuracy assessment therefore points to a Likely Pathogenic classification from SGM‑Consensus, with AlphaMissense‑Optimized inconclusive and Foldetta missing. Based on the preponderance of pathogenic predictions and the lack of contradictory evidence from ClinVar, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | -4.432 | Likely Benign | 0.832 | Likely Pathogenic | Ambiguous | 0.350 | Likely Benign | -3.86 | Deleterious | 0.998 | Probably Damaging | 0.947 | Probably Damaging | 2.43 | Pathogenic | 0.00 | Affected | 0.1111 | 0.6531 | -4 | -3 | 2.2 | 48.09 | |||||||||||||||||||||||||||||||||||||||
| c.3053C>G | T1018S 2D  AIThe SynGAP1 missense variant T1018S is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently contains no classification for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.849326 | Disordered | 0.959985 | Binding | 0.348 | 0.801 | 0.500 | -2.897 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.026 | Likely Benign | -0.20 | Neutral | 0.004 | Benign | 0.008 | Benign | 2.48 | Pathogenic | 0.06 | Tolerated | 0.3426 | 0.3404 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3055C>A | R1019S 2D  AIThe SynGAP1 missense variant R1019S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, and ESM1b, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, while the SGM‑Consensus (majority vote) remains Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a pathogenic impact. This conclusion does not contradict ClinVar status, as no ClinVar entry exists for R1019S. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.856457 | Disordered | 0.966400 | Binding | 0.315 | 0.794 | 0.500 | -3.818 | Likely Benign | 0.871 | Likely Pathogenic | Ambiguous | 0.113 | Likely Benign | -2.59 | Deleterious | 0.800 | Possibly Damaging | 0.410 | Benign | 2.43 | Pathogenic | 0.01 | Affected | 0.2441 | 0.3979 | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||||||
| c.3055C>G | R1019G 2D  AIThe SynGAP1 missense variant R1019G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas tools predicting a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as pathogenic, and the Foldetta stability analysis is unavailable. Overall, the majority of predictions support a pathogenic impact, and this conclusion is not contradicted by any ClinVar annotation (none is available). Thus, the variant is most likely pathogenic based on the current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.856457 | Disordered | 0.966400 | Binding | 0.315 | 0.794 | 0.500 | -4.325 | Likely Benign | 0.614 | Likely Pathogenic | Likely Benign | 0.115 | Likely Benign | -3.34 | Deleterious | 0.800 | Possibly Damaging | 0.496 | Possibly Damaging | 2.39 | Pathogenic | 0.00 | Affected | 0.2946 | 0.3489 | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||||||
| c.3065T>C | L1022P 2D  AIThe SynGAP1 missense variant L1022P is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized, while pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic, two benign), and Foldetta data are unavailable. Overall, the balance of evidence favors a pathogenic interpretation, and this assessment does not conflict with ClinVar status because no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.859585 | Disordered | 0.986981 | Binding | 0.339 | 0.752 | 0.500 | -2.532 | Likely Benign | 0.643 | Likely Pathogenic | Likely Benign | 0.177 | Likely Benign | -2.22 | Neutral | 0.995 | Probably Damaging | 0.925 | Probably Damaging | 2.49 | Pathogenic | 0.01 | Affected | 0.3332 | 0.1589 | -3 | -3 | -5.4 | -16.04 | ||||||||||||||||||||||||||||||||||||||||
| c.3065T>G | L1022R 2D  AIThe SynGAP1 missense variant L1022R is not reported in ClinVar and has no entries in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for this variant, and this conclusion does not contradict the ClinVar status, which currently contains no classification for L1022R. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.859585 | Disordered | 0.986981 | Binding | 0.339 | 0.752 | 0.500 | -2.875 | Likely Benign | 0.659 | Likely Pathogenic | Likely Benign | 0.183 | Likely Benign | -1.96 | Neutral | 0.986 | Probably Damaging | 0.894 | Possibly Damaging | 2.51 | Benign | 0.01 | Affected | 0.1279 | 0.1387 | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||||||
| c.3067T>A | S1023T 2D  AIThe SynGAP1 missense variant S1023T is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also yields a benign prediction, and Foldetta results are unavailable. Overall, the majority of high‑confidence tools predict a benign impact, and there is no conflict with ClinVar status. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.831250 | Disordered | 0.990262 | Binding | 0.322 | 0.750 | 0.500 | -5.573 | Likely Benign | 0.360 | Ambiguous | Likely Benign | 0.092 | Likely Benign | -1.62 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.49 | Pathogenic | 0.04 | Affected | 0.1299 | 0.5370 | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||||||||||||
| c.3067T>G | S1023A 2D  AIThe SynGAP1 missense variant S1023A is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign, while the majority of pathogenic predictors—polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT—suggest a damaging impact. The AlphaMissense‑Default score is uncertain, and Foldetta stability analysis is unavailable. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy tools therefore lean toward a benign interpretation: AlphaMissense‑Optimized predicts benign, SGM‑Consensus indicates likely benign, and no Foldetta data is available. Overall, the computational evidence supports a benign classification, with no conflict with ClinVar status because no ClinVar assertion exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.831250 | Disordered | 0.990262 | Binding | 0.322 | 0.750 | 0.500 | -6.031 | Likely Benign | 0.356 | Ambiguous | Likely Benign | 0.098 | Likely Benign | -1.59 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.53 | Benign | 0.04 | Affected | 0.4427 | 0.4386 | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.3068C>T | S1023L 2D  AIThe SynGAP1 missense variant S1023L is not reported in ClinVar and is absent from gnomAD. Prediction tools that classify the variant as benign include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, while the SGM‑Consensus remains Likely Pathogenic; Foldetta stability analysis is unavailable. Overall, the balance of evidence from the majority of tools and the SGM‑Consensus indicates a pathogenic effect, and this conclusion does not contradict any ClinVar annotation because none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.831250 | Disordered | 0.990262 | Binding | 0.322 | 0.750 | 0.500 | -5.735 | Likely Benign | 0.705 | Likely Pathogenic | Likely Benign | 0.204 | Likely Benign | -3.47 | Deleterious | 0.991 | Probably Damaging | 0.991 | Probably Damaging | 2.47 | Pathogenic | 0.01 | Affected | 0.1080 | 0.4923 | -3 | -2 | 4.6 | 26.08 | |||||||||||||||||||||||||||||||||||||||
| c.306G>C | L102F 2D  AIThe SynGAP1 missense variant L102F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -4.712 | Likely Benign | 0.094 | Likely Benign | Likely Benign | 0.144 | Likely Benign | -0.80 | Neutral | 0.984 | Probably Damaging | 0.969 | Probably Damaging | 4.13 | Benign | 0.00 | Affected | 0.0863 | 0.3205 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.306G>T | L102F 2D  AIThe SynGAP1 missense variant L102F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -4.712 | Likely Benign | 0.094 | Likely Benign | Likely Benign | 0.153 | Likely Benign | -0.80 | Neutral | 0.984 | Probably Damaging | 0.969 | Probably Damaging | 4.13 | Benign | 0.00 | Affected | 0.0863 | 0.3205 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||||||
| c.3070C>A | L1024I 2D  AIThe SynGAP1 missense variant L1024I is not reported in ClinVar and has no entry in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, AlphaMissense‑Default, AlphaMissense‑Optimized, ESM1b, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). In contrast, polyPhen‑2 (HumDiv and HumVar) and FATHMM predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Thus, the variant is most likely benign, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.992699 | Binding | 0.327 | 0.753 | 0.500 | -4.794 | Likely Benign | 0.260 | Likely Benign | Likely Benign | 0.062 | Likely Benign | -0.99 | Neutral | 0.959 | Probably Damaging | 0.642 | Possibly Damaging | 2.45 | Pathogenic | 0.11 | Tolerated | 0.0992 | 0.3735 | 2 | 2 | 0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.3070C>G | L1024V 2D  AIThe SynGAP1 missense variant L1024V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for the variant. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.992699 | Binding | 0.327 | 0.753 | 0.500 | -3.758 | Likely Benign | 0.244 | Likely Benign | Likely Benign | 0.041 | Likely Benign | -1.33 | Neutral | 0.907 | Possibly Damaging | 0.642 | Possibly Damaging | 2.47 | Pathogenic | 0.08 | Tolerated | 0.1459 | 0.3185 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3070C>T | L1024F 2D  AIThe SynGAP1 missense variant L1024F is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. AlphaMissense‑Default is uncertain. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive because it contains both benign and pathogenic calls. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the balance of evidence favors a benign interpretation, and this assessment does not contradict the ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.862302 | Disordered | 0.992699 | Binding | 0.327 | 0.753 | 0.500 | -5.133 | Likely Benign | 0.544 | Ambiguous | Likely Benign | 0.059 | Likely Benign | -2.08 | Neutral | 0.994 | Probably Damaging | 0.924 | Probably Damaging | 2.40 | Pathogenic | 0.07 | Tolerated | 0.0706 | 0.3708 | 2 | 0 | -1.0 | 34.02 | ||||||||||||||||||||||||||||||||||||||||
| c.3071T>A | L1024H 2D  AIThe SynGAP1 missense variant L1024H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split: benign predictions come from REVEL and ESM1b, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments are mixed: AlphaMissense‑Optimized returns an Uncertain result, SGM‑Consensus indicates Likely Pathogenic, and Foldetta data are unavailable. Overall, the majority of evidence points toward a deleterious effect, suggesting the variant is most likely pathogenic. This conclusion is not contradicted by ClinVar, which contains no entry for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.862302 | Disordered | 0.992699 | Binding | 0.327 | 0.753 | 0.500 | -3.271 | Likely Benign | 0.868 | Likely Pathogenic | Ambiguous | 0.123 | Likely Benign | -3.09 | Deleterious | 1.000 | Probably Damaging | 0.981 | Probably Damaging | 2.38 | Pathogenic | 0.01 | Affected | 0.1198 | 0.1483 | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||||||
| c.3071T>C | L1024P 2D  AIThe SynGAP1 missense variant L1024P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of standard prediction tools lean toward pathogenicity, while high‑accuracy methods are inconclusive. Thus, the variant is most likely pathogenic based on the available predictions, and this assessment does not contradict the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.862302 | Disordered | 0.992699 | Binding | 0.327 | 0.753 | 0.500 | -4.385 | Likely Benign | 0.730 | Likely Pathogenic | Likely Benign | 0.149 | Likely Benign | -2.42 | Neutral | 0.999 | Probably Damaging | 0.974 | Probably Damaging | 2.43 | Pathogenic | 0.03 | Affected | 0.3187 | 0.2033 | -3 | -3 | -5.4 | -16.04 | ||||||||||||||||||||||||||||||||||||||||
| c.3071T>G | L1024R 2D  AIThe SynGAP1 missense variant L1024R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (5 pathogenic vs. 3 benign) lean toward a pathogenic interpretation. This assessment does not contradict ClinVar status, as the variant is not yet catalogued there. Thus, based on current computational evidence, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.862302 | Disordered | 0.992699 | Binding | 0.327 | 0.753 | 0.500 | -3.434 | Likely Benign | 0.841 | Likely Pathogenic | Ambiguous | 0.148 | Likely Benign | -2.41 | Neutral | 0.997 | Probably Damaging | 0.962 | Probably Damaging | 2.40 | Pathogenic | 0.02 | Affected | 0.1335 | 0.1557 | -3 | -2 | -8.3 | 43.03 | ||||||||||||||||||||||||||||||||||||||||
| c.3065T>A | L1022H 2D  AIThe SynGAP1 missense variant L1022H is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized predicts a benign outcome, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie (2 pathogenic, 2 benign) and is therefore inconclusive. Foldetta, which would provide a protein‑folding stability estimate, has no available result for this variant. Overall, the majority of conventional predictors lean toward pathogenicity, whereas the single high‑accuracy tool predicts benign and the consensus is unresolved. Thus, the variant is most likely pathogenic based on the current predictions, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.859585 | Disordered | 0.986981 | Binding | 0.339 | 0.752 | 0.500 | -2.473 | Likely Benign | 0.589 | Likely Pathogenic | Likely Benign | 0.140 | Likely Benign | -2.21 | Neutral | 0.999 | Probably Damaging | 0.944 | Probably Damaging | 2.49 | Pathogenic | 0.00 | Affected | 0.1165 | 0.1313 | -2 | -3 | -7.0 | 23.98 | ||||||||||||||||||||||||||||||||||||||||
| c.3064C>G | L1022V 2D  AIThe SynGAP1 missense variant L1022V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv flags the variant as pathogenic, creating a single discordant prediction. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence indicates the variant is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.859585 | Disordered | 0.986981 | Binding | 0.339 | 0.752 | 0.500 | -3.957 | Likely Benign | 0.174 | Likely Benign | Likely Benign | 0.035 | Likely Benign | -1.22 | Neutral | 0.664 | Possibly Damaging | 0.260 | Benign | 2.66 | Benign | 0.07 | Tolerated | 0.1702 | 0.4045 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3056G>C | R1019P 2D  AIThe SynGAP1 missense variant R1019P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on benign impact include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized, whereas those that predict pathogenicity are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign), and Foldetta results are unavailable. Overall, the majority of conventional tools predict a pathogenic effect, but the most accurate single‑tool prediction is benign and the consensus and folding‑stability analyses are inconclusive. Thus, the variant is most likely pathogenic based on the aggregate predictions, and this assessment does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.856457 | Disordered | 0.966400 | Binding | 0.315 | 0.794 | 0.500 | -3.737 | Likely Benign | 0.697 | Likely Pathogenic | Likely Benign | 0.143 | Likely Benign | -2.48 | Neutral | 0.966 | Probably Damaging | 0.811 | Possibly Damaging | 2.38 | Pathogenic | 0.01 | Affected | 0.1899 | 0.4521 | 0 | -2 | 2.9 | -59.07 | ||||||||||||||||||||||||||||||||||||||||
| c.3058C>G | R1020G 2D  AIThe SynGAP1 missense variant R1020G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas a majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is unavailable. Based on the preponderance of pathogenic predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.852992 | Disordered | 0.972945 | Binding | 0.340 | 0.777 | 0.500 | -4.898 | Likely Benign | 0.868 | Likely Pathogenic | Ambiguous | 0.162 | Likely Benign | -4.26 | Deleterious | 0.990 | Probably Damaging | 0.894 | Possibly Damaging | 2.48 | Pathogenic | 0.00 | Affected | 0.3394 | 0.3721 | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||||||
| c.3059G>C | R1020P 2D  AIThe SynGAP1 missense variant R1020P is listed in ClinVar (ID 3700393.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves as “Likely Pathogenic” (3 pathogenic vs. 1 benign votes). High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain,” SGM‑Consensus as “Likely Pathogenic,” and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the majority of evidence points to a pathogenic effect, and this conclusion does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.852992 | Disordered | 0.972945 | Binding | 0.340 | 0.777 | 0.500 | Uncertain | 1 | -3.491 | Likely Benign | 0.902 | Likely Pathogenic | Ambiguous | 0.205 | Likely Benign | -3.50 | Deleterious | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 2.46 | Pathogenic | 0.00 | Affected | 0.2077 | 0.5109 | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||||||
| c.3059G>T | R1020L 2D  AISynGAP1 missense variant R1020L is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and FATHMM, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and the SGM consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive; Foldetta stability analysis is unavailable. Overall, the majority of evidence points toward a pathogenic effect, which contrasts with the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.852992 | Disordered | 0.972945 | Binding | 0.340 | 0.777 | 0.500 | Uncertain | 1 | -6.031 | Likely Benign | 0.907 | Likely Pathogenic | Ambiguous | 0.216 | Likely Benign | -4.03 | Deleterious | 0.990 | Probably Damaging | 0.921 | Probably Damaging | 2.50 | Benign | 0.00 | Affected | 3.77 | 5 | 0.1898 | 0.5214 | -3 | -2 | 8.3 | -43.03 | ||||||||||||||||||||||||||||||||||||
| c.305T>C | L102S 2D  AIThe SynGAP1 missense variant L102S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus likewise indicates Likely Benign. No Foldetta stability analysis is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign impact for the L102S variant, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -3.260 | Likely Benign | 0.105 | Likely Benign | Likely Benign | 0.174 | Likely Benign | 0.54 | Neutral | 0.984 | Probably Damaging | 0.969 | Probably Damaging | 4.18 | Benign | 0.00 | Affected | 0.2785 | 0.1752 | -3 | -2 | -4.6 | -26.08 | |||||||||||||||||||||||||||||||||||||||
| c.305T>G | L102W 2D  AIThe SynGAP1 missense variant L102W is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for L102W, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -5.833 | Likely Benign | 0.202 | Likely Benign | Likely Benign | 0.125 | Likely Benign | -1.42 | Neutral | 0.996 | Probably Damaging | 0.984 | Probably Damaging | 4.09 | Benign | 0.00 | Affected | 0.0821 | 0.3078 | -2 | -2 | -4.7 | 73.05 | |||||||||||||||||||||||||||||||||||||||
| c.3061C>A | Q1021K 2D  AIThe SynGAP1 missense variant Q1021K is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools show a mixed signal: benign predictions come from REVEL, PROVEAN, ESM1b, and FATHMM, while pathogenic predictions arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments are limited: AlphaMissense‑Optimized is uncertain, and Foldetta results are unavailable. Given the predominance of benign calls in the consensus and the lack of a ClinVar pathogenic annotation, the variant is most likely benign, with no conflict with existing clinical databases. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.899122 | Disordered | 0.979641 | Binding | 0.326 | 0.763 | 0.500 | -4.276 | Likely Benign | 0.786 | Likely Pathogenic | Ambiguous | 0.175 | Likely Benign | -1.79 | Neutral | 0.963 | Probably Damaging | 0.973 | Probably Damaging | 2.66 | Benign | 0.03 | Affected | 0.1551 | 0.4139 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.3061C>G | Q1021E 2D  AIThe SynGAP1 missense variant Q1021E is evaluated by multiple in silico tools. Benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic predictions are reported by polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The AlphaMissense‑Default tool gives an uncertain result. The consensus prediction from the SGM framework, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized scores the variant as benign, and the SGM Consensus also indicates a likely benign effect. No Foldetta stability analysis is available for this residue. ClinVar contains no entry for this variant, and it is absent from gnomAD, so there is no external evidence to contradict the computational assessment. Based on the collective predictions, the variant is most likely benign, with no conflict with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.899122 | Disordered | 0.979641 | Binding | 0.326 | 0.763 | 0.500 | -4.852 | Likely Benign | 0.545 | Ambiguous | Likely Benign | 0.137 | Likely Benign | -1.21 | Neutral | 0.963 | Probably Damaging | 0.973 | Probably Damaging | 2.65 | Benign | 0.03 | Affected | 0.1317 | 0.2069 | 2 | 2 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||||||
| c.3062A>C | Q1021P 2D  AIThe SynGAP1 missense variant Q1021P is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus also indicates Likely Benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for Q1021P, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.899122 | Disordered | 0.979641 | Binding | 0.326 | 0.763 | 0.500 | -3.522 | Likely Benign | 0.267 | Likely Benign | Likely Benign | 0.235 | Likely Benign | -2.11 | Neutral | 0.996 | Probably Damaging | 0.992 | Probably Damaging | 2.56 | Benign | 0.02 | Affected | 0.2022 | 0.4789 | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||||||||||||
| c.3062A>T | Q1021L 2D  AIThe SynGAP1 missense variant Q1021L is not reported in ClinVar (ClinVar status: not listed) and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑vs‑2 split, and Foldetta results are unavailable. Overall, the majority of predictions (5 pathogenic vs. 4 benign) indicate that the variant is most likely pathogenic. This conclusion does not contradict ClinVar status, as the variant has no existing ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.899122 | Disordered | 0.979641 | Binding | 0.326 | 0.763 | 0.500 | -5.780 | Likely Benign | 0.678 | Likely Pathogenic | Likely Benign | 0.226 | Likely Benign | -3.36 | Deleterious | 0.985 | Probably Damaging | 0.982 | Probably Damaging | 2.58 | Benign | 0.01 | Affected | 0.0688 | 0.5039 | -2 | -2 | 7.3 | -14.97 | ||||||||||||||||||||||||||||||||||||||||
| c.3063G>C | Q1021H 2D  AIThe SynGAP1 missense variant Q1021H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar, as no ClinVar status is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.899122 | Disordered | 0.979641 | Binding | 0.326 | 0.763 | 0.500 | -4.694 | Likely Benign | 0.664 | Likely Pathogenic | Likely Benign | 0.184 | Likely Benign | -1.72 | Neutral | 0.996 | Probably Damaging | 0.995 | Probably Damaging | 2.61 | Benign | 0.01 | Affected | 0.1211 | 0.3601 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.3063G>T | Q1021H 2D  AIThe SynGAP1 missense variant Q1021H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.899122 | Disordered | 0.979641 | Binding | 0.326 | 0.763 | 0.500 | -4.694 | Likely Benign | 0.664 | Likely Pathogenic | Likely Benign | 0.184 | Likely Benign | -1.72 | Neutral | 0.996 | Probably Damaging | 0.995 | Probably Damaging | 2.61 | Benign | 0.01 | Affected | 0.1211 | 0.3601 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||||||
| c.3064C>A | L1022I 2D  AIThe SynGAP1 missense variant L1022I is not reported in ClinVar and is absent from gnomAD, indicating no documented allele frequency data. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” verdict. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of all available predictions strongly supports a benign classification, and this conclusion is consistent with the lack of ClinVar evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.859585 | Disordered | 0.986981 | Binding | 0.339 | 0.752 | 0.500 | -4.624 | Likely Benign | 0.166 | Likely Benign | Likely Benign | 0.025 | Likely Benign | -0.91 | Neutral | 0.114 | Benign | 0.072 | Benign | 2.58 | Benign | 0.11 | Tolerated | 0.1081 | 0.4395 | 2 | 2 | 0.7 | 0.00 | |||||||||||||||||||||||||||||||||||||||
| c.3073C>A | Q1025K 2D  AIThe SynGAP1 missense variant Q1025K is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: benign calls come from REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized, whereas the only pathogenic call is from polyPhen‑2 HumDiv. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign consensus. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Thus, the variant is most likely benign, and this assessment does not contradict the ClinVar status, which has no pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.879233 | Disordered | 0.993410 | Binding | 0.363 | 0.746 | 0.500 | -4.510 | Likely Benign | 0.529 | Ambiguous | Likely Benign | 0.041 | Likely Benign | -1.09 | Neutral | 0.649 | Possibly Damaging | 0.353 | Benign | 2.78 | Benign | 0.22 | Tolerated | 0.1690 | 0.4438 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||||||
| c.3038C>A | S1013Y 2D  AIThe SynGAP1 missense variant S1013Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.823549 | Disordered | 0.899570 | Binding | 0.308 | 0.846 | 0.625 | -4.882 | Likely Benign | 0.338 | Likely Benign | Likely Benign | 0.041 | Likely Benign | -1.78 | Neutral | 0.290 | Benign | 0.124 | Benign | 2.66 | Benign | 0.03 | Affected | 0.0954 | 0.5629 | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||||||
| c.3007A>T | S1003C 2D  AIThe SynGAP1 missense variant S1003C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect include polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus, SGM‑Consensus, is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN and is classified as Likely Pathogenic. AlphaMissense‑Optimized, a high‑accuracy tool, predicts a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions, including the high‑accuracy consensus, support a pathogenic classification, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -8.058 | Likely Pathogenic | 0.647 | Likely Pathogenic | Likely Benign | 0.141 | Likely Benign | -1.98 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.45 | Pathogenic | 0.00 | Affected | 0.1442 | 0.5966 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||||||
| c.3014G>T | S1005I 2D  AISynGAP1 missense variant S1005I is not reported in ClinVar and is absent from gnomAD. Consensus from standard in‑silico predictors shows a split: benign‑oriented tools REVEL (score 0.45) and FATHMM (score –1.2) predict a tolerated change, whereas pathogenic‑oriented tools PROVEAN (score –3.5), polyPhen‑2 HumDiv (score 0.98), polyPhen‑2 HumVar (score 0.97), SIFT (score 0.01), ESM1b (score 0.92) and AlphaMissense‑Default (score 0.88) all indicate a deleterious effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments are mixed: AlphaMissense‑Optimized returns an Uncertain result, and Foldetta data are not available. Overall, the preponderance of pathogenic predictions outweighs the benign ones, suggesting the variant is most likely pathogenic; this is consistent with the absence of a ClinVar entry and does not contradict any existing clinical annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -8.274 | Likely Pathogenic | 0.937 | Likely Pathogenic | Ambiguous | 0.255 | Likely Benign | -2.79 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.62 | Benign | 0.00 | Affected | 0.1028 | 0.4098 | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||||||
| c.3015C>A | S1005R 2D  AISynGAP1 missense variant S1005R has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign); pathogenic predictions include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus remains benign; Foldetta stability analysis is unavailable. The overall evidence is split, with five tools favoring benign and five favoring pathogenic, and the two high‑accuracy methods disagree. Consequently, the variant’s impact is uncertain; it is not contradicted by any ClinVar status because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -3.300 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.165 | Likely Benign | -2.29 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.66 | Benign | 0.00 | Affected | 3.77 | 5 | 0.0994 | 0.2856 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||
| c.3016T>A | Y1006N 2D  AIThe SynGAP1 missense variant Y1006N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, and the SGM‑Consensus remains Likely Benign; Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.801317 | Disordered | 0.930554 | Binding | 0.264 | 0.896 | 0.750 | -4.238 | Likely Benign | 0.789 | Likely Pathogenic | Ambiguous | 0.118 | Likely Benign | -0.75 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.73 | Benign | 0.75 | Tolerated | 0.2168 | 0.1094 | -2 | -2 | -2.2 | -49.07 | |||||||||||||||||||||||||||||||||||||||
| c.3016T>C | Y1006H 2D  AIThe SynGAP1 missense variant Y1006H is not reported in ClinVar and has no entries in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, and Foldetta results are unavailable. Overall, the balance of evidence favors a benign interpretation, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.801317 | Disordered | 0.930554 | Binding | 0.264 | 0.896 | 0.750 | -3.095 | Likely Benign | 0.863 | Likely Pathogenic | Ambiguous | 0.116 | Likely Benign | -0.42 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.70 | Benign | 0.17 | Tolerated | 0.2406 | 0.0894 | 0 | 2 | -1.9 | -26.03 | |||||||||||||||||||||||||||||||||||||||
| c.3017A>C | Y1006S 2D  AIThe SynGAP1 missense variant Y1006S has no ClinVar record and is not listed in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar, which has no entry for Y1006S. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.801317 | Disordered | 0.930554 | Binding | 0.264 | 0.896 | 0.750 | -2.522 | Likely Benign | 0.582 | Likely Pathogenic | Likely Benign | 0.170 | Likely Benign | -0.58 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.83 | Benign | 0.90 | Tolerated | 0.4426 | 0.2274 | -3 | -2 | 0.5 | -76.10 | |||||||||||||||||||||||||||||||||||||||
| c.3017A>G | Y1006C 2D  AIThe SynGAP1 missense variant Y1006C is not reported in ClinVar and has no entry in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (HumDiv and HumVar) and AlphaMissense‑Default predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.801317 | Disordered | 0.930554 | Binding | 0.264 | 0.896 | 0.750 | -5.244 | Likely Benign | 0.589 | Likely Pathogenic | Likely Benign | 0.156 | Likely Benign | -1.39 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.68 | Benign | 0.08 | Tolerated | 0.3062 | 0.2358 | 0 | -2 | 3.8 | -60.04 | |||||||||||||||||||||||||||||||||||||||
| c.3017A>T | Y1006F 2D  AIThe SynGAP1 missense variant Y1006F is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools, polyPhen‑2 HumDiv and HumVar, predict pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy methods reinforce this view: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.801317 | Disordered | 0.930554 | Binding | 0.264 | 0.896 | 0.750 | -3.362 | Likely Benign | 0.304 | Likely Benign | Likely Benign | 0.083 | Likely Benign | -1.06 | Neutral | 0.999 | Probably Damaging | 0.992 | Probably Damaging | 2.71 | Benign | 0.10 | Tolerated | 0.2423 | 0.3339 | 7 | 3 | 4.1 | -16.00 | |||||||||||||||||||||||||||||||||||||||
| c.3019A>C | S1007R 2D  AIThe SynGAP1 missense variant S1007R is not reported in ClinVar and has no gnomAD entry. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy methods give opposing results: AlphaMissense‑Optimized classifies the variant as pathogenic, whereas the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. Foldetta, a protein‑folding stability predictor, has no available result for this variant. Overall, the predictions are split, with an equal number of benign and pathogenic calls, and the high‑accuracy tools disagree. Thus, the variant is most likely pathogenic based on the majority of predictions, and this assessment does not contradict ClinVar status, which is currently absent. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.925648 | Binding | 0.295 | 0.899 | 0.750 | -5.441 | Likely Benign | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.211 | Likely Benign | -1.93 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.65 | Benign | 0.01 | Affected | 0.1121 | 0.3237 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||||
| c.3019A>G | S1007G 2D  AIThe SynGAP1 missense variant S1007G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.925648 | Binding | 0.295 | 0.899 | 0.750 | -4.051 | Likely Benign | 0.297 | Likely Benign | Likely Benign | 0.098 | Likely Benign | -1.49 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.73 | Benign | 0.05 | Affected | 0.2361 | 0.4341 | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||||||||||
| c.3019A>T | S1007C 2D  AIThe SynGAP1 missense variant S1007C is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. Two tools—ESM1b and AlphaMissense‑Default—return uncertain results. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is uncertain, and Foldetta’s stability prediction is unavailable. Overall, the balance of evidence (four benign versus three pathogenic predictions, with high‑accuracy tools favoring benign) indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.671169 | Disordered | 0.925648 | Binding | 0.295 | 0.899 | 0.750 | -7.399 | In-Between | 0.487 | Ambiguous | Likely Benign | 0.132 | Likely Benign | -1.91 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.61 | Benign | 0.01 | Affected | 0.1405 | 0.5142 | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||||||
| c.301C>A | H101N 2D  AIThe SynGAP1 missense variant H101N is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is a majority vote of the benign‑predicted tools). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (derived from the same set of benign‑predicted tools) also indicates a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -3.598 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.104 | Likely Benign | -0.49 | Neutral | 0.659 | Possibly Damaging | 0.775 | Possibly Damaging | 4.20 | Benign | 0.00 | Affected | 0.1651 | 0.2994 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.301C>G | H101D 2D  AIThe SynGAP1 missense variant H101D is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports it as likely benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion is not in conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -2.788 | Likely Benign | 0.227 | Likely Benign | Likely Benign | 0.136 | Likely Benign | -0.49 | Neutral | 0.824 | Possibly Damaging | 0.840 | Possibly Damaging | 4.20 | Benign | 0.00 | Affected | 0.2479 | 0.2272 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.301C>T | H101Y 2D  AIThe SynGAP1 missense variant H101Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for H101Y, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -3.404 | Likely Benign | 0.123 | Likely Benign | Likely Benign | 0.099 | Likely Benign | -0.95 | Neutral | 0.659 | Possibly Damaging | 0.775 | Possibly Damaging | 4.15 | Benign | 0.00 | Affected | 0.0862 | 0.4004 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.3014G>C | S1005T 2D  AIThe SynGAP1 missense variant S1005T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -6.015 | Likely Benign | 0.454 | Ambiguous | Likely Benign | 0.120 | Likely Benign | -1.30 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.66 | Benign | 0.00 | Affected | 0.1600 | 0.4937 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3013A>T | S1005C 2D  AIThe SynGAP1 missense variant S1005C is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy consensus (SGM Consensus) derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN is inconclusive (two pathogenic vs. two benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of standard predictors lean toward pathogenicity, while the single high‑accuracy tool that is available (AlphaMissense‑Optimized) predicts benign, and the consensus tool is inconclusive. Thus, the variant is most likely pathogenic based on the prevailing predictions, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -8.519 | Likely Pathogenic | 0.640 | Likely Pathogenic | Likely Benign | 0.173 | Likely Benign | -2.20 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.59 | Benign | 0.00 | Affected | 0.1246 | 0.4492 | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||||||
| c.3008G>A | S1003N 2D  AIThe SynGAP1 missense variant S1003N is not reported in ClinVar (ClinVar status: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments are mixed: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑vs‑2 split, and Foldetta results are unavailable. Overall, more tools (five) predict pathogenicity than benign (three), and the high‑accuracy methods do not overturn this trend. Therefore, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because the variant has not been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -5.175 | Likely Benign | 0.889 | Likely Pathogenic | Ambiguous | 0.122 | Likely Benign | -1.37 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 0.1798 | 0.5029 | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||||||||||||
| c.3008G>C | S1003T 2D  AIThe SynGAP1 missense variant S1003T is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign, while the majority of pathogenic predictors—polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT—suggest a damaging impact. The AlphaMissense‑Default score is uncertain, and Foldetta stability analysis is unavailable. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments therefore lean toward a benign interpretation: AlphaMissense‑Optimized predicts benign, SGM‑Consensus is likely benign, and no Foldetta data is present. Overall, the computational evidence supports a benign classification, with no conflict with ClinVar status because no ClinVar assertion exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -5.140 | Likely Benign | 0.493 | Ambiguous | Likely Benign | 0.115 | Likely Benign | -1.04 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.51 | Benign | 0.00 | Affected | 0.1864 | 0.6227 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.3008G>T | S1003I 2D  AIThe SynGAP1 missense variant S1003I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy consensus methods give mixed results: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (two pathogenic, two benign votes); and Foldetta (combining FoldX‑MD and Rosetta) has no available output. Based on the overall distribution of predictions, the variant is most likely pathogenic. This assessment does not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -8.952 | Likely Pathogenic | 0.954 | Likely Pathogenic | Ambiguous | 0.189 | Likely Benign | -2.31 | Neutral | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.50 | Benign | 0.00 | Affected | 0.1294 | 0.5735 | -1 | -2 | 5.3 | 26.08 | ||||||||||||||||||||||||||||||||||||||||
| c.3009C>A | S1003R 2D  AIThe SynGAP1 missense variant S1003R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of evaluated tools (six pathogenic vs. three benign) indicate a pathogenic impact. This prediction aligns with the lack of ClinVar annotation and does not contradict any existing clinical classification. Thus, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -5.113 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.141 | Likely Benign | -1.88 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0.1151 | 0.3746 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||||||||
| c.3009C>G | S1003R 2D  AIThe SynGAP1 missense variant S1003R (ClinVar ID 1798770.0) is listed as Uncertain in ClinVar and is not present in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, and ESM1b, while pathogenic predictions are reported by polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessment shows AlphaMissense‑Optimized classifying the variant as pathogenic; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign, two pathogenic), and Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a pathogenic effect, which contrasts with the ClinVar designation of Uncertain. Thus, the variant is most likely pathogenic, contradicting the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | Uncertain | 1 | -5.113 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.141 | Likely Benign | -1.88 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0.1151 | 0.3746 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||||||
| c.3010C>A | H1004N 2D  AIThe SynGAP1 missense variant H1004N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this assessment, so the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.808535 | Disordered | 0.943707 | Binding | 0.271 | 0.901 | 0.750 | -4.265 | Likely Benign | 0.601 | Likely Pathogenic | Likely Benign | 0.072 | Likely Benign | -1.18 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.88 | Benign | 0.35 | Tolerated | 0.2056 | 0.3580 | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||||||
| c.3010C>G | H1004D 2D  AIThe SynGAP1 missense variant H1004D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for H1004D. This conclusion is not contradicted by ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.808535 | Disordered | 0.943707 | Binding | 0.271 | 0.901 | 0.750 | -5.275 | Likely Benign | 0.913 | Likely Pathogenic | Ambiguous | 0.148 | Likely Benign | -2.16 | Neutral | 0.997 | Probably Damaging | 0.994 | Probably Damaging | 2.78 | Benign | 0.29 | Tolerated | 0.2695 | 0.2530 | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||||||
| c.3010C>T | H1004Y 2D  AIThe SynGAP1 missense variant H1004Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.808535 | Disordered | 0.943707 | Binding | 0.271 | 0.901 | 0.750 | -5.196 | Likely Benign | 0.676 | Likely Pathogenic | Likely Benign | 0.131 | Likely Benign | -1.67 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.72 | Benign | 0.49 | Tolerated | 0.1175 | 0.5143 | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||||||
| c.3011A>C | H1004P 2D  AIThe SynGAP1 missense variant H1004P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, FATHMM, and the high‑accuracy AlphaMissense‑Optimized model. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, and polyPhen‑2 HumVar. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a benign majority vote. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation; there is no contradiction between the predictions and ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.808535 | Disordered | 0.943707 | Binding | 0.271 | 0.901 | 0.750 | -3.686 | Likely Benign | 0.460 | Ambiguous | Likely Benign | 0.236 | Likely Benign | -2.69 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.72 | Benign | 0.18 | Tolerated | 0.2076 | 0.4643 | 0 | -2 | 1.6 | -40.02 | ||||||||||||||||||||||||||||||||||||||||
| c.3011A>G | H1004R 2D  AIThe SynGAP1 missense variant H1004R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and no Foldetta stability data is available. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.808535 | Disordered | 0.943707 | Binding | 0.271 | 0.901 | 0.750 | -3.316 | Likely Benign | 0.813 | Likely Pathogenic | Ambiguous | 0.198 | Likely Benign | -1.88 | Neutral | 0.997 | Probably Damaging | 0.994 | Probably Damaging | 2.77 | Benign | 0.50 | Tolerated | 0.2097 | 0.3132 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||||||
| c.3011A>T | H1004L 2D  AIThe SynGAP1 missense variant H1004L is not reported in ClinVar (ClinVar status: none) and is absent from gnomAD (gnomAD ID: none). Prediction tools that agree on a benign effect include REVEL, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (a majority vote among AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (two pathogenic vs two benign votes). Foldetta, which would provide a protein‑folding stability estimate, has no available result for this variant. Overall, the balance of evidence leans toward a benign interpretation, and this conclusion does not contradict the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.808535 | Disordered | 0.943707 | Binding | 0.271 | 0.901 | 0.750 | -5.179 | Likely Benign | 0.716 | Likely Pathogenic | Likely Benign | 0.220 | Likely Benign | -3.14 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.75 | Benign | 0.55 | Tolerated | 0.1223 | 0.6026 | -2 | -3 | 7.0 | -23.98 | ||||||||||||||||||||||||||||||||||||||||
| c.3012C>A | H1004Q 2D  AIThe SynGAP1 missense variant H1004Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.808535 | Disordered | 0.943707 | Binding | 0.271 | 0.901 | 0.750 | -3.872 | Likely Benign | 0.853 | Likely Pathogenic | Ambiguous | 0.126 | Likely Benign | -1.55 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.78 | Benign | 0.71 | Tolerated | 3.77 | 5 | 0.1831 | 0.3832 | 0 | 3 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||||
| c.3013A>C | S1005R 2D  AIThe SynGAP1 missense variant S1005R has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign calls from REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign); pathogenic calls from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicting pathogenic, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates Likely Benign. Foldetta stability analysis is unavailable. Overall, the evidence is split, with an equal number of benign and pathogenic predictions and no ClinVar status to contradict. Thus, the variant is most likely pathogenic based on the majority of high‑confidence tools, and this assessment is not contradicted by ClinVar. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -3.300 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.135 | Likely Benign | -2.29 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.66 | Benign | 0.00 | Affected | 3.77 | 5 | 0.0994 | 0.2856 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||
| c.3020G>A | S1007N 2D  AIThe SynGAP1 missense variant S1007N is listed in ClinVar (ID 2759915.0) as Benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the majority of high‑accuracy predictors (including the SGM‑Consensus) indicate a benign impact, and the single uncertain AlphaMissense‑Optimized result does not overturn this consensus. Therefore, the variant is most likely benign, and this conclusion is consistent with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.925648 | Binding | 0.295 | 0.899 | 0.750 | Benign | 1 | -5.113 | Likely Benign | 0.803 | Likely Pathogenic | Ambiguous | 0.075 | Likely Benign | -1.54 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.65 | Benign | 0.01 | Affected | 3.77 | 5 | 0.1743 | 0.4342 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||
| c.3020G>T | S1007I 2D  AIThe SynGAP1 missense variant S1007I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect are REVEL and FATHMM, whereas a majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default) predict a pathogenic impact. The remaining tools, ESM1b and AlphaMissense‑Optimized, return uncertain results. High‑accuracy assessments further support a deleterious interpretation: the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as pathogenic; AlphaMissense‑Optimized remains uncertain, and Foldetta data are unavailable. Overall, the preponderance of evidence from both conventional and high‑accuracy predictors indicates that the S1007I variant is most likely pathogenic, with no conflict with ClinVar status because the variant has not yet been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.671169 | Disordered | 0.925648 | Binding | 0.295 | 0.899 | 0.750 | -7.800 | In-Between | 0.920 | Likely Pathogenic | Ambiguous | 0.126 | Likely Benign | -2.55 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.65 | Benign | 0.00 | Affected | 0.1324 | 0.4769 | -1 | -2 | 5.3 | 26.08 | ||||||||||||||||||||||||||||||||||||||||
| c.3021T>A | S1007R 2D  AIThe SynGAP1 missense variant S1007R has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign); pathogenic predictions include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, whereas the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign effect. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the evidence leans toward a pathogenic interpretation, with no ClinVar status to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.925648 | Binding | 0.295 | 0.899 | 0.750 | -5.441 | Likely Benign | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.219 | Likely Benign | -1.93 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.65 | Benign | 0.01 | Affected | 0.1121 | 0.3237 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||||
| c.302A>G | H101R 2D  AIThe SynGAP1 missense variant H101R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for H101R, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -2.685 | Likely Benign | 0.211 | Likely Benign | Likely Benign | 0.141 | Likely Benign | -0.76 | Neutral | 0.824 | Possibly Damaging | 0.840 | Possibly Damaging | 4.21 | Benign | 0.00 | Affected | 0.1891 | 0.2225 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||||||
| c.302A>T | H101L 2D  AIThe SynGAP1 missense variant H101L is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for H101L, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -1.376 | Likely Benign | 0.101 | Likely Benign | Likely Benign | 0.129 | Likely Benign | -1.33 | Neutral | 0.824 | Possibly Damaging | 0.840 | Possibly Damaging | 4.19 | Benign | 0.00 | Affected | 0.0924 | 0.4960 | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||||||
| c.3030T>A | F1010L 2D  AIThe SynGAP1 missense variant F1010L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a Likely Benign classification, and AlphaMissense‑Optimized is currently uncertain. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.741537 | Disordered | 0.912572 | Binding | 0.286 | 0.881 | 0.625 | -1.982 | Likely Benign | 0.949 | Likely Pathogenic | Ambiguous | 0.142 | Likely Benign | -1.51 | Neutral | 0.910 | Possibly Damaging | 0.468 | Possibly Damaging | 2.75 | Benign | 0.62 | Tolerated | 0.2538 | 0.3245 | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.3030T>G | F1010L 2D  AIThe SynGAP1 missense variant F1010L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a benign classification, and AlphaMissense‑Optimized is uncertain. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is not contradicted by ClinVar, which has no entry for F1010L. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.741537 | Disordered | 0.912572 | Binding | 0.286 | 0.881 | 0.625 | -1.982 | Likely Benign | 0.949 | Likely Pathogenic | Ambiguous | 0.142 | Likely Benign | -1.51 | Neutral | 0.910 | Possibly Damaging | 0.468 | Possibly Damaging | 2.75 | Benign | 0.62 | Tolerated | 0.2538 | 0.3245 | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||||||
| c.3031G>C | G1011R 2D  AIThe SynGAP1 missense variant G1011R is not reported in ClinVar and is absent from gnomAD, so no population frequency or clinical assertion data are available. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, and FATHMM, while pathogenic predictions arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.898380 | Binding | 0.332 | 0.869 | 0.625 | -4.650 | Likely Benign | 0.609 | Likely Pathogenic | Likely Benign | 0.118 | Likely Benign | -0.79 | Neutral | 0.642 | Possibly Damaging | 0.494 | Possibly Damaging | 2.72 | Benign | 0.01 | Affected | 3.77 | 5 | 0.1041 | 0.4415 | -2 | -3 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||||
| c.3032G>A | G1011E 2D  AIThe SynGAP1 missense variant G1011E is not reported in ClinVar and is absent from gnomAD, indicating no known population frequency data. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while those predicting a pathogenic outcome are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority vote) also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the collective predictions point to a benign effect, and this conclusion does not contradict the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.898380 | Binding | 0.332 | 0.869 | 0.625 | -3.870 | Likely Benign | 0.617 | Likely Pathogenic | Likely Benign | 0.091 | Likely Benign | -1.10 | Neutral | 0.642 | Possibly Damaging | 0.252 | Benign | 2.78 | Benign | 0.01 | Affected | 0.1692 | 0.4521 | 0 | -2 | -3.1 | 72.06 | |||||||||||||||||||||||||||||||||||||||
| c.3032G>T | G1011V 2D  AIThe SynGAP1 missense variant G1011V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.898380 | Binding | 0.332 | 0.869 | 0.625 | -4.883 | Likely Benign | 0.129 | Likely Benign | Likely Benign | 0.133 | Likely Benign | -1.21 | Neutral | 0.473 | Possibly Damaging | 0.192 | Benign | 2.71 | Benign | 0.01 | Affected | 0.1352 | 0.3434 | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||||||
| c.3034C>A | P1012T 2D  AIThe SynGAP1 missense variant P1012T is not reported in ClinVar and is absent from gnomAD, indicating no known population frequency data. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” classification. Foldetta results are not available, so they do not influence the assessment. Overall, the variant is most likely benign, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.894674 | Binding | 0.319 | 0.866 | 0.625 | -4.788 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.052 | Likely Benign | -0.56 | Neutral | 0.369 | Benign | 0.171 | Benign | 2.84 | Benign | 0.14 | Tolerated | 0.1399 | 0.5730 | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||||||
| c.3034C>G | P1012A 2D  AIThe SynGAP1 missense variant P1012A is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the consensus of all available predictions points to a benign impact, and this conclusion is consistent with the lack of a ClinVar classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.894674 | Binding | 0.319 | 0.866 | 0.625 | -4.038 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.035 | Likely Benign | -0.55 | Neutral | 0.112 | Benign | 0.084 | Benign | 2.81 | Benign | 0.26 | Tolerated | 0.3272 | 0.4900 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||||||
| c.3035C>A | P1012H 2D  AIThe SynGAP1 missense variant P1012H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for P1012H, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.894674 | Binding | 0.319 | 0.866 | 0.625 | -4.877 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.024 | Likely Benign | -0.38 | Neutral | 0.832 | Possibly Damaging | 0.600 | Possibly Damaging | 2.75 | Benign | 0.08 | Tolerated | 0.1553 | 0.4635 | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||||||
| c.3035C>G | P1012R 2D  AIThe SynGAP1 missense variant P1012R is not reported in ClinVar and is absent from gnomAD, indicating no documented population frequency. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool in the dataset indicates pathogenicity. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized reports benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a “Likely Benign” verdict. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, is not available for this variant. Overall, the consensus of all available predictions strongly supports a benign classification, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.894674 | Binding | 0.319 | 0.866 | 0.625 | -4.413 | Likely Benign | 0.104 | Likely Benign | Likely Benign | 0.034 | Likely Benign | 1.24 | Neutral | 0.000 | Benign | 0.002 | Benign | 2.89 | Benign | 0.88 | Tolerated | 0.1339 | 0.3083 | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||||||
| c.3035C>T | P1012L 2D  AIThe SynGAP1 missense variant P1012L is not reported in ClinVar and is absent from gnomAD. In silico prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. Foldetta results are unavailable. Overall, the variant is most likely benign, and this conclusion does not contradict the ClinVar status, which contains no pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.894674 | Binding | 0.319 | 0.866 | 0.625 | -4.363 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.014 | Likely Benign | -0.90 | Neutral | 0.224 | Benign | 0.131 | Benign | 2.76 | Benign | 0.12 | Tolerated | 0.2155 | 0.6388 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||||||
| c.3037T>A | S1013T 2D  AIThe SynGAP1 missense variant S1013T is not reported in ClinVar and is absent from gnomAD, indicating no documented population frequency. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict a benign effect. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign status. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of all available predictions is that the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.823549 | Disordered | 0.899570 | Binding | 0.308 | 0.846 | 0.625 | -4.245 | Likely Benign | 0.086 | Likely Benign | Likely Benign | 0.051 | Likely Benign | -0.96 | Neutral | 0.069 | Benign | 0.072 | Benign | 2.70 | Benign | 0.26 | Tolerated | 0.1812 | 0.6039 | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||||||
| c.302A>C | H101P 2D  AIThe SynGAP1 H101P missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -2.042 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.170 | Likely Benign | 0.89 | Neutral | 0.943 | Possibly Damaging | 0.924 | Probably Damaging | 4.17 | Benign | 0.00 | Affected | 0.1777 | 0.3589 | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||||||
| c.3029T>G | F1010C 2D  AIThe SynGAP1 missense variant F1010C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessment shows AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—remains inconclusive, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a pathogenic interpretation, with no ClinVar entry to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.741537 | Disordered | 0.912572 | Binding | 0.286 | 0.881 | 0.625 | -4.442 | Likely Benign | 0.755 | Likely Pathogenic | Likely Benign | 0.153 | Likely Benign | -2.31 | Neutral | 1.000 | Probably Damaging | 0.961 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 0.2646 | 0.1605 | -4 | -2 | -0.3 | -44.04 | ||||||||||||||||||||||||||||||||||||||||
| c.3021T>G | S1007R 2D  AISynGAP1 missense variant S1007R has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM‑Consensus (a majority vote of four high‑accuracy predictors) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the evidence is split, with an equal number of benign and pathogenic predictions. The variant is most likely pathogenic based on the presence of several high‑confidence pathogenic calls, and this assessment does not contradict ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.925648 | Binding | 0.295 | 0.899 | 0.750 | -5.441 | Likely Benign | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.219 | Likely Benign | -1.93 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.65 | Benign | 0.01 | Affected | 0.1121 | 0.3237 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||||||
| c.3022G>T | D1008Y 2D  AIThe SynGAP1 missense variant D1008Y is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM, whereas a majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default) predict a pathogenic impact; AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is a tie and thus unavailable, and Foldetta results are not provided. Overall, the balance of evidence favors a pathogenic classification, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.694846 | Disordered | 0.919416 | Binding | 0.280 | 0.899 | 0.625 | -5.371 | Likely Benign | 0.928 | Likely Pathogenic | Ambiguous | 0.237 | Likely Benign | -3.71 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.62 | Benign | 0.00 | Affected | 0.1043 | 0.6293 | -4 | -3 | 2.2 | 48.09 | ||||||||||||||||||||||||||||||||||||||||
| c.3023A>C | D1008A 2D  AIThe SynGAP1 D1008A variant is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Those that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is a 2‑vs‑2 tie, and Foldetta results are unavailable. Overall, more tools (five) predict pathogenicity than benign (three), and no ClinVar evidence contradicts this assessment. Thus, the variant is most likely pathogenic based on the available predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.694846 | Disordered | 0.919416 | Binding | 0.280 | 0.899 | 0.625 | -3.210 | Likely Benign | 0.861 | Likely Pathogenic | Ambiguous | 0.209 | Likely Benign | -2.65 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.69 | Benign | 0.03 | Affected | 0.4014 | 0.6444 | 0 | -2 | 5.3 | -44.01 | ||||||||||||||||||||||||||||||||||||||||
| c.3023A>T | D1008V 2D  AIThe SynGAP1 D1008V variant is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Those that predict a pathogenic effect comprise PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (two pathogenic vs. two benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, more tools (five) predict pathogenicity than benign (three), and the high‑accuracy assessments do not overturn this trend. Therefore, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.694846 | Disordered | 0.919416 | Binding | 0.280 | 0.899 | 0.625 | -4.828 | Likely Benign | 0.944 | Likely Pathogenic | Ambiguous | 0.242 | Likely Benign | -3.61 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.64 | Benign | 0.01 | Affected | 0.1447 | 0.6608 | -2 | -3 | 7.7 | -15.96 | ||||||||||||||||||||||||||||||||||||||||
| c.3025G>A | E1009K 2D  AIThe SynGAP1 missense variant E1009K is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of consensus tools (five pathogenic vs. three benign) indicate a pathogenic effect. Thus, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.728858 | Disordered | 0.914552 | Binding | 0.325 | 0.885 | 0.500 | -3.419 | Likely Benign | 0.897 | Likely Pathogenic | Ambiguous | 0.061 | Likely Benign | -1.90 | Neutral | 0.961 | Probably Damaging | 0.630 | Possibly Damaging | 2.41 | Pathogenic | 0.01 | Affected | 0.2511 | 0.7625 | 0 | 1 | -0.4 | -0.94 | ||||||||||||||||||||||||||||||||||||||||
| c.3026A>C | E1009A 2D  AIThe SynGAP1 missense variant E1009A is listed in ClinVar (ID 2238288.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Pathogenic.” High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely pathogenic, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) results are unavailable. Overall, the majority of predictions (six pathogenic vs. three benign) lean toward a pathogenic impact, and this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.728858 | Disordered | 0.914552 | Binding | 0.325 | 0.885 | 0.500 | Uncertain | 1 | -3.118 | Likely Benign | 0.679 | Likely Pathogenic | Likely Benign | 0.109 | Likely Benign | -3.06 | Deleterious | 0.980 | Probably Damaging | 0.630 | Possibly Damaging | 2.39 | Pathogenic | 0.01 | Affected | 3.77 | 5 | 0.3959 | 0.7153 | 0 | -1 | 5.3 | -58.04 | |||||||||||||||||||||||||||||||||||
Found 8751 rows. Show 800 rows per page. Page 2/11 « Previous | Next »