

Table of SynGAP1 Isoform α2 (UniProt Q96PV0-1) Missense Variants.
| c.dna | Variant | SGM Consensus | Domain | IUPred2 | ANCHOR2 | AlphaFold | MobiDB | ClinVar | gnomAD | ESM1b | AlphaMissense | REVEL | PSMutPred | FoldX | Rosetta | Foldetta | PremPS | PROVEAN | PolyPhen-2 HumDiv | PolyPhen-2 HumVar | FATHMM | SIFT | PAM | Physical | SASA | Normalized B-factor backbone | Normalized B-factor sidechain | SynGAP Structural Annotation | DOI | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Prediction | Score | Prediction | pLDDT | disorder | disorder | Clinical Status | Review | Subm. | ID | Allele count | Allele freq. | LLR score | Prediction | Pathogenicity | Class | Optimized | Score | Prediction | IP RF | SP RF | Prediction | Average ΔΔG | Prediction | StdDev | ΔΔG | Prediction | ΔΔG | Prediction | ΔΔG | Prediction | Score | Prediction | pph2_prob | Prediction | pph2_prob | Prediction | Nervous System Score | Prediction | Prediction | Status | Conservation | Sequences | PAM250 | PAM120 | Hydropathy Δ | MW Δ | Average | Δ | Δ | StdDev | Δ | StdDev | Secondary | Tertiary bonds | Inside out | GAP-Ras interface | At membrane | No effect | MD Alert | Verdict | Description | |||||
| c.225G>C | E75D 2D  AIThe SynGAP1 missense variant E75D is reported in gnomAD (6‑33425833‑G‑C) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is “Likely Benign.” No Foldetta stability prediction is available. Overall, the preponderance of evidence indicates that E75D is most likely benign, and this conclusion is not contradicted by ClinVar status, which is currently absent. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.595080 | Disordered | 0.443881 | Uncertain | 0.303 | 0.822 | 0.500 | 6-33425833-G-C | 1 | 6.20e-7 | -3.710 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.058 | Likely Benign | 0.2026 | 0.3833 | -0.27 | Neutral | 0.001 | Benign | 0.000 | Benign | 4.25 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 3 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||
| c.225G>T | E75D 2D  AIThe SynGAP1 missense variant E75D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.595080 | Disordered | 0.443881 | Uncertain | 0.303 | 0.822 | 0.500 | -3.710 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.058 | Likely Benign | 0.2026 | 0.3833 | -0.27 | Neutral | 0.001 | Benign | 0.000 | Benign | 4.25 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 3 | 0.0 | -14.03 | |||||||||||||||||||||||||||||||||
| c.226T>A | S76T 2D  AIThe SynGAP1 missense variant S76T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.444487 | Uncertain | 0.279 | 0.826 | 0.500 | -4.000 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.038 | Likely Benign | 0.1117 | 0.4774 | -0.73 | Neutral | 0.805 | Possibly Damaging | 0.483 | Possibly Damaging | 3.78 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.226T>C | S76P 2D  AIThe SynGAP1 missense variant S76P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect for S76P, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.444487 | Uncertain | 0.279 | 0.826 | 0.500 | -3.833 | Likely Benign | 0.076 | Likely Benign | Likely Benign | 0.058 | Likely Benign | 0.1790 | 0.4138 | -1.64 | Neutral | 0.909 | Possibly Damaging | 0.665 | Possibly Damaging | 3.77 | Benign | 0.00 | Affected | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||
| c.226T>G | S76A 2D  AIThe SynGAP1 missense variant S76A is reported in gnomAD (ID 6‑33425834‑T‑G) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.444487 | Uncertain | 0.279 | 0.826 | 0.500 | 6-33425834-T-G | 1 | 6.20e-7 | -3.230 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.048 | Likely Benign | 0.4543 | 0.3619 | -1.10 | Neutral | 0.643 | Possibly Damaging | 0.277 | Benign | 3.86 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | 2.6 | -16.00 | ||||||||||||||||||||||||||||||
| c.227C>A | S76Y 2D  AIThe SynGAP1 missense variant S76Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for S76Y, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.444487 | Uncertain | 0.279 | 0.826 | 0.500 | -4.243 | Likely Benign | 0.209 | Likely Benign | Likely Benign | 0.119 | Likely Benign | 0.0540 | 0.4753 | -2.35 | Neutral | 0.972 | Probably Damaging | 0.831 | Possibly Damaging | 3.71 | Benign | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||
| c.227C>G | S76C 2D  AIThe SynGAP1 missense variant S76C is listed in ClinVar with an “Uncertain” status (ClinVar ID 1951273.0) and is present in the gnomAD database (gnomAD ID 6‑33425835‑C‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as benign; Foldetta results are not available for this variant. Overall, the majority of computational evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.444487 | Uncertain | 0.279 | 0.826 | 0.500 | Uncertain | 1 | 6-33425835-C-G | 2 | 1.24e-6 | -5.408 | Likely Benign | 0.100 | Likely Benign | Likely Benign | 0.076 | Likely Benign | 0.0807 | 0.4787 | -1.78 | Neutral | 0.992 | Probably Damaging | 0.869 | Possibly Damaging | 3.71 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||
| c.227C>T | S76F 2D  AIThe SynGAP1 missense variant S76F is reported in ClinVar as “Not submitted” and is present in gnomAD (variant ID 6-33425835-C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently contains no pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.517562 | Disordered | 0.444487 | Uncertain | 0.279 | 0.826 | 0.500 | 6-33425835-C-T | 4 | 2.48e-6 | -4.557 | Likely Benign | 0.197 | Likely Benign | Likely Benign | 0.082 | Likely Benign | 0.0501 | 0.4887 | -2.40 | Neutral | 0.972 | Probably Damaging | 0.831 | Possibly Damaging | 3.71 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -3 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||
| c.2291A>T | N764I 2D  AIThe SynGAP1 missense variant N764I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Those that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is a 2‑vs‑2 tie, and Foldetta results are unavailable. Overall, more tools predict pathogenicity (five) than benignity (three), and no ClinVar evidence contradicts this assessment. Thus, the variant is most likely pathogenic based on the available predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.380708 | Structured | 0.919527 | Binding | 0.305 | 0.861 | 0.250 | -6.879 | Likely Benign | 0.883 | Likely Pathogenic | Ambiguous | 0.115 | Likely Benign | 0.0581 | 0.4483 | -2.58 | Deleterious | 0.906 | Possibly Damaging | 0.679 | Possibly Damaging | 2.58 | Benign | 0.00 | Affected | -2 | -3 | 8.0 | -0.94 | ||||||||||||||||||||||||||||||||||||
| c.2297C>A | S766Y 2D  AIThe SynGAP1 missense variant S766Y is reported in gnomAD (ID 6‑33442455‑C‑A) but has no ClinVar entry. Functional prediction tools show mixed results: benign calls come from REVEL, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves as Likely Pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts benign, but the SGM‑Consensus (majority vote) predicts pathogenic, and no Foldetta stability data are available. Overall, the majority of evidence points to a pathogenic impact, and this conclusion does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.405110 | Structured | 0.923125 | Binding | 0.338 | 0.874 | 0.250 | 6-33442455-C-A | -8.636 | Likely Pathogenic | 0.641 | Likely Pathogenic | Likely Benign | 0.222 | Likely Benign | 0.0794 | 0.5609 | -2.67 | Deleterious | 0.990 | Probably Damaging | 0.856 | Possibly Damaging | 4.09 | Benign | 0.00 | Affected | 3.64 | 6 | -2 | -3 | -0.5 | 76.10 | ||||||||||||||||||||||||||||||||
| c.2297C>G | S766C 2D  AIThe SynGAP1 missense variant S766C is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. ESM1b is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for S766C, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.405110 | Structured | 0.923125 | Binding | 0.338 | 0.874 | 0.250 | -7.681 | In-Between | 0.326 | Likely Benign | Likely Benign | 0.192 | Likely Benign | 0.1049 | 0.6110 | -2.02 | Neutral | 0.997 | Probably Damaging | 0.889 | Possibly Damaging | 4.07 | Benign | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2297C>T | S766F 2D  AIThe SynGAP1 missense variant S766F is listed in gnomAD (ID 6‑33442455‑C‑T) but has no ClinVar entry. Functional prediction tools show a split verdict: benign calls come from REVEL, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Pathogenic.” High‑accuracy assessments further reveal AlphaMissense‑Optimized as benign, while the SGM‑Consensus remains pathogenic; Foldetta results are unavailable. Overall, the majority of evidence points toward a pathogenic effect, and this conclusion does not contradict ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.405110 | Structured | 0.923125 | Binding | 0.338 | 0.874 | 0.250 | 6-33442455-C-T | -8.944 | Likely Pathogenic | 0.709 | Likely Pathogenic | Likely Benign | 0.233 | Likely Benign | 0.0770 | 0.5887 | -2.87 | Deleterious | 0.990 | Probably Damaging | 0.856 | Possibly Damaging | 4.08 | Benign | 0.00 | Affected | 3.64 | 6 | -2 | -3 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||||
| c.229A>C | S77R 2D  AIThe SynGAP1 missense variant S77R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority of the high‑accuracy tools) also indicates Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the collective evidence points to a benign effect for S77R, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.446124 | Uncertain | 0.310 | 0.855 | 0.375 | -2.823 | Likely Benign | 0.482 | Ambiguous | Likely Benign | 0.011 | Likely Benign | 0.0752 | 0.3247 | -1.22 | Neutral | 0.198 | Benign | 0.015 | Benign | 4.09 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.229A>G | S77G 2D  AIThe SynGAP1 missense variant S77G is reported in gnomAD (variant ID 6‑33425837‑A‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods points to a benign impact for S77G. This conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.446124 | Uncertain | 0.310 | 0.855 | 0.375 | 6-33425837-A-G | 2 | 1.24e-6 | -3.571 | Likely Benign | 0.064 | Likely Benign | Likely Benign | 0.014 | Likely Benign | 0.2204 | 0.3866 | -1.23 | Neutral | 0.022 | Benign | 0.003 | Benign | 4.09 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 1 | 0.4 | -30.03 | ||||||||||||||||||||||||||||||
| c.229A>T | S77C 2D  AIThe SynGAP1 missense variant S77C is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools (polyPhen‑2 HumDiv and SIFT) predict pathogenicity, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.446124 | Uncertain | 0.310 | 0.855 | 0.375 | -5.549 | Likely Benign | 0.061 | Likely Benign | Likely Benign | 0.022 | Likely Benign | 0.0954 | 0.5144 | -0.94 | Neutral | 0.953 | Possibly Damaging | 0.129 | Benign | 4.04 | Benign | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.22A>C | I8L 2D 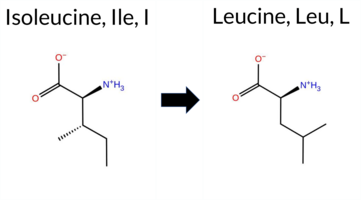 AIThe SynGAP1 missense variant I8L is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote from the four high‑accuracy tools) is benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that the I8L variant is most likely benign, and this conclusion does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.543080 | Binding | 0.341 | 0.916 | 0.625 | -1.752 | Likely Benign | 0.083 | Likely Benign | Likely Benign | 0.124 | Likely Benign | 0.0707 | 0.3776 | 0.08 | Neutral | 0.002 | Benign | 0.000 | Benign | 4.20 | Benign | 0.00 | Affected | 2 | 2 | -0.7 | 0.00 | |||||||||||||||||||||||||||||||||||
| c.22A>G | I8V 2D 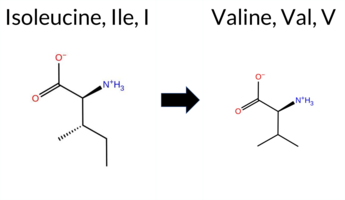 AIThe SynGAP1 missense variant I8V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.543080 | Binding | 0.341 | 0.916 | 0.625 | -2.723 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.122 | Likely Benign | 0.1031 | 0.3470 | -0.01 | Neutral | 0.005 | Benign | 0.001 | Benign | 4.22 | Benign | 0.00 | Affected | 4 | 3 | -0.3 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.22A>T | I8F 2D 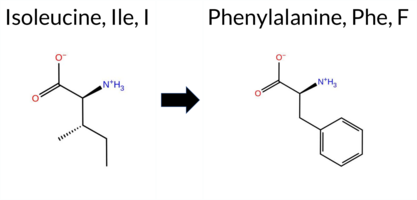 AIThe SynGAP1 missense variant I8F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.543080 | Binding | 0.341 | 0.916 | 0.625 | -3.000 | Likely Benign | 0.092 | Likely Benign | Likely Benign | 0.116 | Likely Benign | 0.0483 | 0.2871 | -0.29 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.02 | Benign | 0.00 | Affected | 1 | 0 | -1.7 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.2306T>A | L769H 2D  AIThe SynGAP1 missense variant L769H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, all of which classify the variant as benign. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT, all of which report the variant as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.411940 | Structured | 0.928432 | Binding | 0.367 | 0.883 | 0.250 | -6.038 | Likely Benign | 0.422 | Ambiguous | Likely Benign | 0.235 | Likely Benign | 0.1016 | 0.0828 | -1.96 | Neutral | 0.977 | Probably Damaging | 0.721 | Possibly Damaging | 3.90 | Benign | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||
| c.2306T>C | L769P 2D  AIThe SynGAP1 missense variant L769P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign impact for this variant, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.411940 | Structured | 0.928432 | Binding | 0.367 | 0.883 | 0.250 | -6.261 | Likely Benign | 0.338 | Likely Benign | Likely Benign | 0.200 | Likely Benign | 0.3573 | 0.1323 | -1.75 | Neutral | 0.925 | Possibly Damaging | 0.427 | Benign | 3.90 | Benign | 0.00 | Affected | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||||||
| c.2306T>G | L769R 2D  AIThe SynGAP1 missense variant L769R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus as likely benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) is unavailable for this variant. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.411940 | Structured | 0.928432 | Binding | 0.367 | 0.883 | 0.250 | -6.245 | Likely Benign | 0.493 | Ambiguous | Likely Benign | 0.206 | Likely Benign | 0.1286 | 0.0702 | -1.66 | Neutral | 0.003 | Benign | 0.006 | Benign | 3.91 | Benign | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||
| c.230G>A | S77N 2D  AIThe SynGAP1 missense variant S77N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.446124 | Uncertain | 0.310 | 0.855 | 0.375 | -4.597 | Likely Benign | 0.101 | Likely Benign | Likely Benign | 0.063 | Likely Benign | 0.1088 | 0.3774 | -0.78 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.09 | Benign | 0.00 | Affected | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||
| c.230G>C | S77T 2D  AIThe SynGAP1 missense variant S77T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority vote) also indicates Likely Benign. Foldetta results are not available, so they do not influence the assessment. Overall, the majority of computational evidence points to a benign effect, and this is consistent with the lack of any ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.446124 | Uncertain | 0.310 | 0.855 | 0.375 | -4.204 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.058 | Likely Benign | 0.1180 | 0.5003 | -0.03 | Neutral | 0.092 | Benign | 0.007 | Benign | 4.19 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.230G>T | S77I 2D  AIThe SynGAP1 missense variant S77I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar entry to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.446124 | Uncertain | 0.310 | 0.855 | 0.375 | -3.918 | Likely Benign | 0.140 | Likely Benign | Likely Benign | 0.038 | Likely Benign | 0.0758 | 0.5165 | -1.41 | Neutral | 0.604 | Possibly Damaging | 0.029 | Benign | 4.07 | Benign | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||
| c.231T>A | S77R 2D  AIThe SynGAP1 missense variant S77R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority of the high‑accuracy tools) is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.446124 | Uncertain | 0.310 | 0.855 | 0.375 | -2.823 | Likely Benign | 0.482 | Ambiguous | Likely Benign | 0.026 | Likely Benign | 0.0752 | 0.3247 | -1.22 | Neutral | 0.198 | Benign | 0.015 | Benign | 4.09 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.231T>G | S77R 2D  AIThe SynGAP1 missense variant S77R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority of the high‑accuracy tools) is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.446124 | Uncertain | 0.310 | 0.855 | 0.375 | -2.823 | Likely Benign | 0.482 | Ambiguous | Likely Benign | 0.026 | Likely Benign | 0.0752 | 0.3247 | -1.22 | Neutral | 0.198 | Benign | 0.015 | Benign | 4.09 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.232C>A | R78S 2D  AIThe SynGAP1 missense variant R78S is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized returns an uncertain result. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments are: AlphaMissense‑Optimized (Uncertain), SGM‑Consensus (Likely Benign), and Foldetta (no data available). Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict the ClinVar status, which contains no pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.505461 | Disordered | 0.448183 | Uncertain | 0.304 | 0.866 | 0.500 | -3.680 | Likely Benign | 0.792 | Likely Pathogenic | Ambiguous | 0.063 | Likely Benign | 0.3081 | 0.3274 | -1.11 | Neutral | 0.385 | Benign | 0.015 | Benign | 3.86 | Benign | 0.00 | Affected | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||
| c.232C>G | R78G 2D  AIThe SynGAP1 R78G missense variant has no ClinVar entry and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign status: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Taken together, the preponderance of evidence from both general and high‑accuracy predictors indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.505461 | Disordered | 0.448183 | Uncertain | 0.304 | 0.866 | 0.500 | -3.972 | Likely Benign | 0.480 | Ambiguous | Likely Benign | 0.072 | Likely Benign | 0.3285 | 0.2986 | -1.94 | Neutral | 0.385 | Benign | 0.028 | Benign | 3.83 | Benign | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||
| c.232C>T | R78C 2D  AIThe SynGAP1 missense variant R78C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for R78C, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.505461 | Disordered | 0.448183 | Uncertain | 0.304 | 0.866 | 0.500 | -6.079 | Likely Benign | 0.467 | Ambiguous | Likely Benign | 0.114 | Likely Benign | 0.3255 | 0.2804 | -2.13 | Neutral | 0.991 | Probably Damaging | 0.194 | Benign | 3.80 | Benign | 0.00 | Affected | -4 | -3 | 7.0 | -53.05 | |||||||||||||||||||||||||||||||||||
| c.2330T>A | L777H 2D  AIThe SynGAP1 missense variant L777H has no ClinVar record and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized, while those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors a benign outcome. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the balance of evidence—including the consensus of high‑accuracy tools—suggests that the variant is most likely benign, and this assessment does not contradict the ClinVar status, which currently contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.408655 | Structured | 0.876129 | Binding | 0.336 | 0.882 | 0.250 | -6.719 | Likely Benign | 0.492 | Ambiguous | Likely Benign | 0.230 | Likely Benign | 0.1052 | 0.0972 | -2.51 | Deleterious | 0.997 | Probably Damaging | 0.993 | Probably Damaging | 3.95 | Benign | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | ||||||||||||||||||||||||||||||||||||
| c.2330T>C | L777P 2D  AIThe SynGAP1 missense variant L777P is catalogued in gnomAD (6‑33442488‑T‑C) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Pathogenic predictions are reported by polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments reinforce the benign trend: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus also indicates a likely benign outcome. No Foldetta stability analysis is available, so it does not influence the assessment. Overall, the preponderance of evidence points to a benign effect for the variant, and this conclusion is not contradicted by any ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.408655 | Structured | 0.876129 | Binding | 0.336 | 0.882 | 0.250 | 6-33442488-T-C | -5.807 | Likely Benign | 0.361 | Ambiguous | Likely Benign | 0.255 | Likely Benign | 0.3732 | 0.1664 | -2.13 | Neutral | 0.991 | Probably Damaging | 0.985 | Probably Damaging | 3.94 | Benign | 0.00 | Affected | 3.64 | 6 | -3 | -3 | -5.4 | -16.04 | ||||||||||||||||||||||||||||||||
| c.2330T>G | L777R 2D  AIThe SynGAP1 missense variant L777R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.408655 | Structured | 0.876129 | Binding | 0.336 | 0.882 | 0.250 | -6.084 | Likely Benign | 0.650 | Likely Pathogenic | Likely Benign | 0.227 | Likely Benign | 0.1249 | 0.0846 | -2.20 | Neutral | 0.991 | Probably Damaging | 0.985 | Probably Damaging | 3.97 | Benign | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||
| c.233G>A | R78H 2D  AIThe SynGAP1 R78H missense variant has no ClinVar entry and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. In contrast, polyPhen‑2 HumDiv and SIFT predict a pathogenic impact. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.505461 | Disordered | 0.448183 | Uncertain | 0.304 | 0.866 | 0.500 | -4.851 | Likely Benign | 0.250 | Likely Benign | Likely Benign | 0.070 | Likely Benign | 0.2453 | 0.1601 | -1.38 | Neutral | 0.908 | Possibly Damaging | 0.108 | Benign | 3.83 | Benign | 0.00 | Affected | 2 | 0 | 1.3 | -19.05 | |||||||||||||||||||||||||||||||||||
| c.233G>C | R78P 2D  AIThe SynGAP1 missense variant R78P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus, REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for R78P, and this conclusion does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.505461 | Disordered | 0.448183 | Uncertain | 0.304 | 0.866 | 0.500 | -4.049 | Likely Benign | 0.611 | Likely Pathogenic | Likely Benign | 0.115 | Likely Benign | 0.2083 | 0.3750 | -1.72 | Neutral | 0.817 | Possibly Damaging | 0.123 | Benign | 3.82 | Benign | 0.00 | Affected | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||||
| c.233G>T | R78L 2D  AIThe SynGAP1 missense variant R78L is listed in ClinVar (ID 3390541.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus score (which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.505461 | Disordered | 0.448183 | Uncertain | 0.304 | 0.866 | 0.500 | Uncertain | 1 | -3.389 | Likely Benign | 0.635 | Likely Pathogenic | Likely Benign | 0.062 | Likely Benign | 0.1445 | 0.4276 | -1.59 | Neutral | 0.385 | Benign | 0.021 | Benign | 3.84 | Benign | 0.00 | Affected | -3 | -2 | 8.3 | -43.03 | |||||||||||||||||||||||||||||||||
| c.2344G>C | D782H 2D  AIThe SynGAP1 missense variant D782H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score (Likely Pathogenic). Only REVEL predicts a benign outcome, while AlphaMissense‑Optimized is uncertain. High‑accuracy assessments show the SGM‑Consensus as Likely Pathogenic, whereas AlphaMissense‑Optimized remains inconclusive and Foldetta data are unavailable. Taken together, the majority of evidence supports a pathogenic interpretation, and this is consistent with the absence of a ClinVar assertion. Therefore, the variant is most likely pathogenic, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.768342 | Binding | 0.285 | 0.883 | 0.625 | -8.528 | Likely Pathogenic | 0.937 | Likely Pathogenic | Ambiguous | 0.311 | Likely Benign | 0.1333 | 0.7286 | -2.63 | Deleterious | 1.000 | Probably Damaging | 0.989 | Probably Damaging | 1.93 | Pathogenic | 0.00 | Affected | 1 | -1 | 0.3 | 22.05 | |||||||||||||||||||||||||||||||||||
| c.2344G>T | D782Y 2D 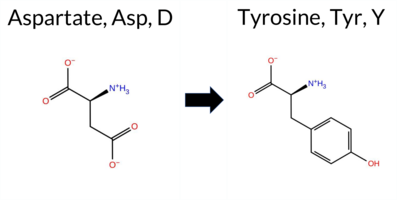 AIThe SynGAP1 missense variant D782Y is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools largely converge on a deleterious effect: pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default, while only REVEL predicts a benign outcome. High‑accuracy assessments reinforce this trend: AlphaMissense‑Optimized is uncertain, but the SGM‑Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is classified as Likely Pathogenic. Foldetta, a protein‑folding stability predictor that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.768342 | Binding | 0.285 | 0.883 | 0.625 | -8.785 | Likely Pathogenic | 0.919 | Likely Pathogenic | Ambiguous | 0.382 | Likely Benign | 0.0559 | 0.6202 | -3.75 | Deleterious | 1.000 | Probably Damaging | 0.989 | Probably Damaging | 1.91 | Pathogenic | 0.00 | Affected | -4 | -3 | 2.2 | 48.09 | |||||||||||||||||||||||||||||||||||
| c.2345A>T | D782V 2D  AIThe SynGAP1 missense variant D782V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (which reports “Likely Pathogenic”). The high‑accuracy AlphaMissense‑Optimized tool yields an uncertain result, and the Foldetta stability assessment is unavailable. Overall, the consensus of the available predictions strongly favors a pathogenic effect for D782V. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical database status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.768342 | Binding | 0.285 | 0.883 | 0.625 | -8.250 | Likely Pathogenic | 0.931 | Likely Pathogenic | Ambiguous | 0.462 | Likely Benign | 0.0803 | 0.6477 | -3.59 | Deleterious | 0.999 | Probably Damaging | 0.979 | Probably Damaging | 1.92 | Pathogenic | 0.00 | Affected | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||
| c.2353C>G | R785G 2D  AIThe SynGAP1 missense variant R785G is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split: benign predictions come from REVEL, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic predictions are reported by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates a likely pathogenic outcome. High‑accuracy assessments further reveal that AlphaMissense‑Optimized predicts a benign effect, while the SGM‑Consensus again suggests pathogenicity; Foldetta stability analysis is not available for this variant. Overall, the majority of predictions lean toward pathogenicity, and this is consistent with the SGM‑Consensus result. Because the variant is not present in ClinVar, there is no existing clinical classification to contradict; thus, based on the available computational evidence, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.859585 | Disordered | 0.681730 | Binding | 0.325 | 0.896 | 0.625 | -3.684 | Likely Benign | 0.697 | Likely Pathogenic | Likely Benign | 0.190 | Likely Benign | 0.3695 | 0.3686 | -3.44 | Deleterious | 0.980 | Probably Damaging | 0.818 | Possibly Damaging | 2.25 | Pathogenic | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | ||||||||||||||||||||||||||||||||||
| c.2353C>T | R785C 2D  AIThe SynGAP1 R785C missense variant is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33442905‑C‑T). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—indicates a likely pathogenic outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points toward a pathogenic impact, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.859585 | Disordered | 0.681730 | Binding | 0.325 | 0.896 | 0.625 | Uncertain | 1 | 6-33442905-C-T | 29 | 1.80e-5 | -5.887 | Likely Benign | 0.662 | Likely Pathogenic | Likely Benign | 0.126 | Likely Benign | 0.3775 | 0.3530 | -5.06 | Deleterious | 0.144 | Benign | 0.046 | Benign | 2.22 | Pathogenic | 0.00 | Affected | 3.64 | 6 | -4 | -3 | 7.0 | -53.05 | |||||||||||||||||||||||||||
| c.2356C>A | L786I 2D  AIThe SynGAP1 missense variant L786I is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence, especially from the high‑accuracy methods, points to a benign impact. This conclusion is not contradicted by ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.882776 | Disordered | 0.655253 | Binding | 0.341 | 0.895 | 0.750 | -5.464 | Likely Benign | 0.215 | Likely Benign | Likely Benign | 0.087 | Likely Benign | 0.1056 | 0.4199 | -1.12 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 1.93 | Pathogenic | 0.00 | Affected | 2 | 2 | 0.7 | 0.00 | ||||||||||||||||||||||||||||||||||
| c.2356C>G | L786V 2D  AIThe SynGAP1 missense variant L786V is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact for this variant, and this conclusion does not contradict the ClinVar status, which currently has no classification for L786V. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.882776 | Disordered | 0.655253 | Binding | 0.341 | 0.895 | 0.750 | -4.607 | Likely Benign | 0.310 | Likely Benign | Likely Benign | 0.099 | Likely Benign | 0.1683 | 0.4049 | -1.66 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.00 | Pathogenic | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | ||||||||||||||||||||||||||||||||||
| c.2356C>T | L786F 2D  AIThe SynGAP1 missense variant L786F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas a majority of tools (SGM‑Consensus, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely pathogenic outcome; Foldetta results are unavailable. Overall, the balance of evidence leans toward pathogenicity, and this conclusion does not contradict the ClinVar status, which currently contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.882776 | Disordered | 0.655253 | Binding | 0.341 | 0.895 | 0.750 | -4.949 | Likely Benign | 0.578 | Likely Pathogenic | Likely Benign | 0.112 | Likely Benign | 0.0765 | 0.3847 | -2.53 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.83 | Pathogenic | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | ||||||||||||||||||||||||||||||||||
| c.2357T>A | L786H 2D  AIThe SynGAP1 missense variant L786H is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools show a split: benign calls come from REVEL and ESM1b, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score. Grouping by agreement, two tools predict benign, seven predict pathogenic, and AlphaMissense‑Optimized remains uncertain. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is inconclusive, but the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a pathogenic impact, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.882776 | Disordered | 0.655253 | Binding | 0.341 | 0.895 | 0.750 | -5.892 | Likely Benign | 0.836 | Likely Pathogenic | Ambiguous | 0.168 | Likely Benign | 0.1309 | 0.1214 | -3.72 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.79 | Pathogenic | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | ||||||||||||||||||||||||||||||||||
| c.2357T>C | L786P 2D  AISynGAP1 missense variant L786P is reported in gnomAD (ID 6‑33442909‑T‑C) but has no ClinVar entry. Functional prediction tools show discordant results: benign predictions come from REVEL, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further highlight this split: AlphaMissense‑Optimized reports a benign effect, SGM‑Consensus confirms a likely pathogenic outcome, and Foldetta results are unavailable. Overall, the majority of conventional tools and the SGM‑Consensus support a pathogenic interpretation, while one high‑accuracy tool suggests benign. No ClinVar status is present, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.882776 | Disordered | 0.655253 | Binding | 0.341 | 0.895 | 0.750 | 6-33442909-T-C | -3.217 | Likely Benign | 0.656 | Likely Pathogenic | Likely Benign | 0.219 | Likely Benign | 0.3343 | 0.1814 | -4.06 | Deleterious | 0.999 | Probably Damaging | 0.999 | Probably Damaging | 1.79 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||
| c.2357T>G | L786R 2D  AIThe SynGAP1 missense variant L786R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score. AlphaMissense‑Optimized is uncertain, and no Foldetta stability assessment is available. The high‑accuracy consensus from SGM (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) favors pathogenicity, and the lack of a Foldetta result does not alter this conclusion. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because the variant has not yet been catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.882776 | Disordered | 0.655253 | Binding | 0.341 | 0.895 | 0.750 | -4.989 | Likely Benign | 0.842 | Likely Pathogenic | Ambiguous | 0.169 | Likely Benign | 0.1403 | 0.1288 | -3.07 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.79 | Pathogenic | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | ||||||||||||||||||||||||||||||||||
| c.235A>C | N79H 2D  AIThe SynGAP1 missense variant N79H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar classification because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.457064 | Uncertain | 0.290 | 0.876 | 0.375 | -2.090 | Likely Benign | 0.110 | Likely Benign | Likely Benign | 0.045 | Likely Benign | 0.1247 | 0.4718 | -0.72 | Neutral | 0.939 | Possibly Damaging | 0.114 | Benign | 4.16 | Benign | 0.00 | Affected | 2 | 1 | 0.3 | 23.04 | |||||||||||||||||||||||||||||||||||
| c.235A>G | N79D 2D  AIThe SynGAP1 missense variant N79D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.457064 | Uncertain | 0.290 | 0.876 | 0.375 | -3.746 | Likely Benign | 0.281 | Likely Benign | Likely Benign | 0.021 | Likely Benign | 0.1893 | 0.2535 | -0.92 | Neutral | 0.371 | Benign | 0.018 | Benign | 4.19 | Benign | 0.00 | Affected | 2 | 1 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||
| c.235A>T | N79Y 2D  AIThe SynGAP1 missense variant N79Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.457064 | Uncertain | 0.290 | 0.876 | 0.375 | -2.844 | Likely Benign | 0.250 | Likely Benign | Likely Benign | 0.039 | Likely Benign | 0.0510 | 0.4389 | -1.35 | Neutral | 0.939 | Possibly Damaging | 0.114 | Benign | 4.13 | Benign | 0.00 | Affected | -2 | -2 | 2.2 | 49.07 | |||||||||||||||||||||||||||||||||||
| c.2363C>A | S788Y 2D  AIThe SynGAP1 missense variant S788Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD. Prediction tools that agree on a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score—consistently predict a pathogenic or likely pathogenic impact. High‑accuracy assessments show that the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely pathogenic effect, AlphaMissense‑Optimized is uncertain (treated as unavailable), and Foldetta results are not provided. Based on the collective predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.956248 | Disordered | 0.573557 | Binding | 0.349 | 0.895 | 0.750 | -8.745 | Likely Pathogenic | 0.795 | Likely Pathogenic | Ambiguous | 0.251 | Likely Benign | 0.0844 | 0.5551 | -4.56 | Deleterious | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | ||||||||||||||||||||||||||||||||||
| c.2363C>G | S788C 2D  AIThe SynGAP1 missense variant S788C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are REVEL and AlphaMissense‑Optimized, while five tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM) predict a pathogenic outcome. Two tools (ESM1b and AlphaMissense‑Default) return uncertain results. High‑accuracy assessments show AlphaMissense‑Optimized as benign, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—classifies the variant as pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion does not contradict any ClinVar status because the variant has not yet been classified in that database. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.956248 | Disordered | 0.573557 | Binding | 0.349 | 0.895 | 0.750 | -7.935 | In-Between | 0.472 | Ambiguous | Likely Benign | 0.269 | Likely Benign | 0.1393 | 0.6073 | -3.90 | Deleterious | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2363C>T | S788F 2D  AIThe SynGAP1 missense variant S788F is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL and AlphaMissense‑Optimized, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score (Likely Pathogenic). ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates likely pathogenic; Foldetta stability analysis is unavailable. Overall, the preponderance of predictions points to a pathogenic effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.956248 | Disordered | 0.573557 | Binding | 0.349 | 0.895 | 0.750 | -7.870 | In-Between | 0.749 | Likely Pathogenic | Likely Benign | 0.275 | Likely Benign | 0.0781 | 0.5824 | -4.73 | Deleterious | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||||||
| c.2365C>A | P789T 2D  AIThe SynGAP1 P789T missense variant has no ClinVar entry and is not present in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and AlphaMissense‑Optimized, while pathogenic predictions arise from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default remains uncertain. High‑accuracy assessments further clarify the variant’s likely effect: AlphaMissense‑Optimized predicts a benign outcome, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—leans pathogenic (2 pathogenic vs. 1 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so its status is unavailable. Overall, the preponderance of evidence from both general and high‑accuracy tools indicates that P789T is most likely pathogenic, and this assessment does not contradict any ClinVar status, as none is reported. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.963420 | Disordered | 0.541575 | Binding | 0.398 | 0.903 | 0.750 | -5.242 | Likely Benign | 0.356 | Ambiguous | Likely Benign | 0.224 | Likely Benign | 0.1371 | 0.3837 | -4.77 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.06 | Pathogenic | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.2365C>G | P789A 2D  AIThe SynGAP1 P789A missense variant is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized; pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a tie (2 benign, 2 pathogenic) and is therefore inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus remains inconclusive and Foldetta data are unavailable. Overall, the majority of tools (five out of nine) predict pathogenicity, but the presence of four benign predictions and the inconclusive high‑accuracy results suggest uncertainty. The variant is most likely pathogenic based on the current predictions, and this assessment does not contradict any ClinVar annotation because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.963420 | Disordered | 0.541575 | Binding | 0.398 | 0.903 | 0.750 | -4.777 | Likely Benign | 0.235 | Likely Benign | Likely Benign | 0.258 | Likely Benign | 0.3120 | 0.3052 | -4.92 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.11 | Pathogenic | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.2365C>T | P789S 2D  AIThe SynGAP1 P789S variant has no ClinVar entry (ClinVar status: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that classify it as benign include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—predicts pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the balance of evidence leans toward pathogenicity, with five tools supporting a deleterious effect versus three supporting benignity. This prediction does not contradict ClinVar status, which currently has no classification for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.963420 | Disordered | 0.541575 | Binding | 0.398 | 0.903 | 0.750 | -4.793 | Likely Benign | 0.409 | Ambiguous | Likely Benign | 0.221 | Likely Benign | 0.3100 | 0.3436 | -4.64 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.19 | Pathogenic | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||
| c.2366C>A | P789Q 2D  AIThe SynGAP1 P789Q missense change is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) favors pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a pathogenic impact, and this conclusion does not contradict the ClinVar status, which currently has no classification for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.963420 | Disordered | 0.541575 | Binding | 0.398 | 0.903 | 0.750 | -5.191 | Likely Benign | 0.465 | Ambiguous | Likely Benign | 0.235 | Likely Benign | 0.1284 | 0.3215 | -4.53 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.03 | Pathogenic | 0.00 | Affected | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||||||||||
| c.2366C>G | P789R 2D  AIThe SynGAP1 missense variant P789R is not reported in ClinVar (ClinVar status: none) and is absent from gnomAD (gnomAD ID: none). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. In contrast, the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus remains Likely Pathogenic; Foldetta results are unavailable. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion does not contradict the absence of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.963420 | Disordered | 0.541575 | Binding | 0.398 | 0.903 | 0.750 | -2.503 | Likely Benign | 0.668 | Likely Pathogenic | Likely Benign | 0.354 | Likely Benign | 0.1353 | 0.2129 | -5.04 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.03 | Pathogenic | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||
| c.2366C>T | P789L 2D  AIThe SynGAP1 P789L missense variant has no ClinVar entry and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and AlphaMissense‑Optimized, while pathogenic predictions arise from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default remains uncertain. High‑accuracy assessments further refine the picture: AlphaMissense‑Optimized predicts benign, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—leans pathogenic (2 pathogenic vs. 1 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence (five pathogenic vs. three benign predictions, with the SGM Consensus supporting pathogenicity) indicates that P789L is most likely pathogenic, and this assessment does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.963420 | Disordered | 0.541575 | Binding | 0.398 | 0.903 | 0.750 | -4.623 | Likely Benign | 0.457 | Ambiguous | Likely Benign | 0.303 | Likely Benign | 0.1958 | 0.4866 | -5.91 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.02 | Pathogenic | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||
| c.236A>C | N79T 2D  AIThe SynGAP1 missense variant N79T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.457064 | Uncertain | 0.290 | 0.876 | 0.375 | -3.752 | Likely Benign | 0.102 | Likely Benign | Likely Benign | 0.022 | Likely Benign | 0.1133 | 0.4890 | -0.61 | Neutral | 0.371 | Benign | 0.018 | Benign | 4.21 | Benign | 0.00 | Affected | 0 | 0 | 2.8 | -13.00 | |||||||||||||||||||||||||||||||||||
| c.236A>G | N79S 2D  AIThe SynGAP1 missense variant N79S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.457064 | Uncertain | 0.290 | 0.876 | 0.375 | -2.311 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.017 | Likely Benign | 0.3155 | 0.4740 | -0.31 | Neutral | 0.113 | Benign | 0.011 | Benign | 4.29 | Benign | 0.00 | Affected | 1 | 1 | 2.7 | -27.03 | |||||||||||||||||||||||||||||||||||
| c.236A>T | N79I 2D  AIThe SynGAP1 missense variant N79I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.457064 | Uncertain | 0.290 | 0.876 | 0.375 | -3.958 | Likely Benign | 0.337 | Likely Benign | Likely Benign | 0.030 | Likely Benign | 0.0572 | 0.4924 | -1.42 | Neutral | 0.939 | Possibly Damaging | 0.080 | Benign | 4.14 | Benign | 0.00 | Affected | -2 | -3 | 8.0 | -0.94 | |||||||||||||||||||||||||||||||||||
| c.2375A>T | E792V 2D  AIThe SynGAP1 E792V missense change is not listed in ClinVar and has no allele record in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM, while pathogenic predictions arise from PROVEAN, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie and is therefore inconclusive. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the balance of evidence leans toward a benign effect. This conclusion is consistent with the absence of a ClinVar assertion, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.974374 | Disordered | 0.452261 | Uncertain | 0.352 | 0.896 | 0.875 | -4.643 | Likely Benign | 0.640 | Likely Pathogenic | Likely Benign | 0.072 | Likely Benign | 0.0987 | 0.7772 | -3.85 | Deleterious | 0.000 | Benign | 0.001 | Benign | 3.83 | Benign | 0.00 | Affected | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||||||||
| c.237C>A | N79K 2D  AIThe SynGAP1 missense variant N79K is listed in gnomAD (ID 6‑33425845‑C‑A) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized scores benign and the SGM‑Consensus indicates “Likely Benign.” No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the preponderance of evidence points to a benign effect for the variant, and this conclusion is not contradicted by any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.457064 | Uncertain | 0.290 | 0.876 | 0.375 | 6-33425845-C-A | 1 | 6.20e-7 | -2.811 | Likely Benign | 0.422 | Ambiguous | Likely Benign | 0.030 | Likely Benign | 0.1883 | 0.3929 | -0.91 | Neutral | 0.001 | Benign | 0.000 | Benign | 4.22 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 1 | -0.4 | 14.07 | ||||||||||||||||||||||||||||||
| c.237C>G | N79K 2D  AIThe SynGAP1 missense variant N79K is listed in gnomAD (ID 6‑33425845‑C‑G) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar classification to contradict this conclusion. Thus, based on current predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.618285 | Disordered | 0.457064 | Uncertain | 0.290 | 0.876 | 0.375 | 6-33425845-C-G | 1 | 6.20e-7 | -2.811 | Likely Benign | 0.422 | Ambiguous | Likely Benign | 0.030 | Likely Benign | 0.1883 | 0.3929 | -0.91 | Neutral | 0.001 | Benign | 0.000 | Benign | 4.22 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 1 | -0.4 | 14.07 | ||||||||||||||||||||||||||||||
| c.238A>C | K80Q 2D  AIThe SynGAP1 missense variant K80Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates Likely Benign. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence points to a benign impact, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.477530 | Uncertain | 0.331 | 0.873 | 0.500 | -4.475 | Likely Benign | 0.662 | Likely Pathogenic | Likely Benign | 0.064 | Likely Benign | 0.4373 | 0.1132 | -0.81 | Neutral | 0.414 | Benign | 0.040 | Benign | 3.93 | Benign | 0.00 | Affected | 1 | 1 | 0.4 | -0.04 | |||||||||||||||||||||||||||||||||||
| c.238A>G | K80E 2D  AIThe SynGAP1 K80E missense variant has no ClinVar record and is not listed in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The high‑accuracy consensus (SGM‑Consensus) is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yielding a “Likely Benign” classification. AlphaMissense‑Optimized returns an uncertain result, and Foldetta (which would combine FoldX‑MD and Rosetta outputs) has no available data for this variant. Overall, the balance of evidence favors a benign impact, and this assessment does not contradict any ClinVar status because no ClinVar entry exists for K80E. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.477530 | Uncertain | 0.331 | 0.873 | 0.500 | -5.605 | Likely Benign | 0.943 | Likely Pathogenic | Ambiguous | 0.034 | Likely Benign | 0.3850 | 0.0952 | -0.91 | Neutral | 0.225 | Benign | 0.027 | Benign | 3.92 | Benign | 0.00 | Affected | 0 | 1 | 0.4 | 0.94 | |||||||||||||||||||||||||||||||||||
| c.2392C>A | P798T 2D  AIThe SynGAP1 missense variant P798T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence—including the high‑accuracy tools—points to a benign effect for P798T, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.492709 | Uncertain | 0.426 | 0.899 | 0.875 | -5.926 | Likely Benign | 0.062 | Likely Benign | Likely Benign | 0.039 | Likely Benign | 0.1293 | 0.4854 | -0.38 | Neutral | 0.901 | Possibly Damaging | 0.534 | Possibly Damaging | 4.24 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | ||||||||||||||||||||||||||||||||||
| c.2392C>G | P798A 2D  AIThe SynGAP1 missense variant P798A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: benign predictions come from REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, whereas only polyPhen‑2 HumDiv and SIFT predict pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized reports benign, and the SGM‑Consensus also indicates benign. Foldetta results are not available, so they do not influence the assessment. Overall, the consensus of available predictions indicates that P798A is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.492709 | Uncertain | 0.426 | 0.899 | 0.875 | -4.841 | Likely Benign | 0.050 | Likely Benign | Likely Benign | 0.045 | Likely Benign | 0.3060 | 0.4030 | -0.57 | Neutral | 0.790 | Possibly Damaging | 0.353 | Benign | 4.27 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | ||||||||||||||||||||||||||||||||||
| c.2392C>T | P798S 2D  AIThe SynGAP1 missense variant P798S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and SIFT—suggest a pathogenic impact. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized reports benign, and the SGM‑Consensus (majority vote) also indicates benign. Foldetta results are not available, so they do not influence the assessment. Overall, the majority of evidence points to a benign effect, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.492709 | Uncertain | 0.426 | 0.899 | 0.875 | -5.382 | Likely Benign | 0.065 | Likely Benign | Likely Benign | 0.058 | Likely Benign | 0.2950 | 0.4425 | 0.14 | Neutral | 0.818 | Possibly Damaging | 0.353 | Benign | 4.28 | Benign | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||||||
| c.2393C>A | P798Q 2D  AIThe SynGAP1 missense variant P798Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for P798Q, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.492709 | Uncertain | 0.426 | 0.899 | 0.875 | -5.944 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.068 | Likely Benign | 0.1290 | 0.3864 | -0.53 | Neutral | 0.988 | Probably Damaging | 0.780 | Possibly Damaging | 4.25 | Benign | 0.00 | Affected | 0 | -1 | -1.9 | 31.01 | ||||||||||||||||||||||||||||||||||
| c.2393C>G | P798R 2D  AIThe SynGAP1 missense variant P798R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for P798R, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.492709 | Uncertain | 0.426 | 0.899 | 0.875 | -5.232 | Likely Benign | 0.162 | Likely Benign | Likely Benign | 0.065 | Likely Benign | 0.1397 | 0.2840 | -0.83 | Neutral | 0.976 | Probably Damaging | 0.780 | Possibly Damaging | 4.25 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||
| c.2393C>T | P798L 2D  AIThe SynGAP1 missense variant P798L is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33442945‑C‑T). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Benign; a Foldetta stability prediction is not available. Overall, the majority of evidence points to a benign effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.492709 | Uncertain | 0.426 | 0.899 | 0.875 | Uncertain | 2 | 6-33442945-C-T | 6 | 3.72e-6 | -5.640 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.042 | Likely Benign | 0.2039 | 0.5555 | -0.86 | Neutral | 0.981 | Probably Damaging | 0.631 | Possibly Damaging | 4.21 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||
| c.2395C>A | P799T 2D  AIThe SynGAP1 missense variant P799T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for P799T, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.537892 | Binding | 0.400 | 0.894 | 0.750 | -5.470 | Likely Benign | 0.063 | Likely Benign | Likely Benign | 0.033 | Likely Benign | 0.1522 | 0.5257 | -0.82 | Neutral | 0.951 | Possibly Damaging | 0.738 | Possibly Damaging | 4.24 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | ||||||||||||||||||||||||||||||||||
| c.2395C>G | P799A 2D  AIThe SynGAP1 missense variant P799A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign) score; pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available, so it does not influence the conclusion. Overall, the majority of evidence points to a benign effect for P799A, and this assessment is consistent with the absence of a ClinVar pathogenic classification. Thus, the variant is most likely benign, and this conclusion does not contradict the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.537892 | Binding | 0.400 | 0.894 | 0.750 | -4.660 | Likely Benign | 0.048 | Likely Benign | Likely Benign | 0.054 | Likely Benign | 0.3488 | 0.4279 | -0.72 | Neutral | 0.798 | Possibly Damaging | 0.489 | Possibly Damaging | 4.27 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | ||||||||||||||||||||||||||||||||||
| c.2395C>T | P799S 2D  AIThe SynGAP1 missense variant P799S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for P799S, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.537892 | Binding | 0.400 | 0.894 | 0.750 | -4.635 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.065 | Likely Benign | 0.3411 | 0.4464 | -0.73 | Neutral | 0.951 | Possibly Damaging | 0.593 | Possibly Damaging | 4.26 | Benign | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||||||
| c.2396C>A | P799H 2D  AIThe SynGAP1 missense variant P799H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree that the substitution is benign: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all indicate a benign effect. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence supports a benign classification for P799H, and this conclusion does not contradict any ClinVar annotation, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.537892 | Binding | 0.400 | 0.894 | 0.750 | -5.611 | Likely Benign | 0.108 | Likely Benign | Likely Benign | 0.089 | Likely Benign | 0.1684 | 0.4192 | -0.80 | Neutral | 0.999 | Probably Damaging | 0.933 | Probably Damaging | 4.20 | Benign | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | ||||||||||||||||||||||||||||||||||
| c.2396C>G | P799R 2D  AIThe SynGAP1 missense variant P799R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a “Likely Benign” outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) likewise indicates benign. No Foldetta stability analysis is available, so it does not influence the assessment. Overall, the preponderance of evidence points to the variant being most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.537892 | Binding | 0.400 | 0.894 | 0.750 | -5.100 | Likely Benign | 0.141 | Likely Benign | Likely Benign | 0.029 | Likely Benign | 0.1425 | 0.2905 | -0.49 | Neutral | 0.029 | Benign | 0.022 | Benign | 4.27 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||
| c.2396C>T | P799L 2D  AIThe SynGAP1 missense variant P799L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for P799L, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.871313 | Disordered | 0.537892 | Binding | 0.400 | 0.894 | 0.750 | -5.296 | Likely Benign | 0.090 | Likely Benign | Likely Benign | 0.043 | Likely Benign | 0.2078 | 0.6285 | -0.93 | Neutral | 0.905 | Possibly Damaging | 0.670 | Possibly Damaging | 4.27 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||
| c.239A>C | K80T 2D  AIThe SynGAP1 missense variant K80T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which classifies the variant as Likely Benign. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized prediction is uncertain, and the Foldetta protein‑folding stability assessment is unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of a ClinVar entry, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.477530 | Uncertain | 0.331 | 0.873 | 0.500 | -4.987 | Likely Benign | 0.830 | Likely Pathogenic | Ambiguous | 0.072 | Likely Benign | 0.2139 | 0.2321 | -1.60 | Neutral | 0.588 | Possibly Damaging | 0.036 | Benign | 3.89 | Benign | 0.00 | Affected | 0 | -1 | 3.2 | -27.07 | |||||||||||||||||||||||||||||||||||
| c.239A>G | K80R 2D  AIThe SynGAP1 missense variant K80R is reported in gnomAD (ID 6‑33425847‑A‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments reinforce this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.477530 | Uncertain | 0.331 | 0.873 | 0.500 | 6-33425847-A-G | 1 | 6.20e-7 | -2.919 | Likely Benign | 0.146 | Likely Benign | Likely Benign | 0.077 | Likely Benign | 0.4481 | 0.0858 | 0.11 | Neutral | 0.001 | Benign | 0.001 | Benign | 4.33 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 3 | -0.6 | 28.01 | ||||||||||||||||||||||||||||||
| c.239A>T | K80I 2D  AIThe SynGAP1 missense variant K80I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM, while those that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized remains uncertain, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is a 2‑vs‑2 tie, and Foldetta data are unavailable. Consequently, the variant’s predicted impact is ambiguous, with an equal split between benign and pathogenic signals and no ClinVar entry to contradict the computational assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.637480 | Disordered | 0.477530 | Uncertain | 0.331 | 0.873 | 0.500 | -5.320 | Likely Benign | 0.947 | Likely Pathogenic | Ambiguous | 0.083 | Likely Benign | 0.1095 | 0.2981 | -2.54 | Deleterious | 0.939 | Possibly Damaging | 0.164 | Benign | 3.86 | Benign | 0.00 | Affected | -2 | -3 | 8.4 | -15.01 | ||||||||||||||||||||||||||||||||||||
| c.23T>A | I8N 2D  AIThe SynGAP1 missense variant I8N is not reported in ClinVar and has no entry in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools (polyPhen‑2 HumDiv and SIFT) predict pathogenicity, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the consensus of the majority of predictors, including the high‑accuracy methods, points to a benign impact. This conclusion is consistent with the absence of any ClinVar classification, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.543080 | Binding | 0.341 | 0.916 | 0.625 | -3.979 | Likely Benign | 0.163 | Likely Benign | Likely Benign | 0.120 | Likely Benign | 0.0803 | 0.0540 | 0.05 | Neutral | 0.561 | Possibly Damaging | 0.032 | Benign | 3.99 | Benign | 0.00 | Affected | -2 | -3 | -8.0 | 0.94 | |||||||||||||||||||||||||||||||||||
| c.23T>C | I8T 2D  AIThe SynGAP1 missense variant I8T is reported in gnomAD (variant ID 6‑33420287‑T‑C) but has no ClinVar entry. In silico prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.543080 | Binding | 0.341 | 0.916 | 0.625 | 6-33420287-T-C | -3.149 | Likely Benign | 0.239 | Likely Benign | Likely Benign | 0.108 | Likely Benign | 0.0949 | 0.1019 | -0.10 | Neutral | 0.047 | Benign | 0.006 | Benign | 4.03 | Benign | 0.00 | Affected | 4.32 | 1 | -1 | 0 | -5.2 | -12.05 | ||||||||||||||||||||||||||||||||
| c.23T>G | I8S 2D  AIThe SynGAP1 missense variant I8S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign prediction: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.543080 | Binding | 0.341 | 0.916 | 0.625 | -1.577 | Likely Benign | 0.122 | Likely Benign | Likely Benign | 0.266 | Likely Benign | 0.2726 | 0.0910 | 0.18 | Neutral | 0.107 | Benign | 0.006 | Benign | 4.02 | Benign | 0.00 | Affected | -1 | -2 | -5.3 | -26.08 | |||||||||||||||||||||||||||||||||||
| c.2404G>T | G802C 2D  AIThe SynGAP1 missense variant G802C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; Foldetta results are not available. Overall, the majority of evidence—including the consensus of multiple independent predictors and the high‑accuracy tools—supports a benign classification for G802C. This conclusion is consistent with the lack of any ClinVar pathogenic annotation, so there is no contradiction with existing clinical database status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.894241 | Disordered | 0.681966 | Binding | 0.294 | 0.898 | 0.625 | -6.332 | Likely Benign | 0.127 | Likely Benign | Likely Benign | 0.127 | Likely Benign | 0.1426 | 0.4126 | -1.60 | Neutral | 0.983 | Probably Damaging | 0.819 | Possibly Damaging | 2.65 | Benign | 0.00 | Affected | -3 | -3 | 2.9 | 46.09 | ||||||||||||||||||||||||||||||||||
| c.2405G>T | G802V 2D  AIThe SynGAP1 missense variant G802V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the only pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign. No Foldetta stability analysis is available for this variant. Overall, the consensus of the available predictions indicates that G802V is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.894241 | Disordered | 0.681966 | Binding | 0.294 | 0.898 | 0.625 | -3.871 | Likely Benign | 0.126 | Likely Benign | Likely Benign | 0.078 | Likely Benign | 0.1345 | 0.3533 | -1.49 | Neutral | 0.411 | Benign | 0.321 | Benign | 2.67 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | ||||||||||||||||||||||||||||||||||
| c.2407A>C | K803Q 2D  AIThe SynGAP1 missense variant K803Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of high‑confidence predictions lean toward a benign impact, and there is no ClinVar entry to contradict this assessment. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.827927 | Disordered | 0.733908 | Binding | 0.349 | 0.900 | 0.625 | -2.792 | Likely Benign | 0.305 | Likely Benign | Likely Benign | 0.108 | Likely Benign | 0.4810 | 0.1398 | -2.01 | Neutral | 0.984 | Probably Damaging | 0.773 | Possibly Damaging | 2.36 | Pathogenic | 0.00 | Affected | 1 | 1 | 0.4 | -0.04 | ||||||||||||||||||||||||||||||||||
| c.2407A>G | K803E 2D  AIThe SynGAP1 missense variant K803E is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs 2 benign) and Foldetta results are unavailable. Overall, the majority of tools (five pathogenic vs. four benign) predict a pathogenic impact. Thus, the variant is most likely pathogenic, and this prediction does not contradict any ClinVar status because the variant has not yet been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.827927 | Disordered | 0.733908 | Binding | 0.349 | 0.900 | 0.625 | -3.889 | Likely Benign | 0.650 | Likely Pathogenic | Likely Benign | 0.172 | Likely Benign | 0.4043 | 0.1357 | -2.06 | Neutral | 0.896 | Possibly Damaging | 0.596 | Possibly Damaging | 2.38 | Pathogenic | 0.00 | Affected | 0 | 1 | 0.4 | 0.94 | |||||||||||||||||||||||||||||||||||
| c.2408A>C | K803T 2D  AIThe SynGAP1 missense variant K803T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. In contrast, the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score (which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicate it is likely pathogenic. High‑accuracy assessments further support this view: AlphaMissense‑Optimized classifies the variant as benign, whereas the SGM‑Consensus indicates it is likely pathogenic; a Foldetta stability analysis is not available. Overall, the balance of evidence points to a pathogenic effect for K803T, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.827927 | Disordered | 0.733908 | Binding | 0.349 | 0.900 | 0.625 | -3.571 | Likely Benign | 0.584 | Likely Pathogenic | Likely Benign | 0.121 | Likely Benign | 0.2417 | 0.4395 | -2.90 | Deleterious | 0.946 | Possibly Damaging | 0.741 | Possibly Damaging | 2.40 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.2 | -27.07 | ||||||||||||||||||||||||||||||||||
| c.2408A>G | K803R 2D  AIThe SynGAP1 missense variant K803R is listed in ClinVar (ID 834618.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: SGM‑Consensus, REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—SIFT and FATHMM—predict pathogenicity. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates the variant is most likely benign, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.827927 | Disordered | 0.733908 | Binding | 0.349 | 0.900 | 0.625 | Uncertain | 1 | -2.281 | Likely Benign | 0.097 | Likely Benign | Likely Benign | 0.018 | Likely Benign | 0.4857 | 0.1715 | -1.52 | Neutral | 0.103 | Benign | 0.038 | Benign | 2.38 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 3 | 2 | -0.6 | 28.01 | ||||||||||||||||||||||||||||||
| c.2408A>T | K803I 2D  AIThe SynGAP1 K803I missense variant is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score. The high‑accuracy AlphaMissense‑Optimized assessment is uncertain, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely pathogenic outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion does not contradict the ClinVar status, which currently contains no classification for K803I. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.827927 | Disordered | 0.733908 | Binding | 0.349 | 0.900 | 0.625 | -5.207 | Likely Benign | 0.894 | Likely Pathogenic | Ambiguous | 0.196 | Likely Benign | 0.1425 | 0.3889 | -4.06 | Deleterious | 0.995 | Probably Damaging | 0.913 | Probably Damaging | 2.31 | Pathogenic | 0.00 | Affected | -2 | -3 | 8.4 | -15.01 | ||||||||||||||||||||||||||||||||||
| c.2409A>C | K803N 2D  AIThe SynGAP1 K803N missense variant is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool rates the variant as uncertain, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive because it yields a 2‑to‑2 split. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, more tools predict pathogenicity (five) than benignity (three), and the high‑accuracy assessments do not overturn this trend. Therefore, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.827927 | Disordered | 0.733908 | Binding | 0.349 | 0.900 | 0.625 | -5.039 | Likely Benign | 0.807 | Likely Pathogenic | Ambiguous | 0.045 | Likely Benign | 0.3805 | 0.2035 | -2.13 | Neutral | 0.896 | Possibly Damaging | 0.673 | Possibly Damaging | 2.34 | Pathogenic | 0.00 | Affected | 1 | 0 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||||
| c.2409A>T | K803N 2D  AIThe SynGAP1 K803N missense variant is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive because it yields a 2‑to‑2 split. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, more tools (five) predict pathogenicity than benign (three), and the high‑accuracy assessments do not overturn this trend. Therefore, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.827927 | Disordered | 0.733908 | Binding | 0.349 | 0.900 | 0.625 | -5.039 | Likely Benign | 0.807 | Likely Pathogenic | Ambiguous | 0.045 | Likely Benign | 0.3805 | 0.2035 | -2.13 | Neutral | 0.896 | Possibly Damaging | 0.673 | Possibly Damaging | 2.34 | Pathogenic | 0.00 | Affected | 1 | 0 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||||
| c.240A>C | K80N 2D  AIThe SynGAP1 K80N missense variant has no ClinVar record and is not reported in gnomAD. Functional prediction tools that agree on benign impact include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while those that predict pathogenicity are polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized predicting pathogenicity, whereas the SGM‑Consensus (majority vote) predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Based on the overall distribution of predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.477530 | Uncertain | 0.331 | 0.873 | 0.500 | -5.072 | Likely Benign | 0.958 | Likely Pathogenic | Likely Pathogenic | 0.078 | Likely Benign | 0.3749 | 0.1237 | -1.07 | Neutral | 0.588 | Possibly Damaging | 0.054 | Benign | 3.89 | Benign | 0.00 | Affected | 1 | 0 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||||
| c.240A>T | K80N 2D  AIThe SynGAP1 K80N missense variant has no ClinVar record and is not listed in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote) remains benign; Foldetta results are unavailable. Based on the overall distribution of predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.477530 | Uncertain | 0.331 | 0.873 | 0.500 | -5.072 | Likely Benign | 0.958 | Likely Pathogenic | Likely Pathogenic | 0.078 | Likely Benign | 0.3749 | 0.1237 | -1.07 | Neutral | 0.588 | Possibly Damaging | 0.054 | Benign | 3.89 | Benign | 0.00 | Affected | 1 | 0 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||||
| c.2413C>A | L805M 2D  AIThe SynGAP1 missense variant L805M is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, and no Foldetta stability data are available. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical databases. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.775545 | Disordered | 0.827669 | Binding | 0.341 | 0.903 | 0.625 | -4.229 | Likely Benign | 0.133 | Likely Benign | Likely Benign | 0.034 | Likely Benign | 0.0911 | 0.3867 | -0.67 | Neutral | 0.029 | Benign | 0.033 | Benign | 2.39 | Pathogenic | 0.00 | Affected | 4 | 2 | -1.9 | 18.03 | ||||||||||||||||||||||||||||||||||
| c.2413C>G | L805V 2D  AIThe SynGAP1 missense variant L805V is not reported in ClinVar and is absent from gnomAD. Prediction tools that assess pathogenicity largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote) is also benign. Foldetta results are not available, so they do not influence the conclusion. Overall, the preponderance of evidence indicates that the variant is most likely benign, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.775545 | Disordered | 0.827669 | Binding | 0.341 | 0.903 | 0.625 | -2.445 | Likely Benign | 0.094 | Likely Benign | Likely Benign | 0.027 | Likely Benign | 0.1553 | 0.3493 | -1.16 | Neutral | 0.003 | Benign | 0.008 | Benign | 2.65 | Benign | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | ||||||||||||||||||||||||||||||||||
| c.2414T>A | L805Q 2D  AIThe SynGAP1 missense variant L805Q is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and AlphaMissense‑Optimized, while pathogenic predictions arise from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default remains uncertain. High‑accuracy assessments further clarify the picture: AlphaMissense‑Optimized predicts a benign effect, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—leans pathogenic (2 pathogenic vs. 1 benign, 1 uncertain). Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the balance of evidence (five pathogenic vs. three benign predictions, with a pathogenic SGM Consensus) indicates that the variant is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.775545 | Disordered | 0.827669 | Binding | 0.341 | 0.903 | 0.625 | -6.244 | Likely Benign | 0.427 | Ambiguous | Likely Benign | 0.152 | Likely Benign | 0.1215 | 0.1265 | -2.66 | Deleterious | 0.927 | Possibly Damaging | 0.690 | Possibly Damaging | 2.37 | Pathogenic | 0.00 | Affected | -2 | -2 | -7.3 | 14.97 | |||||||||||||||||||||||||||||||||||
| c.2414T>C | L805P 2D  AIThe SynGAP1 missense variant L805P is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, ESM1b, and AlphaMissense‑Optimized, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default remains uncertain. High‑accuracy assessments give a benign result from AlphaMissense‑Optimized, a pathogenic outcome from the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), and no Foldetta data are available. Overall, the majority of predictions lean toward pathogenicity, and this conclusion does not conflict with the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.775545 | Disordered | 0.827669 | Binding | 0.341 | 0.903 | 0.625 | Uncertain | 1 | -4.661 | Likely Benign | 0.444 | Ambiguous | Likely Benign | 0.272 | Likely Benign | 0.3128 | 0.1650 | -3.40 | Deleterious | 0.975 | Probably Damaging | 0.767 | Possibly Damaging | 2.36 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||
| c.2414T>G | L805R 2D  AIThe SynGAP1 missense variant L805R is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. In contrast, the majority of tools predict a pathogenic impact: SGM‑Consensus, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, whereas the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) labels it as likely pathogenic; Foldetta results are unavailable. Overall, the balance of evidence from the broader set of predictors leans toward pathogenicity, and this conclusion does not contradict any existing ClinVar annotation, as none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.775545 | Disordered | 0.827669 | Binding | 0.341 | 0.903 | 0.625 | -6.640 | Likely Benign | 0.569 | Likely Pathogenic | Likely Benign | 0.196 | Likely Benign | 0.1372 | 0.0908 | -3.00 | Deleterious | 0.927 | Possibly Damaging | 0.617 | Possibly Damaging | 2.37 | Pathogenic | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | ||||||||||||||||||||||||||||||||||
| c.2417T>C | F806S 2D  AIThe SynGAP1 missense variant F806S is catalogued in gnomAD (ID 6‑33442969‑T‑C) but has no ClinVar entry. Functional prediction tools split into two groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessment shows AlphaMissense‑Optimized as uncertain, whereas the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) resolves as likely pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a pathogenic effect, and this conclusion does not contradict any ClinVar status because none is reported. Thus, the variant is most likely pathogenic based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.736850 | Disordered | 0.847454 | Binding | 0.276 | 0.904 | 0.500 | 6-33442969-T-C | 1 | 6.20e-7 | -6.959 | Likely Benign | 0.911 | Likely Pathogenic | Ambiguous | 0.269 | Likely Benign | 0.5067 | 0.0391 | Weaken | -4.13 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.21 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -2 | -3 | -3.6 | -60.10 | ||||||||||||||||||||||||||||
| c.2417T>G | F806C 2D  AIThe SynGAP1 missense variant F806C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. All other evaluated tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default—predict a pathogenic impact, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, while SGM‑Consensus remains likely pathogenic; Foldetta results are unavailable. Based on the predominance of pathogenic predictions and the SGM‑Consensus outcome, the variant is most likely pathogenic. This assessment does not contradict ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.736850 | Disordered | 0.847454 | Binding | 0.276 | 0.904 | 0.500 | -8.565 | Likely Pathogenic | 0.809 | Likely Pathogenic | Ambiguous | 0.266 | Likely Benign | 0.3071 | 0.1125 | -4.46 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.11 | Pathogenic | 0.00 | Affected | -4 | -2 | -0.3 | -44.04 | ||||||||||||||||||||||||||||||||||
| c.2419T>A | Y807N 2D  AIThe SynGAP1 missense variant Y807N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split assessment: benign predictions come from REVEL and AlphaMissense‑Optimized, whereas pathogenic predictions are returned by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score, which is labeled Likely Pathogenic. ESM1b is uncertain. High‑accuracy methods give a clearer picture: AlphaMissense‑Optimized predicts a benign effect, while the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates pathogenicity. Foldetta, a protein‑folding stability approach that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions lean toward pathogenicity, and this is consistent with the lack of ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.699094 | Disordered | 0.853760 | Binding | 0.336 | 0.901 | 0.500 | -7.795 | In-Between | 0.591 | Likely Pathogenic | Likely Benign | 0.154 | Likely Benign | 0.2274 | 0.0704 | -4.01 | Deleterious | 0.934 | Possibly Damaging | 0.773 | Possibly Damaging | 2.43 | Pathogenic | 0.00 | Affected | -2 | -2 | -2.2 | -49.07 | ||||||||||||||||||||||||||||||||||
| c.2419T>C | Y807H 2D  AIThe SynGAP1 missense variant Y807H is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that indicate a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default is uncertain. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as pathogenic. AlphaMissense‑Optimized remains benign, while Foldetta results are unavailable. Overall, the majority of high‑accuracy and consensus predictors (five out of six) suggest a pathogenic impact, whereas only three predict benign. Consequently, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.699094 | Disordered | 0.853760 | Binding | 0.336 | 0.901 | 0.500 | -5.879 | Likely Benign | 0.413 | Ambiguous | Likely Benign | 0.152 | Likely Benign | 0.2530 | 0.0704 | -2.74 | Deleterious | 0.989 | Probably Damaging | 0.913 | Probably Damaging | 2.44 | Pathogenic | 0.00 | Affected | 0 | 2 | -1.9 | -26.03 | |||||||||||||||||||||||||||||||||||
| c.2419T>G | Y807D 2D  AIThe SynGAP1 missense variant Y807D is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect are limited to REVEL, while the majority of algorithms (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic impact. Uncertain calls come from ESM1b and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as pathogenic, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a pathogenic effect for Y807D, and this conclusion does not contradict the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.699094 | Disordered | 0.853760 | Binding | 0.336 | 0.901 | 0.500 | -7.598 | In-Between | 0.862 | Likely Pathogenic | Ambiguous | 0.186 | Likely Benign | 0.3944 | 0.0704 | -4.40 | Deleterious | 0.966 | Probably Damaging | 0.773 | Possibly Damaging | 2.42 | Pathogenic | 0.00 | Affected | -4 | -3 | -2.2 | -48.09 | ||||||||||||||||||||||||||||||||||
| c.241C>A | L81M 2D  AIThe SynGAP1 missense variant L81M is listed in ClinVar with no submitted interpretation and is present in gnomAD (variant ID 6‑33425849‑C‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; no Foldetta stability result is available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which remains unclassified. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.502033 | Binding | 0.291 | 0.878 | 0.250 | 6-33425849-C-A | 1 | 6.20e-7 | -5.351 | Likely Benign | 0.254 | Likely Benign | Likely Benign | 0.039 | Likely Benign | 0.0692 | 0.2432 | -0.27 | Neutral | 0.789 | Possibly Damaging | 0.165 | Benign | 3.95 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 4 | -1.9 | 18.03 | ||||||||||||||||||||||||||||||
| c.241C>G | L81V 2D  AIThe SynGAP1 missense variant L81V is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign status. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence supports a benign classification for L81V, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.502033 | Binding | 0.291 | 0.878 | 0.250 | -5.207 | Likely Benign | 0.289 | Likely Benign | Likely Benign | 0.038 | Likely Benign | 0.1423 | 0.2653 | -0.51 | Neutral | 0.178 | Benign | 0.014 | Benign | 4.04 | Benign | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2422G>A | V808I 2D  AIThe SynGAP1 missense variant V808I is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Pathogenic predictions come from polyPhen‑2 HumDiv, SIFT, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign and the SGM‑Consensus (majority vote) is likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates the variant is most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.699094 | Disordered | 0.856438 | Binding | 0.289 | 0.903 | 0.500 | -4.190 | Likely Benign | 0.151 | Likely Benign | Likely Benign | 0.051 | Likely Benign | 0.0935 | 0.4282 | -0.67 | Neutral | 0.659 | Possibly Damaging | 0.191 | Benign | 2.33 | Pathogenic | 0.00 | Affected | 4 | 3 | 0.3 | 14.03 | ||||||||||||||||||||||||||||||||||
| c.2422G>C | V808L 2D  AIThe SynGAP1 missense variant V808L is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact for V808L, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.699094 | Disordered | 0.856438 | Binding | 0.289 | 0.903 | 0.500 | -4.723 | Likely Benign | 0.306 | Likely Benign | Likely Benign | 0.188 | Likely Benign | 0.1166 | 0.4745 | -1.82 | Neutral | 0.911 | Possibly Damaging | 0.621 | Possibly Damaging | 2.33 | Pathogenic | 0.00 | Affected | 2 | 1 | -0.4 | 14.03 | ||||||||||||||||||||||||||||||||||
| c.2422G>T | V808L 2D  AIThe SynGAP1 missense variant V808L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | 0.699094 | Disordered | 0.856438 | Binding | 0.289 | 0.903 | 0.500 | -4.723 | Likely Benign | 0.306 | Likely Benign | Likely Benign | 0.188 | Likely Benign | 0.1166 | 0.4745 | -1.82 | Neutral | 0.911 | Possibly Damaging | 0.621 | Possibly Damaging | 2.33 | Pathogenic | 0.00 | Affected | 2 | 1 | -0.4 | 14.03 | ||||||||||||||||||||||||||||||||||
| c.2423T>A | V808E 2D  AIThe SynGAP1 missense variant V808E is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: the single benign prediction comes from REVEL, while the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus—indicate pathogenicity. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized returns an uncertain result, SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) labels the variant as Likely Pathogenic, and Foldetta data are not available. Based on the preponderance of pathogenic predictions and the SGM‑Consensus outcome, the variant is most likely pathogenic; this assessment does not contradict any ClinVar status, as none exists for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.699094 | Disordered | 0.856438 | Binding | 0.289 | 0.903 | 0.500 | -9.078 | Likely Pathogenic | 0.888 | Likely Pathogenic | Ambiguous | 0.307 | Likely Benign | 0.1129 | 0.2787 | -2.84 | Deleterious | 0.999 | Probably Damaging | 0.958 | Probably Damaging | 2.28 | Pathogenic | 0.00 | Affected | -2 | -2 | -7.7 | 29.98 | ||||||||||||||||||||||||||||||||||
| c.2423T>C | V808A 2D  AIThe SynGAP1 missense variant V808A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores the variant as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a benign verdict, while Foldetta results are unavailable. Taken together, the majority of evidence points to a benign impact, and this conclusion does not contradict the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.699094 | Disordered | 0.856438 | Binding | 0.289 | 0.903 | 0.500 | -5.091 | Likely Benign | 0.541 | Ambiguous | Likely Benign | 0.177 | Likely Benign | 0.2748 | 0.2809 | -1.96 | Neutral | 0.953 | Possibly Damaging | 0.621 | Possibly Damaging | 2.31 | Pathogenic | 0.00 | Affected | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||||||
| c.2423T>G | V808G 2D  AIThe SynGAP1 V808G missense variant is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, SIFT, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) predicts pathogenic. Foldetta results are unavailable. Overall, the majority of individual predictors (five benign vs. three pathogenic) support a benign classification, and this is not contradicted by any ClinVar annotation. Thus, based on the available predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.699094 | Disordered | 0.856438 | Binding | 0.289 | 0.903 | 0.500 | -6.635 | Likely Benign | 0.460 | Ambiguous | Likely Benign | 0.268 | Likely Benign | 0.1925 | 0.3336 | -2.94 | Deleterious | 0.069 | Benign | 0.135 | Benign | 2.27 | Pathogenic | 0.00 | Affected | -1 | -3 | -4.6 | -42.08 | |||||||||||||||||||||||||||||||||||
| c.2428C>T | R810C 2D  AIThe SynGAP1 missense variant R810C is listed in gnomAD (6‑33442980‑C‑T) but has no ClinVar entry. Prediction tools that agree on a benign effect include REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default—consistently predict pathogenicity. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely pathogenic outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the majority of evidence points to a pathogenic effect, and this conclusion does not contradict any ClinVar status because none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.486429 | Structured | 0.851848 | Binding | 0.263 | 0.907 | 0.375 | 6-33442980-C-T | 2 | 1.24e-6 | -8.925 | Likely Pathogenic | 0.839 | Likely Pathogenic | Ambiguous | 0.245 | Likely Benign | 0.3517 | 0.4263 | -4.91 | Deleterious | 1.000 | Probably Damaging | 0.991 | Probably Damaging | 2.32 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -3 | -4 | 7.0 | -53.05 | |||||||||||||||||||||||||||||
| c.242T>A | L81Q 2D  AIThe SynGAP1 missense variant L81Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.502033 | Binding | 0.291 | 0.878 | 0.250 | -5.055 | Likely Benign | 0.517 | Ambiguous | Likely Benign | 0.052 | Likely Benign | 0.1046 | 0.0888 | -1.22 | Neutral | 0.919 | Possibly Damaging | 0.226 | Benign | 3.93 | Benign | 0.00 | Affected | -2 | -2 | -7.3 | 14.97 | |||||||||||||||||||||||||||||||||||
| c.242T>C | L81P 2D  AIThe SynGAP1 missense variant L81P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as likely benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) is not available for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.502033 | Binding | 0.291 | 0.878 | 0.250 | -4.413 | Likely Benign | 0.827 | Likely Pathogenic | Ambiguous | 0.095 | Likely Benign | 0.3062 | 0.1818 | -1.51 | Neutral | 0.919 | Possibly Damaging | 0.226 | Benign | 3.92 | Benign | 0.00 | Affected | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||||||
| c.242T>G | L81R 2D  AIThe SynGAP1 missense variant L81R is not reported in ClinVar and has no entries in gnomAD. Consensus from multiple in silico predictors shows a split: benign calls from REVEL, PROVEAN, polyPhen2_HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic calls come from polyPhen2_HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy tools further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. No Foldetta stability assessment is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.632174 | Disordered | 0.502033 | Binding | 0.291 | 0.878 | 0.250 | -4.451 | Likely Benign | 0.639 | Likely Pathogenic | Likely Benign | 0.053 | Likely Benign | 0.1179 | 0.0688 | -1.22 | Neutral | 0.919 | Possibly Damaging | 0.226 | Benign | 3.94 | Benign | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||
| c.2435C>A | P812H 2D  AIThe SynGAP1 missense variant P812H is listed in ClinVar with an uncertain significance and is present in the gnomAD database (ID 6‑33442987‑C‑A). Prediction tools that agree on a benign effect include REVEL, FATHMM, and AlphaMissense‑Optimized, whereas a majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default) predict a pathogenic outcome; ESM1b remains uncertain. High‑accuracy methods give a benign result from AlphaMissense‑Optimized, a pathogenic consensus from the SGM approach (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN), and Foldetta data are unavailable. Overall, the balance of evidence points to a pathogenic effect, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | 0.414856 | Structured | 0.842442 | Binding | 0.388 | 0.901 | 0.125 | Uncertain | 2 | 6-33442987-C-A | 3 | 1.86e-6 | -7.470 | In-Between | 0.698 | Likely Pathogenic | Likely Benign | 0.272 | Likely Benign | 0.1568 | 0.4923 | -2.81 | Deleterious | 1.000 | Probably Damaging | 0.995 | Probably Damaging | 2.68 | Benign | 0.00 | Affected | 4.32 | 4 | 0 | -2 | -1.6 | 40.02 | ||||||||||||||||||||||||||||
| c.2443C>T | R815C 2D  AIThe SynGAP1 missense variant R815C is listed in ClinVar (ID 660618.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33442995‑C‑T). Prediction tools that agree on a benign effect include REVEL and FATHMM, whereas the majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default) predict a pathogenic impact. The high‑accuracy AlphaMissense‑Optimized result is “Uncertain.” The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Pathogenic.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of predictions indicates a pathogenic effect, which does not contradict the ClinVar “Uncertain” classification but suggests that the variant is more likely pathogenic rather than benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | SH3-binding motif | 0.394753 | Structured | 0.780568 | Binding | 0.278 | 0.907 | 0.250 | Uncertain | 1 | 6-33442995-C-T | 5 | 3.10e-6 | -9.373 | Likely Pathogenic | 0.828 | Likely Pathogenic | Ambiguous | 0.174 | Likely Benign | 0.3389 | 0.3682 | -3.89 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.59 | Benign | 0.00 | Affected | 4.32 | 4 | -4 | -3 | 7.0 | -53.05 | |||||||||||||||||||||||||||
| c.2449T>A | S817T 2D  AIThe SynGAP1 missense variant S817T is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also leans benign, and Foldetta results are unavailable. Overall, the balance of evidence from both general and high‑accuracy predictors points to a benign impact for S817T. This conclusion does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.490133 | Structured | 0.727082 | Binding | 0.314 | 0.901 | 0.625 | -6.544 | Likely Benign | 0.492 | Ambiguous | Likely Benign | 0.157 | Likely Benign | 0.1622 | 0.6482 | -1.95 | Neutral | 0.990 | Probably Damaging | 0.846 | Possibly Damaging | 2.43 | Pathogenic | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||||||||
| c.2449T>C | S817P 2D  AIThe SynGAP1 missense variant S817P is not reported in ClinVar and is absent from gnomAD. Prediction tools that classify the variant as benign include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Overall, the majority of tools and the consensus score indicate a pathogenic effect, and this conclusion does not contradict any ClinVar annotation because no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.490133 | Structured | 0.727082 | Binding | 0.314 | 0.901 | 0.625 | -4.633 | Likely Benign | 0.724 | Likely Pathogenic | Likely Benign | 0.201 | Likely Benign | 0.2272 | 0.6023 | -3.26 | Deleterious | 0.999 | Probably Damaging | 0.966 | Probably Damaging | 2.39 | Pathogenic | 0.00 | Affected | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||
| c.2449T>G | S817A 2D  AIThe SynGAP1 missense variant S817A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also resolves to benign, while Foldetta results are unavailable. Overall, the majority of high‑confidence predictions indicate a benign impact. This conclusion does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.490133 | Structured | 0.727082 | Binding | 0.314 | 0.901 | 0.625 | -6.321 | Likely Benign | 0.429 | Ambiguous | Likely Benign | 0.116 | Likely Benign | 0.5207 | 0.5866 | Strenghten | -1.86 | Neutral | 0.977 | Probably Damaging | 0.846 | Possibly Damaging | 2.45 | Pathogenic | 0.00 | Affected | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||
| c.244C>A | L82M 2D  AIThe SynGAP1 missense variant L82M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which collectively classify the variant as likely benign. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized prediction is uncertain, and no Foldetta stability result is available. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of a ClinVar assertion; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.517720 | Binding | 0.284 | 0.890 | 0.375 | -4.608 | Likely Benign | 0.888 | Likely Pathogenic | Ambiguous | 0.082 | Likely Benign | 0.0744 | 0.3278 | -0.68 | Neutral | 0.939 | Possibly Damaging | 0.114 | Benign | 3.67 | Benign | 0.00 | Affected | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||
| c.244C>G | L82V 2D  AIThe SynGAP1 missense variant L82V is not reported in ClinVar (ClinVar status: not listed) but is present in gnomAD (ID 6‑33425852‑C‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Those that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain, and no Foldetta stability result is available. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments therefore indicate a benign likelihood: AlphaMissense‑Optimized is inconclusive, SGM‑Consensus is likely benign, and Foldetta data are missing. Overall, the majority of predictions support a benign impact, and this is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.517720 | Binding | 0.284 | 0.890 | 0.375 | 6-33425852-C-G | -6.701 | Likely Benign | 0.914 | Likely Pathogenic | Ambiguous | 0.065 | Likely Benign | 0.1467 | 0.2353 | -1.13 | Neutral | 0.371 | Benign | 0.024 | Benign | 3.72 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 2 | 0.4 | -14.03 | ||||||||||||||||||||||||||||||||
| c.2450C>T | S817L 2D  AIThe SynGAP1 missense variant S817L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and AlphaMissense‑Optimized, whereas a majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic impact; ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports the variant as Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect. This conclusion does not contradict ClinVar status, as no ClinVar classification exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.490133 | Structured | 0.727082 | Binding | 0.314 | 0.901 | 0.625 | -7.612 | In-Between | 0.659 | Likely Pathogenic | Likely Benign | 0.226 | Likely Benign | 0.1226 | 0.5662 | -3.90 | Deleterious | 0.997 | Probably Damaging | 0.945 | Probably Damaging | 2.41 | Pathogenic | 0.00 | Affected | -3 | -2 | 4.6 | 26.08 | |||||||||||||||||||||||||||||||||||
| c.2456C>A | A819E 2D  AIThe SynGAP1 missense variant A819E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. In contrast, the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as pathogenic. ESM1b is uncertain, and no Foldetta stability assessment is available. High‑accuracy methods give a consistent pathogenic signal: AlphaMissense‑Optimized predicts pathogenic, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as Likely Pathogenic, and Foldetta data are unavailable. Based on the preponderance of pathogenic predictions and the lack of any benign consensus, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.694846 | Disordered | 0.707644 | Binding | 0.317 | 0.892 | 0.625 | -7.333 | In-Between | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.292 | Likely Benign | 0.1189 | 0.2037 | -2.85 | Deleterious | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 2.19 | Pathogenic | 0.00 | Affected | 0 | -1 | -5.3 | 58.04 | |||||||||||||||||||||||||||||||||||
| c.245T>A | L82Q 2D  AIThe SynGAP1 missense variant L82Q is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized; ESM1b remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, whereas the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields a benign prediction. Foldetta results are unavailable. Overall, the majority of conventional tools lean toward benign, and the SGM Consensus supports this, but the AlphaMissense‑Optimized prediction introduces a pathogenic signal. Consequently, the variant is most likely benign based on the prevailing evidence, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.637480 | Disordered | 0.517720 | Binding | 0.284 | 0.890 | 0.375 | -7.576 | In-Between | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.079 | Likely Benign | 0.1150 | 0.0790 | -2.16 | Neutral | 0.939 | Possibly Damaging | 0.114 | Benign | 3.71 | Benign | 0.00 | Affected | -2 | -2 | -7.3 | 14.97 | ||||||||||||||||||||||||||||||||||||
| c.245T>C | L82P 2D  AIThe SynGAP1 missense variant L82P is not reported in ClinVar (ClinVar status: none) but is present in gnomAD (ID 6‑33425853‑T‑C). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, and FATHMM, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized; ESM1b remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic and the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors pathogenicity. Foldetta, a protein‑folding stability method, did not provide a result for this variant. Overall, the majority of evidence points to a pathogenic impact. This conclusion is not contradicted by ClinVar status, which has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.637480 | Disordered | 0.517720 | Binding | 0.284 | 0.890 | 0.375 | 6-33425853-T-C | 1 | 6.20e-7 | -7.667 | In-Between | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.125 | Likely Benign | 0.3477 | 0.1118 | -2.73 | Deleterious | 0.939 | Possibly Damaging | 0.162 | Benign | 3.64 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||
| c.245T>G | L82R 2D  AIThe SynGAP1 missense variant L82R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation, and this is not contradicted by any ClinVar annotation. Thus, based on the current computational evidence, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.517720 | Binding | 0.284 | 0.890 | 0.375 | -6.345 | Likely Benign | 0.974 | Likely Pathogenic | Likely Pathogenic | 0.077 | Likely Benign | 0.1338 | 0.0590 | -2.18 | Neutral | 0.939 | Possibly Damaging | 0.114 | Benign | 3.67 | Benign | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||
| c.2467A>C | S823R 2D  AIThe SynGAP1 missense variant S823R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) all indicate likely pathogenicity. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus also reports it as likely pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy prediction tools points to a pathogenic effect for S823R, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.685117 | Disordered | 0.627336 | Binding | 0.358 | 0.884 | 0.750 | -6.612 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.293 | Likely Benign | 0.0893 | 0.4046 | -3.47 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.93 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2467A>G | S823G 2D  AIThe SynGAP1 missense variant S823G is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, and the Foldetta stability analysis is unavailable. Based on the collective evidence, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.685117 | Disordered | 0.627336 | Binding | 0.358 | 0.884 | 0.750 | -6.034 | Likely Benign | 0.822 | Likely Pathogenic | Ambiguous | 0.202 | Likely Benign | 0.2855 | 0.5287 | -2.59 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.96 | Pathogenic | 0.00 | Affected | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||||||
| c.2467A>T | S823C 2D  AIThe SynGAP1 missense variant S823C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, whereas the majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic impact. Two tools give uncertain results: ESM1b and AlphaMissense‑Optimized. High‑accuracy assessments show that the SGM Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Pathogenic, while AlphaMissense‑Optimized remains uncertain. No Foldetta stability prediction is available, so it does not contribute to the assessment. Overall, the preponderance of evidence points to a pathogenic effect for S823C, and this conclusion does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.685117 | Disordered | 0.627336 | Binding | 0.358 | 0.884 | 0.750 | -7.881 | In-Between | 0.911 | Likely Pathogenic | Ambiguous | 0.332 | Likely Benign | 0.1019 | 0.6137 | -3.80 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.91 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2468G>A | S823N 2D  AIThe SynGAP1 missense variant S823N is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b. Tools that agree on a pathogenic effect include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic, two benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Based on the available predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.685117 | Disordered | 0.627336 | Binding | 0.358 | 0.884 | 0.750 | -6.880 | Likely Benign | 0.974 | Likely Pathogenic | Likely Pathogenic | 0.170 | Likely Benign | 0.1370 | 0.5172 | -1.76 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 1.93 | Pathogenic | 0.00 | Affected | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||||||||
| c.2468G>C | S823T 2D  AIThe SynGAP1 missense variant S823T is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus (SGM Consensus) derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN is inconclusive (two benign, two pathogenic). AlphaMissense‑Optimized predicts benign, while Foldetta (combining FoldX‑MD and Rosetta) has no available result for this variant. Overall, the majority of standard predictors indicate a pathogenic effect, and this assessment does not contradict the lack of ClinVar annotation. Thus, the variant is most likely pathogenic based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.685117 | Disordered | 0.627336 | Binding | 0.358 | 0.884 | 0.750 | -6.036 | Likely Benign | 0.717 | Likely Pathogenic | Likely Benign | 0.165 | Likely Benign | 0.1453 | 0.6580 | -2.02 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.05 | Pathogenic | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||||||||
| c.2468G>T | S823I 2D  AIThe SynGAP1 missense variant S823I is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a deleterious effect: pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only REVEL predicts a benign outcome, while ESM1b remains uncertain. High‑accuracy assessments reinforce the pathogenic signal: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is labeled Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of prediction tools indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.685117 | Disordered | 0.627336 | Binding | 0.358 | 0.884 | 0.750 | -7.332 | In-Between | 0.990 | Likely Pathogenic | Likely Pathogenic | 0.287 | Likely Benign | 0.0964 | 0.5848 | -4.26 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.92 | Pathogenic | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||
| c.2469C>A | S823R 2D  AIThe SynGAP1 missense variant S823R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a pathogenic effect, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.685117 | Disordered | 0.627336 | Binding | 0.358 | 0.884 | 0.750 | -6.612 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.196 | Likely Benign | 0.0893 | 0.4046 | -3.47 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.93 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2469C>G | S823R 2D  AIThe SynGAP1 missense variant S823R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a pathogenic effect, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.685117 | Disordered | 0.627336 | Binding | 0.358 | 0.884 | 0.750 | -6.612 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.196 | Likely Benign | 0.0893 | 0.4046 | -3.47 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.93 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2474C>G | S825W 2D  AIThe SynGAP1 missense variant S825W is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that assess pathogenicity largely agree: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all predict a deleterious effect. Only REVEL classifies the variant as benign, representing the sole benign prediction. High‑accuracy methods further support a pathogenic interpretation: AlphaMissense‑Optimized returns a pathogenic score, and the SGM‑Consensus indicates “Likely Pathogenic.” Foldetta, a protein‑folding stability predictor, has no available result for this variant. Overall, the consensus of the majority of tools, including the high‑accuracy predictors, indicates that S825W is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.618614 | Binding | 0.384 | 0.886 | 0.750 | -8.396 | Likely Pathogenic | 0.981 | Likely Pathogenic | Likely Pathogenic | 0.316 | Likely Benign | 0.0818 | 0.6083 | -5.25 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.91 | Pathogenic | 0.00 | Affected | -2 | -3 | -0.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.2476G>C | D826H 2D  AIThe SynGAP1 missense variant D826H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence indicates that D826H is most likely pathogenic, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.666105 | Disordered | 0.627309 | Binding | 0.327 | 0.886 | 0.625 | -6.437 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.345 | Likely Benign | 0.1757 | 0.8651 | -3.00 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.49 | Pathogenic | 0.00 | Affected | 1 | -1 | 0.3 | 22.05 | |||||||||||||||||||||||||||||||||||
| c.2476G>T | D826Y 2D  AIThe SynGAP1 missense variant D826Y is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining predictors—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—label it pathogenic. The consensus score from the SGM framework, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further support this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM Consensus also indicates Likely Pathogenic; Foldetta results are not available. Taken together, the overwhelming majority of evidence points to a pathogenic effect for D826Y. Thus, the variant is most likely pathogenic, and this assessment does not contradict the current ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.666105 | Disordered | 0.627309 | Binding | 0.327 | 0.886 | 0.625 | -8.029 | Likely Pathogenic | 0.988 | Likely Pathogenic | Likely Pathogenic | 0.336 | Likely Benign | 0.0683 | 0.7410 | -4.25 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.47 | Pathogenic | 0.00 | Affected | -4 | -3 | 2.2 | 48.09 | |||||||||||||||||||||||||||||||||||
| c.2477A>T | D826V 2D  AIThe SynGAP1 missense variant D826V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments reinforce the pathogenic signal: AlphaMissense‑Optimized predicts pathogenic, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also indicates likely pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect for D826V, and this conclusion does not conflict with ClinVar, which currently contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.666105 | Disordered | 0.627309 | Binding | 0.327 | 0.886 | 0.625 | -6.918 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.428 | Likely Benign | 0.1017 | 0.8023 | -4.64 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||
| c.247A>G | R83G 2D  AIThe SynGAP1 R83G missense variant is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Tools that agree on a pathogenic effect include PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenicity. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic vs. two benign votes) and is therefore treated as unavailable. Foldetta stability analysis is not provided and is likewise unavailable. Overall, the preponderance of evidence (six pathogenic vs. three benign predictions) indicates that the variant is most likely pathogenic, with no contradiction to ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.637480 | Disordered | 0.522784 | Binding | 0.275 | 0.895 | 0.250 | -4.708 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.111 | Likely Benign | 0.3514 | 0.2537 | -2.60 | Deleterious | 0.909 | Possibly Damaging | 0.587 | Possibly Damaging | 3.18 | Benign | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | ||||||||||||||||||||||||||||||||||||
| c.2485G>A | E829K 2D  AIThe SynGAP1 missense variant E829K is listed in ClinVar as Pathogenic (ClinVar ID 1721258.0) and is not reported in gnomAD. Functional prediction tools largely agree on a deleterious effect: pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only REVEL predicts a benign outcome, while ESM1b and AlphaMissense‑Optimized are uncertain. High‑accuracy assessments show the SGM‑Consensus as Likely Pathogenic, AlphaMissense‑Optimized as uncertain, and Foldetta results are unavailable. Overall, the preponderance of evidence indicates that E829K is most likely pathogenic, and this conclusion aligns with the ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.562014 | Disordered | 0.626045 | Binding | 0.326 | 0.882 | 0.375 | Pathogenic | 1 | -7.527 | In-Between | 0.807 | Likely Pathogenic | Ambiguous | 0.194 | Likely Benign | 0.2400 | 0.7372 | -2.65 | Deleterious | 0.994 | Probably Damaging | 0.900 | Possibly Damaging | 2.27 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | 1 | -0.4 | -0.94 | |||||||||||||||||||||||||||||||
| c.2485G>C | E829Q 2D  AIThe SynGAP1 missense variant E829Q is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also yields a benign prediction. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of reliable tools predict a benign impact, and there is no ClinVar entry to contradict this assessment. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.562014 | Disordered | 0.626045 | Binding | 0.326 | 0.882 | 0.375 | -4.985 | Likely Benign | 0.531 | Ambiguous | Likely Benign | 0.225 | Likely Benign | 0.1321 | 0.7248 | -1.83 | Neutral | 0.994 | Probably Damaging | 0.946 | Probably Damaging | 2.26 | Pathogenic | 0.00 | Affected | 2 | 2 | 0.0 | -0.98 | ||||||||||||||||||||||||||||||||||||
| c.2486A>C | E829A 2D  AIThe SynGAP1 missense variant E829A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which is a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as Likely Pathogenic, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) results are unavailable. Overall, the majority of evidence—including the SGM‑Consensus—points to a pathogenic impact. Thus, the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.562014 | Disordered | 0.626045 | Binding | 0.326 | 0.882 | 0.375 | -4.096 | Likely Benign | 0.574 | Likely Pathogenic | Likely Benign | 0.265 | Likely Benign | 0.4707 | 0.7255 | -3.75 | Deleterious | 0.994 | Probably Damaging | 0.926 | Probably Damaging | 2.26 | Pathogenic | 0.00 | Affected | 0 | -1 | 5.3 | -58.04 | |||||||||||||||||||||||||||||||||||
| c.2486A>G | E829G 2D  AIThe SynGAP1 missense variant E829G is not reported in ClinVar and is absent from gnomAD. Prediction tools that indicate a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas the remaining tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely pathogenic, and the Foldetta stability analysis is unavailable. Overall, the majority of predictions lean toward pathogenicity, with one high‑accuracy tool suggesting benign. No ClinVar annotation exists, so there is no contradiction with clinical database status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.562014 | Disordered | 0.626045 | Binding | 0.326 | 0.882 | 0.375 | -4.152 | Likely Benign | 0.593 | Likely Pathogenic | Likely Benign | 0.316 | Likely Benign | 0.3517 | 0.6557 | -4.52 | Deleterious | 0.994 | Probably Damaging | 0.927 | Probably Damaging | 2.24 | Pathogenic | 0.00 | Affected | 0 | -2 | 3.1 | -72.06 | |||||||||||||||||||||||||||||||||||
| c.2486A>T | E829V 2D  AIThe SynGAP1 missense variant E829V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus (majority of the four high‑accuracy inputs) remains pathogenic; Foldetta results are unavailable. Based on the overall distribution of predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.562014 | Disordered | 0.626045 | Binding | 0.326 | 0.882 | 0.375 | -5.142 | Likely Benign | 0.719 | Likely Pathogenic | Likely Benign | 0.296 | Likely Benign | 0.0835 | 0.7984 | -4.86 | Deleterious | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 2.21 | Pathogenic | 0.00 | Affected | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||||||||
| c.2487G>C | E829D 2D  AIThe SynGAP1 missense variant E829D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence from multiple independent predictors indicates that E829D is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.562014 | Disordered | 0.626045 | Binding | 0.326 | 0.882 | 0.375 | -4.015 | Likely Benign | 0.091 | Likely Benign | Likely Benign | 0.066 | Likely Benign | 0.1939 | 0.4964 | -1.89 | Neutral | 0.217 | Benign | 0.121 | Benign | 2.40 | Pathogenic | 0.00 | Affected | 3 | 2 | 0.0 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2487G>T | E829D 2D  AIThe SynGAP1 missense variant E829D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence from multiple independent predictors indicates that E829D is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.562014 | Disordered | 0.626045 | Binding | 0.326 | 0.882 | 0.375 | -4.015 | Likely Benign | 0.091 | Likely Benign | Likely Benign | 0.066 | Likely Benign | 0.1939 | 0.4964 | -1.89 | Neutral | 0.217 | Benign | 0.121 | Benign | 2.40 | Pathogenic | 0.00 | Affected | 3 | 2 | 0.0 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2489C>A | P830Q 2D  AIThe SynGAP1 missense variant P830Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.590140 | Disordered | 0.618152 | Binding | 0.333 | 0.874 | 0.500 | -4.984 | Likely Benign | 0.304 | Likely Benign | Likely Benign | 0.257 | Likely Benign | 0.1396 | 0.4741 | -3.40 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.68 | Benign | 0.00 | Affected | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||||||||||
| c.2489C>G | P830R 2D  AIThe SynGAP1 missense variant P830R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of reliable predictors and the high‑accuracy consensus indicate a benign impact. This conclusion is not contradicted by any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.590140 | Disordered | 0.618152 | Binding | 0.333 | 0.874 | 0.500 | -5.919 | Likely Benign | 0.515 | Ambiguous | Likely Benign | 0.227 | Likely Benign | 0.1277 | 0.2984 | -3.51 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.72 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||||
| c.2489C>T | P830L 2D  AIThe SynGAP1 missense variant P830L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also predicts benign. Foldetta results are unavailable. Overall, the majority of reliable predictors lean toward a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.590140 | Disordered | 0.618152 | Binding | 0.333 | 0.874 | 0.500 | -3.990 | Likely Benign | 0.362 | Ambiguous | Likely Benign | 0.269 | Likely Benign | 0.2138 | 0.6631 | -5.31 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.65 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||
| c.248G>A | R83K 2D  AIThe SynGAP1 missense variant R83K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign,” while AlphaMissense‑Optimized is “Uncertain.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.522784 | Binding | 0.275 | 0.895 | 0.250 | -3.480 | Likely Benign | 0.930 | Likely Pathogenic | Ambiguous | 0.101 | Likely Benign | 0.4715 | 0.3091 | -0.87 | Neutral | 0.643 | Possibly Damaging | 0.364 | Benign | 3.28 | Benign | 0.00 | Affected | 3 | 2 | 0.6 | -28.01 | |||||||||||||||||||||||||||||||||||
| c.248G>C | R83T 2D  AIThe SynGAP1 missense variant R83T has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign calls (REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus “Likely Benign”) and pathogenic calls (polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, AlphaMissense‑Optimized). High‑accuracy assessments further split the verdict: AlphaMissense‑Optimized predicts pathogenic, whereas the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates likely benign; Foldetta results are unavailable. With five tools supporting benign and five supporting pathogenic, the evidence is evenly divided. Consequently, the variant’s clinical significance remains uncertain and is not contradicted by any ClinVar annotation, which has no classification. Overall, the variant is most likely pathogenic, and this does not contradict ClinVar status, which is currently absent. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.522784 | Binding | 0.275 | 0.895 | 0.250 | -3.585 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.124 | Likely Benign | 0.1671 | 0.3360 | -2.15 | Neutral | 0.909 | Possibly Damaging | 0.587 | Possibly Damaging | 3.18 | Benign | 0.00 | Affected | -1 | -1 | 3.8 | -55.08 | |||||||||||||||||||||||||||||||||||
| c.248G>T | R83I 2D  AIThe SynGAP1 missense variant R83I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic vs. two benign). Foldetta, which would provide a protein‑folding stability estimate, has no available result for this variant. Overall, the preponderance of evidence (seven pathogenic vs. three benign predictions) indicates that R83I is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.637480 | Disordered | 0.522784 | Binding | 0.275 | 0.895 | 0.250 | -4.021 | Likely Benign | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.183 | Likely Benign | 0.1450 | 0.3219 | -3.15 | Deleterious | 0.972 | Probably Damaging | 0.766 | Possibly Damaging | 3.17 | Benign | 0.00 | Affected | -2 | -3 | 9.0 | -43.03 | ||||||||||||||||||||||||||||||||||||
| c.249A>C | R83S 2D  AIThe SynGAP1 R83S missense variant has no ClinVar entry and is present in gnomAD (ID 6‑33425857‑A‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) remains Likely Benign. Foldetta results are unavailable. Overall, the predictions are split, but the consensus‑based high‑accuracy tools lean toward a benign interpretation. Thus, the variant is most likely benign based on current computational evidence, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.522784 | Binding | 0.275 | 0.895 | 0.250 | 6-33425857-A-C | 1 | 6.20e-7 | -2.550 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.094 | Likely Benign | 0.3161 | 0.2734 | -1.87 | Neutral | 0.909 | Possibly Damaging | 0.587 | Possibly Damaging | 3.19 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -1 | 3.7 | -69.11 | ||||||||||||||||||||||||||||||
| c.249A>T | R83S 2D  AISynGAP1 R83S is listed in ClinVar (ID 537001.0) with an uncertain significance and is not reported in gnomAD. Prediction tools that classify the variant as benign include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict pathogenicity are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates likely benign; Foldetta results are unavailable. Overall, the predictions are split, with an equal number of benign and pathogenic calls, and the high‑accuracy tools are discordant. Thus, the variant is most likely pathogenic based on the predominance of pathogenic predictions, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.637480 | Disordered | 0.522784 | Binding | 0.275 | 0.895 | 0.250 | Uncertain | 1 | -2.550 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.094 | Likely Benign | 0.3161 | 0.2734 | -1.87 | Neutral | 0.909 | Possibly Damaging | 0.587 | Possibly Damaging | 3.19 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||
| c.24C>G | I8M 2D  AIThe SynGAP1 missense variant I8M is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus, SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign status. Foldetta, a protein‑folding stability method that combines FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.543080 | Binding | 0.341 | 0.916 | 0.625 | -3.957 | Likely Benign | 0.140 | Likely Benign | Likely Benign | 0.085 | Likely Benign | 0.0626 | 0.3034 | -0.07 | Neutral | 0.296 | Benign | 0.022 | Benign | 4.02 | Benign | 0.00 | Affected | 2 | 1 | -2.6 | 18.03 | |||||||||||||||||||||||||||||||||||
| c.2500A>C | M834L 2D  AIThe SynGAP1 missense variant M834L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | -1.926 | Likely Benign | 0.114 | Likely Benign | Likely Benign | 0.136 | Likely Benign | 0.1087 | 0.3331 | -0.97 | Neutral | 0.000 | Benign | 0.001 | Benign | 3.05 | Benign | 0.00 | Affected | 4 | 2 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||
| c.2500A>G | M834V 2D  AIThe SynGAP1 missense variant M834V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | -2.345 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.059 | Likely Benign | 0.2562 | 0.2705 | -1.10 | Neutral | 0.001 | Benign | 0.001 | Benign | 2.53 | Benign | 0.00 | Affected | 2 | 1 | 2.3 | -32.06 | |||||||||||||||||||||||||||||||||||
| c.2500A>T | M834L 2D  AIThe SynGAP1 missense variant M834L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | -1.926 | Likely Benign | 0.114 | Likely Benign | Likely Benign | 0.136 | Likely Benign | 0.1087 | 0.3331 | -0.97 | Neutral | 0.000 | Benign | 0.001 | Benign | 3.05 | Benign | 0.00 | Affected | 4 | 2 | 1.9 | -18.03 | |||||||||||||||||||||||||||||||||||
| c.2501T>A | M834K 2D  AIThe SynGAP1 missense variant M834K is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also predicts benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation and does not contradict any existing clinical database status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | -3.154 | Likely Benign | 0.453 | Ambiguous | Likely Benign | 0.154 | Likely Benign | 0.1131 | 0.0688 | -2.21 | Neutral | 0.369 | Benign | 0.150 | Benign | 2.43 | Pathogenic | 0.00 | Affected | 0 | -1 | -5.8 | -3.02 | ||||||||||||||||||||||||||||||||||||
| c.2501T>C | M834T 2D  AIThe SynGAP1 missense variant M834T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for M834T, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | -1.525 | Likely Benign | 0.332 | Likely Benign | Likely Benign | 0.137 | Likely Benign | 0.1757 | 0.1799 | -2.03 | Neutral | 0.224 | Benign | 0.085 | Benign | 2.45 | Pathogenic | 0.00 | Affected | -1 | -1 | -2.6 | -30.09 | |||||||||||||||||||||||||||||||||||
| c.2501T>G | M834R 2D  AIThe SynGAP1 missense variant M834R is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) leans toward benign (2 benign vs. 1 pathogenic votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence indicates a benign impact, and this conclusion does not contradict any ClinVar annotation, as none exists for M834R. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | -2.621 | Likely Benign | 0.449 | Ambiguous | Likely Benign | 0.148 | Likely Benign | 0.1310 | 0.0837 | -2.44 | Neutral | 0.812 | Possibly Damaging | 0.284 | Benign | 2.43 | Pathogenic | 0.00 | Affected | 0 | -1 | -6.4 | 24.99 | ||||||||||||||||||||||||||||||||||||
| c.2502G>A | M834I 2D  AIThe SynGAP1 missense variant M834I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | -3.377 | Likely Benign | 0.291 | Likely Benign | Likely Benign | 0.055 | Likely Benign | 0.0976 | 0.2456 | -1.21 | Neutral | 0.026 | Benign | 0.009 | Benign | 2.56 | Benign | 0.00 | Affected | 4.32 | 4 | 1 | 2 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||
| c.2502G>C | M834I 2D  AIThe SynGAP1 missense variant M834I is listed in ClinVar (ID 3007819.0) with an uncertain significance designation and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only SIFT classifies the change as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority‑vote) is likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the collective predictions indicate that M834I is most likely benign, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | Uncertain | 1 | -3.377 | Likely Benign | 0.291 | Likely Benign | Likely Benign | 0.055 | Likely Benign | 0.0976 | 0.2456 | -1.21 | Neutral | 0.026 | Benign | 0.009 | Benign | 2.56 | Benign | 0.00 | Affected | 4.32 | 4 | 1 | 2 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||
| c.2502G>T | M834I 2D  AIThe SynGAP1 missense variant M834I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect; there is no ClinVar entry to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.585406 | Disordered | 0.640801 | Binding | 0.258 | 0.863 | 0.375 | -3.377 | Likely Benign | 0.291 | Likely Benign | Likely Benign | 0.055 | Likely Benign | 0.0976 | 0.2456 | -1.21 | Neutral | 0.026 | Benign | 0.009 | Benign | 2.56 | Benign | 0.00 | Affected | 4.32 | 4 | 1 | 2 | 2.6 | -18.03 | |||||||||||||||||||||||||||||||||
| c.250C>A | R84S 2D  AIThe SynGAP1 missense variant R84S is not reported in ClinVar and has no gnomAD entry. Prediction tools show mixed results: benign predictions come from SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, and FATHMM, whereas pathogenic predictions arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments further highlight this discordance: AlphaMissense‑Optimized predicts pathogenic, SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates likely benign, and Foldetta stability analysis is unavailable. Overall, the majority of tools lean toward a benign interpretation, but a substantial subset predicts pathogenicity, leaving the variant’s effect uncertain. Based on the current predictions, R84S is most likely benign, and this assessment does not contradict any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.666105 | Disordered | 0.529205 | Binding | 0.298 | 0.888 | 0.500 | -6.233 | Likely Benign | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.103 | Likely Benign | 0.2966 | 0.4139 | -2.18 | Neutral | 0.962 | Probably Damaging | 0.726 | Possibly Damaging | 3.71 | Benign | 0.00 | Affected | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||
| c.250C>G | R84G 2D  AIThe SynGAP1 missense variant R84G is listed in ClinVar with an “Uncertain” significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM, whereas those that predict a pathogenic outcome are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic versus two benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Based on the majority of available predictions, the variant is most likely pathogenic, which does not contradict the current ClinVar status of “Uncertain.” Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.529205 | Binding | 0.298 | 0.888 | 0.500 | Uncertain | 1 | -6.627 | Likely Benign | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.139 | Likely Benign | 0.3387 | 0.3391 | -2.64 | Deleterious | 0.962 | Probably Damaging | 0.726 | Possibly Damaging | 3.68 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -2 | 4.1 | -99.14 | ||||||||||||||||||||||||||||||||
| c.250C>T | R84C 2D  AIThe SynGAP1 missense variant R84C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and FATHMM, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus (a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that R84C is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.666105 | Disordered | 0.529205 | Binding | 0.298 | 0.888 | 0.500 | -9.044 | Likely Pathogenic | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.243 | Likely Benign | 0.3281 | 0.3657 | -3.25 | Deleterious | 0.999 | Probably Damaging | 0.876 | Possibly Damaging | 3.66 | Benign | 0.00 | Affected | -4 | -3 | 7.0 | -53.05 | |||||||||||||||||||||||||||||||||||
| c.2516A>T | K839M 2D  AIThe SynGAP1 missense variant K839M is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL indicates a benign likelihood, whereas the remaining predictors—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—classify the change as pathogenic. The consensus score from the SGM framework, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Pathogenic.” High‑accuracy assessments further support this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM consensus also reports a likely pathogenic outcome. Foldetta, a protein‑folding stability method that combines FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion is consistent with the absence of a ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.642678 | Disordered | 0.611185 | Binding | 0.282 | 0.865 | 0.375 | -13.688 | Likely Pathogenic | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.241 | Likely Benign | 0.1253 | 0.4481 | -3.54 | Deleterious | 1.000 | Probably Damaging | 0.983 | Probably Damaging | 2.40 | Pathogenic | 0.00 | Affected | 0 | -1 | 5.8 | 3.02 | |||||||||||||||||||||||||||||||||||
| c.2518A>C | S840R 2D  AIThe SynGAP1 missense variant S840R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining 11 predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) all predict pathogenicity. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as Likely Pathogenic. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus result is consistent. Foldetta, a protein‑folding stability method that combines FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence indicates that S840R is most likely pathogenic, and this conclusion does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.622677 | Disordered | 0.611356 | Binding | 0.259 | 0.865 | 0.250 | -9.366 | Likely Pathogenic | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.282 | Likely Benign | 0.0726 | 0.3328 | -3.27 | Deleterious | 0.993 | Probably Damaging | 0.904 | Possibly Damaging | 1.51 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2518A>G | S840G 2D  AIThe SynGAP1 missense variant S840G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and AlphaMissense‑Optimized, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, while the SGM‑Consensus remains Likely Pathogenic; the Foldetta protein‑folding stability analysis is unavailable. Overall, the preponderance of evidence points to a pathogenic effect for S840G, and this conclusion does not contradict any existing ClinVar annotation, as none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.622677 | Disordered | 0.611356 | Binding | 0.259 | 0.865 | 0.250 | -8.117 | Likely Pathogenic | 0.674 | Likely Pathogenic | Likely Benign | 0.163 | Likely Benign | 0.2354 | 0.3858 | -2.72 | Deleterious | 0.889 | Possibly Damaging | 0.663 | Possibly Damaging | 1.54 | Pathogenic | 0.00 | Affected | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||||||
| c.2518A>T | S840C 2D  AIThe SynGAP1 missense variant S840C is listed in ClinVar (ID 2089808.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include only REVEL, whereas the majority of algorithms—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default—consistently predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain,” SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as “Likely Pathogenic,” and Foldetta results are unavailable. Taken together, the preponderance of evidence points to a pathogenic effect for S840C. This conclusion aligns with the ClinVar designation of uncertainty rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.622677 | Disordered | 0.611356 | Binding | 0.259 | 0.865 | 0.250 | Uncertain | 1 | -8.799 | Likely Pathogenic | 0.904 | Likely Pathogenic | Ambiguous | 0.376 | Likely Benign | 0.0803 | 0.5481 | -3.96 | Deleterious | 0.999 | Probably Damaging | 0.975 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||
| c.2519G>A | S840N 2D  AIThe SynGAP1 missense variant S840N is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, and both polyPhen‑2 HumDiv and HumVar scores. Tools that predict a pathogenic effect are SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is pathogenic, while AlphaMissense‑Optimized is uncertain. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑confidence predictions (including the SGM consensus) indicate a pathogenic impact, and this assessment does not contradict any ClinVar annotation because no ClinVar entry exists. Thus, the variant is most likely pathogenic based on the current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.622677 | Disordered | 0.611356 | Binding | 0.259 | 0.865 | 0.250 | -9.849 | Likely Pathogenic | 0.891 | Likely Pathogenic | Ambiguous | 0.130 | Likely Benign | 0.0934 | 0.4181 | -1.65 | Neutral | 0.206 | Benign | 0.098 | Benign | 1.52 | Pathogenic | 0.00 | Affected | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||
| c.2519G>C | S840T 2D  AIThe SynGAP1 missense variant S840T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default; ESM1b remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is pathogenic. Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs. three benign) indicate that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.622677 | Disordered | 0.611356 | Binding | 0.259 | 0.865 | 0.250 | -7.243 | In-Between | 0.583 | Likely Pathogenic | Likely Benign | 0.170 | Likely Benign | 0.1068 | 0.5494 | -2.30 | Neutral | 0.951 | Possibly Damaging | 0.729 | Possibly Damaging | 1.55 | Pathogenic | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||||||||
| c.2519G>T | S840I 2D  AIThe SynGAP1 missense variant S840I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. All other evaluated predictors—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—classify the variant as pathogenic or likely pathogenic. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus indicates likely pathogenic. Foldetta results are not available, so they do not influence the overall assessment. Based on the consensus of the majority of prediction tools and the high‑accuracy methods, the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.622677 | Disordered | 0.611356 | Binding | 0.259 | 0.865 | 0.250 | -12.509 | Likely Pathogenic | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.357 | Likely Benign | 0.0788 | 0.5251 | -4.31 | Deleterious | 0.998 | Probably Damaging | 0.967 | Probably Damaging | 1.51 | Pathogenic | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||
| c.251G>A | R84H 2D  AIThe SynGAP1 missense variant R84H is not reported in ClinVar (ClinVar status: not reported) and is absent from gnomAD (gnomAD: not present). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM, while those that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. Two tools give uncertain results: ESM1b and AlphaMissense‑Optimized. High‑accuracy assessment shows that AlphaMissense‑Optimized remains uncertain, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) resolves as benign, and Foldetta (combining FoldX‑MD and Rosetta) has no available output. Overall, the majority of standard predictors (four pathogenic vs. three benign) lean toward a pathogenic interpretation, and the high‑accuracy consensus does not overturn this trend. Thus, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.529205 | Binding | 0.298 | 0.888 | 0.500 | -7.333 | In-Between | 0.935 | Likely Pathogenic | Ambiguous | 0.147 | Likely Benign | 0.2739 | 0.2426 | -1.77 | Neutral | 0.995 | Probably Damaging | 0.840 | Possibly Damaging | 3.68 | Benign | 0.00 | Affected | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||||||||||||||||
| c.251G>C | R84P 2D  AIThe SynGAP1 missense variant R84P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are REVEL and FATHMM, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. ESM1b is uncertain and does not influence the consensus. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a pathogenic prediction. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the preponderance of evidence indicates that R84P is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.529205 | Binding | 0.298 | 0.888 | 0.500 | -7.959 | In-Between | 0.994 | Likely Pathogenic | Likely Pathogenic | 0.157 | Likely Benign | 0.1978 | 0.4231 | -2.50 | Deleterious | 0.989 | Probably Damaging | 0.859 | Possibly Damaging | 3.67 | Benign | 0.00 | Affected | 0 | -2 | 2.9 | -59.07 | ||||||||||||||||||||||||||||||||||||
| c.251G>T | R84L 2D  AIThe SynGAP1 missense variant R84L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect are REVEL and FATHMM, while the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. ESM1b remains uncertain. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a pathogenic verdict (2 pathogenic vs. 1 benign). Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the preponderance of evidence indicates that R84L is most likely pathogenic, and this conclusion does not contradict any existing ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.529205 | Binding | 0.298 | 0.888 | 0.500 | -7.579 | In-Between | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.120 | Likely Benign | 0.1595 | 0.4653 | -2.75 | Deleterious | 0.962 | Probably Damaging | 0.726 | Possibly Damaging | 3.70 | Benign | 0.00 | Affected | -3 | -2 | 8.3 | -43.03 | ||||||||||||||||||||||||||||||||||||
| c.2520T>A | S840R 2D  AIThe SynGAP1 missense variant S840R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that assess pathogenicity largely agree: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all predict a deleterious effect. Only REVEL classifies the variant as benign, representing the sole benign prediction. High‑accuracy assessments further support a pathogenic interpretation: AlphaMissense‑Optimized returns a pathogenic score, and the SGM‑Consensus indicates “Likely Pathogenic.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence from multiple in silico tools and high‑accuracy predictors indicates that S840R is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.622677 | Disordered | 0.611356 | Binding | 0.259 | 0.865 | 0.250 | -9.366 | Likely Pathogenic | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.244 | Likely Benign | 0.0726 | 0.3328 | -3.27 | Deleterious | 0.993 | Probably Damaging | 0.904 | Possibly Damaging | 1.51 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2520T>G | S840R 2D  AIThe SynGAP1 missense variant S840R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that assess pathogenicity largely agree: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all predict a deleterious effect. Only REVEL classifies the variant as benign, representing the sole benign prediction. High‑accuracy assessments further support a pathogenic outcome: AlphaMissense‑Optimized returns a pathogenic score, and the SGM‑Consensus indicates “Likely Pathogenic.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence from multiple in silico tools and high‑accuracy predictors indicates that S840R is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.622677 | Disordered | 0.611356 | Binding | 0.259 | 0.865 | 0.250 | -9.366 | Likely Pathogenic | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.244 | Likely Benign | 0.0726 | 0.3328 | -3.27 | Deleterious | 0.993 | Probably Damaging | 0.904 | Possibly Damaging | 1.51 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2522T>A | V841E 2D  AIThe SynGAP1 missense variant V841E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are REVEL and FATHMM. All other evaluated algorithms—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict a pathogenic impact. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from multiple prediction tools and high‑accuracy methods indicates that V841E is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.622677 | Disordered | 0.616495 | Binding | 0.261 | 0.873 | 0.125 | -13.750 | Likely Pathogenic | 0.976 | Likely Pathogenic | Likely Pathogenic | 0.292 | Likely Benign | 0.0939 | 0.1656 | -3.13 | Deleterious | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.52 | Benign | 0.00 | Affected | -2 | -2 | -7.7 | 29.98 | |||||||||||||||||||||||||||||||||||
| c.2522T>G | V841G 2D  AIThe SynGAP1 missense variant V841G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect are REVEL and FATHMM, while the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default—consistently predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, whereas the SGM Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) favors pathogenicity. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect for V841G, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.622677 | Disordered | 0.616495 | Binding | 0.261 | 0.873 | 0.125 | -10.054 | Likely Pathogenic | 0.913 | Likely Pathogenic | Ambiguous | 0.288 | Likely Benign | 0.2351 | 0.2422 | -4.11 | Deleterious | 0.997 | Probably Damaging | 0.999 | Probably Damaging | 2.51 | Benign | 0.00 | Affected | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||
| c.2524T>A | S842T 2D  AIThe SynGAP1 missense variant S842T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, while the SGM‑Consensus (majority of the four high‑accuracy predictors) is Pathogenic; Foldetta results are unavailable. Overall, the majority of evidence points to a pathogenic impact for S842T, and this conclusion does not contradict any ClinVar annotation because no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.617281 | Binding | 0.274 | 0.861 | 0.250 | -13.443 | Likely Pathogenic | 0.725 | Likely Pathogenic | Likely Benign | 0.163 | Likely Benign | 0.0974 | 0.5391 | -2.23 | Neutral | 0.983 | Probably Damaging | 0.702 | Possibly Damaging | 2.15 | Pathogenic | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2524T>C | S842P 2D  AIThe SynGAP1 missense variant S842P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that assess pathogenicity largely agree: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all predict a deleterious effect. Only REVEL classifies the variant as benign. High‑accuracy assessments further support a pathogenic interpretation: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus indicates “Likely Pathogenic.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the consensus of the majority of tools, including the high‑accuracy predictors, indicates that S842P is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.617281 | Binding | 0.274 | 0.861 | 0.250 | -13.890 | Likely Pathogenic | 0.972 | Likely Pathogenic | Likely Pathogenic | 0.309 | Likely Benign | 0.1494 | 0.5342 | -3.43 | Deleterious | 0.995 | Probably Damaging | 0.892 | Possibly Damaging | 1.99 | Pathogenic | 0.00 | Affected | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||
| c.2524T>G | S842A 2D  AIThe SynGAP1 missense variant S842A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Overall, the majority of predictions (seven pathogenic vs. three benign) indicate a pathogenic impact. This conclusion is not contradicted by ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.617281 | Binding | 0.274 | 0.861 | 0.250 | -13.601 | Likely Pathogenic | 0.656 | Likely Pathogenic | Likely Benign | 0.180 | Likely Benign | 0.3971 | 0.4223 | -2.37 | Neutral | 0.889 | Possibly Damaging | 0.614 | Possibly Damaging | 2.09 | Pathogenic | 0.00 | Affected | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||
| c.2525C>A | S842Y 2D  AIThe SynGAP1 missense variant S842Y is listed in ClinVar as Pathogenic (ClinVar ID 624244.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized returns a pathogenic score, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is labeled Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, in agreement with its ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.617281 | Binding | 0.274 | 0.861 | 0.250 | Likely Pathogenic | 1 | -16.124 | Likely Pathogenic | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.191 | Likely Benign | 0.0576 | 0.5403 | -4.28 | Deleterious | 0.944 | Possibly Damaging | 0.676 | Possibly Damaging | 1.97 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||
| c.2525C>G | S842C 2D  AIThe SynGAP1 missense variant S842C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. All other evaluated algorithms—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—predict a pathogenic or likely pathogenic outcome. AlphaMissense‑Optimized is uncertain, providing no definitive direction. High‑accuracy assessments show the SGM‑Consensus as Likely Pathogenic, while the Foldetta stability analysis is unavailable. Based on the collective evidence, the variant is most likely pathogenic; this conclusion does not contradict any ClinVar status, as none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.617281 | Binding | 0.274 | 0.861 | 0.250 | -12.405 | Likely Pathogenic | 0.863 | Likely Pathogenic | Ambiguous | 0.233 | Likely Benign | 0.0806 | 0.5506 | -3.93 | Deleterious | 0.999 | Probably Damaging | 0.944 | Probably Damaging | 1.98 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2525C>T | S842F 2D  AIThe SynGAP1 missense variant S842F is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split: benign calls from REVEL, polyPhen‑2 HumDiv and HumVar, whereas pathogenic calls come from PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default and AlphaMissense‑Optimized. The consensus predictor SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus result is consistent with this. Foldetta, a protein‑folding stability method that combines FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a pathogenic impact, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.604312 | Disordered | 0.617281 | Binding | 0.274 | 0.861 | 0.250 | -14.590 | Likely Pathogenic | 0.988 | Likely Pathogenic | Likely Pathogenic | 0.188 | Likely Benign | 0.0584 | 0.5692 | -4.12 | Deleterious | 0.029 | Benign | 0.043 | Benign | 1.98 | Pathogenic | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||||||||
| c.2528T>A | M843K 2D  AIThe SynGAP1 missense variant M843K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are REVEL and FATHMM, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—all predict a pathogenic or likely pathogenic impact. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus indicates it is likely pathogenic. The Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that M843K is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation because no ClinVar status exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.617934 | Binding | 0.327 | 0.854 | 0.375 | -13.256 | Likely Pathogenic | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.442 | Likely Benign | 0.1763 | 0.0940 | -3.60 | Deleterious | 0.968 | Probably Damaging | 0.969 | Probably Damaging | 2.60 | Benign | 0.00 | Affected | 0 | -1 | -5.8 | -3.02 | |||||||||||||||||||||||||||||||||||
| c.2528T>C | M843T 2D  AIThe SynGAP1 missense variant M843T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions from REVEL and FATHMM, and pathogenic predictions from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. ESM1b remains uncertain. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a pathogenic majority, and Foldetta data are unavailable. Overall, the preponderance of evidence indicates that M843T is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.585406 | Disordered | 0.617934 | Binding | 0.327 | 0.854 | 0.375 | -7.297 | In-Between | 0.990 | Likely Pathogenic | Likely Pathogenic | 0.295 | Likely Benign | 0.2235 | 0.2037 | -3.22 | Deleterious | 0.968 | Probably Damaging | 0.954 | Probably Damaging | 2.62 | Benign | 0.00 | Affected | -1 | -1 | -2.6 | -30.09 | ||||||||||||||||||||||||||||||||||||
| c.2528T>G | M843R 2D  AIThe SynGAP1 missense variant M843R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and FATHMM, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Pathogenic). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that M843R is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.585406 | Disordered | 0.617934 | Binding | 0.327 | 0.854 | 0.375 | -12.044 | Likely Pathogenic | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.430 | Likely Benign | 0.1819 | 0.0922 | -3.78 | Deleterious | 0.968 | Probably Damaging | 0.978 | Probably Damaging | 2.59 | Benign | 0.00 | Affected | 0 | -1 | -6.4 | 24.99 | |||||||||||||||||||||||||||||||||||
| c.2533G>A | D845N 2D  AIThe SynGAP1 missense variant D845N is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools show a split: benign predictions come from REVEL and ESM1b, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. When the high‑accuracy consensus is considered, AlphaMissense‑Optimized remains pathogenic, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also indicates pathogenicity. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors points to a pathogenic effect, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.553315 | Disordered | 0.599971 | Binding | 0.297 | 0.827 | 0.500 | -6.586 | Likely Benign | 0.961 | Likely Pathogenic | Likely Pathogenic | 0.264 | Likely Benign | 0.1216 | 0.7112 | -3.42 | Deleterious | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 1.96 | Pathogenic | 0.00 | Affected | 2 | 1 | 0.0 | -0.98 | |||||||||||||||||||||||||||||||||||
| c.2533G>C | D845H 2D  AIThe SynGAP1 missense variant D845H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL indicates a benign likelihood, whereas the remaining predictors—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—classify the change as pathogenic. The consensus score from the SGM framework, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Pathogenic.” High‑accuracy assessments further support this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM consensus also reports a likely pathogenic status. Foldetta, a protein‑folding stability method that combines FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion is consistent with the absence of a ClinVar entry (no contradictory status). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.553315 | Disordered | 0.599971 | Binding | 0.297 | 0.827 | 0.500 | -8.613 | Likely Pathogenic | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.382 | Likely Benign | 0.1394 | 0.7515 | -5.08 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.92 | Pathogenic | 0.00 | Affected | 1 | -1 | 0.3 | 22.05 | |||||||||||||||||||||||||||||||||||
| c.2533G>T | D845Y 2D  AIThe SynGAP1 missense variant D845Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus—consistently predict a pathogenic or likely pathogenic impact. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as likely pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of evidence from multiple in silico predictors indicates that D845Y is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.553315 | Disordered | 0.599971 | Binding | 0.297 | 0.827 | 0.500 | -9.917 | Likely Pathogenic | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.384 | Likely Benign | 0.0590 | 0.6699 | -6.55 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.91 | Pathogenic | 0.00 | Affected | -4 | -3 | 2.2 | 48.09 | |||||||||||||||||||||||||||||||||||
| c.2534A>C | D845A 2D  AIThe SynGAP1 missense variant D845A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic outcome: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments reinforce this trend: AlphaMissense‑Optimized classifies the variant as pathogenic; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also predicts pathogenicity. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that D845A is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation because no ClinVar entry exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.553315 | Disordered | 0.599971 | Binding | 0.297 | 0.827 | 0.500 | -6.482 | Likely Benign | 0.983 | Likely Pathogenic | Likely Pathogenic | 0.376 | Likely Benign | 0.3999 | 0.6731 | -5.67 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.95 | Pathogenic | 0.00 | Affected | 0 | -2 | 5.3 | -44.01 | |||||||||||||||||||||||||||||||||||
| c.2534A>G | D845G 2D  AIThe SynGAP1 missense variant D845G is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining 11 predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) all predict pathogenicity. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as Likely Pathogenic. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus confirms a Likely Pathogenic status; Foldetta results are not available. Taken together, the preponderance of evidence indicates that D845G is most likely pathogenic, and this assessment does not conflict with the current ClinVar record, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.553315 | Disordered | 0.599971 | Binding | 0.297 | 0.827 | 0.500 | -8.209 | Likely Pathogenic | 0.980 | Likely Pathogenic | Likely Pathogenic | 0.399 | Likely Benign | 0.3953 | 0.6740 | -4.96 | Deleterious | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 2.06 | Pathogenic | 0.00 | Affected | 1 | -1 | 3.1 | -58.04 | |||||||||||||||||||||||||||||||||||
| c.2534A>T | D845V 2D  AIThe SynGAP1 D845V missense variant is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining 11 predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) all predict pathogenicity. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further support a harmful outcome: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus also indicates Likely Pathogenic; Foldetta results are not available. Taken together, the overwhelming majority of evidence points to a pathogenic effect. This conclusion is consistent with the absence of a ClinVar entry, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.553315 | Disordered | 0.599971 | Binding | 0.297 | 0.827 | 0.500 | -8.914 | Likely Pathogenic | 0.994 | Likely Pathogenic | Likely Pathogenic | 0.426 | Likely Benign | 0.0871 | 0.7088 | -6.15 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.91 | Pathogenic | 0.00 | Affected | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||
| c.2535C>A | D845E 2D  AIThe SynGAP1 missense variant D845E is not reported in ClinVar and has no entries in gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas seven tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus) predict a pathogenic outcome. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and Foldetta stability analysis is unavailable. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Pathogenic. Overall, the balance of evidence points to a pathogenic effect for D845E, and this assessment does not conflict with the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.553315 | Disordered | 0.599971 | Binding | 0.297 | 0.827 | 0.500 | -6.979 | Likely Benign | 0.914 | Likely Pathogenic | Ambiguous | 0.196 | Likely Benign | 0.1404 | 0.6998 | -2.67 | Deleterious | 0.992 | Probably Damaging | 0.992 | Probably Damaging | 2.02 | Pathogenic | 0.00 | Affected | 3 | 2 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2535C>G | D845E 2D  AIThe SynGAP1 missense variant D845E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is uncertain, but the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—classifies the variant as Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence points to a pathogenic effect for D845E, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.553315 | Disordered | 0.599971 | Binding | 0.297 | 0.827 | 0.500 | -6.979 | Likely Benign | 0.914 | Likely Pathogenic | Ambiguous | 0.196 | Likely Benign | 0.1404 | 0.6998 | -2.67 | Deleterious | 0.992 | Probably Damaging | 0.992 | Probably Damaging | 2.02 | Pathogenic | 0.00 | Affected | 3 | 2 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2536T>A | L846I 2D  AIThe SynGAP1 missense variant L846I is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. Two tools, ESM1b and AlphaMissense‑Default, return uncertain results. For high‑accuracy assessment, AlphaMissense‑Optimized classifies the variant as benign. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive because the votes are split (one pathogenic, one benign, two uncertain). Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, has no available output for this variant. Overall, the balance of evidence from the majority of prediction tools suggests the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.653063 | Disordered | 0.589606 | Binding | 0.349 | 0.825 | 0.500 | -7.882 | In-Between | 0.474 | Ambiguous | Likely Benign | 0.151 | Likely Benign | 0.0973 | 0.3475 | -1.38 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.18 | Pathogenic | 0.00 | Affected | 2 | 2 | 0.7 | 0.00 | ||||||||||||||||||||||||||||||||||||
| c.2536T>G | L846V 2D  AIThe SynGAP1 missense variant L846V is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect comprise polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Pathogenic, while AlphaMissense‑Optimized remains benign. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a pathogenic impact, and this assessment does not conflict with any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.589606 | Binding | 0.349 | 0.825 | 0.500 | -8.326 | Likely Pathogenic | 0.569 | Likely Pathogenic | Likely Benign | 0.165 | Likely Benign | 0.1540 | 0.3126 | -2.07 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.20 | Pathogenic | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2537T>C | L846S 2D  AIThe SynGAP1 missense variant L846S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining 11 predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) all predict pathogenicity. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as Likely Pathogenic. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus result is consistent with a likely pathogenic classification. Foldetta predictions are unavailable. Taken together, the preponderance of evidence indicates that the variant is most likely pathogenic, and this assessment does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.589606 | Binding | 0.349 | 0.825 | 0.500 | -10.944 | Likely Pathogenic | 0.977 | Likely Pathogenic | Likely Pathogenic | 0.366 | Likely Benign | 0.3128 | 0.1070 | -3.44 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.25 | Pathogenic | 0.00 | Affected | -3 | -2 | -4.6 | -26.08 | |||||||||||||||||||||||||||||||||||
| c.2538A>C | L846F 2D  AIThe SynGAP1 missense variant L846F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. In contrast, the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, and AlphaMissense‑Default all classify the variant as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as Likely Pathogenic. AlphaMissense‑Optimized yields an uncertain result, and no Foldetta (FoldX‑MD/Rosetta) stability assessment is available. Overall, the preponderance of evidence from multiple high‑accuracy predictors indicates that the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because none is assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.589606 | Binding | 0.349 | 0.825 | 0.500 | -10.559 | Likely Pathogenic | 0.862 | Likely Pathogenic | Ambiguous | 0.206 | Likely Benign | 0.0665 | 0.2912 | -2.88 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.14 | Pathogenic | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.2538A>T | L846F 2D  AIThe SynGAP1 missense variant L846F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. All other evaluated tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default—predict a pathogenic or deleterious impact, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Pathogenic. AlphaMissense‑Optimized is uncertain, providing no definitive direction. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Overall, the majority of predictions indicate a pathogenic effect, and this conclusion does not contradict any ClinVar status because none is available. Thus, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.589606 | Binding | 0.349 | 0.825 | 0.500 | -10.559 | Likely Pathogenic | 0.862 | Likely Pathogenic | Ambiguous | 0.206 | Likely Benign | 0.0665 | 0.2912 | -2.88 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.14 | Pathogenic | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.2539C>A | Q847K 2D  AIThe SynGAP1 missense variant Q847K is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show mixed results: benign calls come from REVEL, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic calls come from PROVEAN, polyPhen‑2 HumDiv, SIFT, FATHMM, and AlphaMissense‑Default. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a Likely Pathogenic verdict. High‑accuracy assessments further indicate that AlphaMissense‑Optimized predicts a benign effect, while the SGM Consensus remains Likely Pathogenic; no Foldetta stability data are available. Overall, the majority of predictions lean toward pathogenicity, and this is consistent with the SGM Consensus result. Thus, the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar annotation exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.577677 | Binding | 0.282 | 0.818 | 0.500 | -5.507 | Likely Benign | 0.736 | Likely Pathogenic | Likely Benign | 0.214 | Likely Benign | 0.1694 | 0.4129 | -2.82 | Deleterious | 0.481 | Possibly Damaging | 0.373 | Benign | 2.32 | Pathogenic | 0.00 | Affected | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||
| c.2539C>G | Q847E 2D  AIThe SynGAP1 missense variant Q847E is not reported in ClinVar (ClinVar status: not reported) and is absent from gnomAD (gnomAD: not present). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. Two tools remain uncertain: AlphaMissense‑Default and ESM1b. High‑accuracy assessment shows AlphaMissense‑Optimized predicts a benign outcome, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a tie between benign and pathogenic signals; Foldetta’s protein‑folding stability analysis is unavailable. Overall, the majority of conventional predictors lean toward pathogenicity, whereas the single high‑accuracy tool suggests benign. Based on the collective evidence, the variant is most likely pathogenic, and this assessment does not contradict ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.653063 | Disordered | 0.577677 | Binding | 0.282 | 0.818 | 0.500 | -7.864 | In-Between | 0.377 | Ambiguous | Likely Benign | 0.140 | Likely Benign | 0.1376 | 0.2083 | -2.12 | Neutral | 0.649 | Possibly Damaging | 0.535 | Possibly Damaging | 2.31 | Pathogenic | 0.00 | Affected | 2 | 2 | 0.0 | 0.98 | ||||||||||||||||||||||||||||||||||||
| c.253A>C | T85P 2D  AIThe SynGAP1 missense variant T85P is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, and FATHMM. Tools that agree on a pathogenic effect include polyPhen‑2 HumDiv, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments are mixed: AlphaMissense‑Optimized predicts pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields no clear majority and is therefore unavailable; Foldetta results are not provided. Overall, the majority of available predictions (five pathogenic vs. four benign) indicate a pathogenic effect. There is no ClinVar entry to contradict this assessment, so the variant is most likely pathogenic based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.680603 | Disordered | 0.542004 | Binding | 0.288 | 0.888 | 0.500 | -8.406 | Likely Pathogenic | 0.978 | Likely Pathogenic | Likely Pathogenic | 0.125 | Likely Benign | 0.1499 | 0.4485 | -2.28 | Neutral | 0.813 | Possibly Damaging | 0.053 | Benign | 3.80 | Benign | 0.00 | Affected | 0 | -1 | -0.9 | -3.99 | ||||||||||||||||||||||||||||||||||||
| c.253A>G | T85A 2D  AIThe SynGAP1 missense variant T85A is not reported in ClinVar and is absent from gnomAD. Consensus and most in‑silico predictors classify it as benign: SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM all indicate a benign effect. In contrast, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized predict a pathogenic impact. High‑accuracy assessments further highlight this discordance: AlphaMissense‑Optimized reports a pathogenic change, whereas the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) remains benign. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of tools and the consensus prediction lean toward a benign effect, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.680603 | Disordered | 0.542004 | Binding | 0.288 | 0.888 | 0.500 | -4.803 | Likely Benign | 0.978 | Likely Pathogenic | Likely Pathogenic | 0.101 | Likely Benign | 0.3155 | 0.3517 | -1.79 | Neutral | 0.060 | Benign | 0.004 | Benign | 3.88 | Benign | 0.00 | Affected | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||
| c.253A>T | T85S 2D  AIThe SynGAP1 missense variant T85S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Pathogenic, while the SGM‑Consensus remains Likely Benign; Foldetta results are unavailable. Overall, the majority of conventional predictors lean toward a benign impact, but the high‑accuracy AlphaMissense‑Optimized tool suggests pathogenicity, creating a conflict. No ClinVar entry exists, so there is no reported status to contradict. Based on the collective evidence, the variant is most likely benign, though the high‑accuracy prediction indicates uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.680603 | Disordered | 0.542004 | Binding | 0.288 | 0.888 | 0.500 | -4.171 | Likely Benign | 0.973 | Likely Pathogenic | Likely Pathogenic | 0.103 | Likely Benign | 0.2590 | 0.3569 | -1.37 | Neutral | 0.113 | Benign | 0.011 | Benign | 3.87 | Benign | 0.00 | Affected | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2540A>C | Q847P 2D  AIThe SynGAP1 missense variant Q847P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive because it yields a 2‑vs‑2 split. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑accuracy predictors (AlphaMissense‑Optimized and AlphaMissense‑Default) indicate a benign outcome, while the consensus of other tools is mixed. Thus, the variant is most likely benign based on current predictions, and this assessment does not contradict the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.653063 | Disordered | 0.577677 | Binding | 0.282 | 0.818 | 0.500 | -6.742 | Likely Benign | 0.320 | Likely Benign | Likely Benign | 0.331 | Likely Benign | 0.2290 | 0.4831 | -3.83 | Deleterious | 0.990 | Probably Damaging | 0.892 | Possibly Damaging | 2.26 | Pathogenic | 0.00 | Affected | 0 | -1 | 1.9 | -31.01 | ||||||||||||||||||||||||||||||||||||
| c.2540A>G | Q847R 2D  AIThe SynGAP1 missense variant Q847R has no ClinVar entry and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, and AlphaMissense‑Optimized; pathogenic calls come from PROVEAN, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further reveal AlphaMissense‑Optimized as benign, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Thus, the overall prediction leans toward benign based on the majority of tools, but the high‑accuracy SGM‑Consensus contradicts this by indicating likely pathogenic. No ClinVar annotation exists, so there is no conflict with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.577677 | Binding | 0.282 | 0.818 | 0.500 | -3.232 | Likely Benign | 0.662 | Likely Pathogenic | Likely Benign | 0.256 | Likely Benign | 0.1404 | 0.1979 | -2.63 | Deleterious | 0.014 | Benign | 0.026 | Benign | 2.30 | Pathogenic | 0.00 | Affected | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||||||
| c.2540A>T | Q847L 2D  AIThe SynGAP1 missense variant Q847L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default—predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Pathogenic, and the Foldetta stability analysis is unavailable. Overall, the majority of predictions lean toward pathogenicity, and this conclusion does not contradict any existing ClinVar annotation, as none is available. Thus, the variant is most likely pathogenic based on the current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.577677 | Binding | 0.282 | 0.818 | 0.500 | -6.966 | Likely Benign | 0.625 | Likely Pathogenic | Likely Benign | 0.326 | Likely Benign | 0.0701 | 0.5132 | -4.52 | Deleterious | 0.818 | Possibly Damaging | 0.637 | Possibly Damaging | 2.33 | Pathogenic | 0.00 | Affected | -2 | -2 | 7.3 | -14.97 | |||||||||||||||||||||||||||||||||||
| c.2541G>C | Q847H 2D  AIThe SynGAP1 missense variant Q847H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default—predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely pathogenic, and the Foldetta stability analysis is unavailable. Overall, the majority of predictions (seven pathogenic vs. three benign) and the SGM‑Consensus result point to a pathogenic effect. Thus, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.577677 | Binding | 0.282 | 0.818 | 0.500 | -4.208 | Likely Benign | 0.720 | Likely Pathogenic | Likely Benign | 0.204 | Likely Benign | 0.1429 | 0.3634 | -2.82 | Deleterious | 0.990 | Probably Damaging | 0.925 | Probably Damaging | 2.27 | Pathogenic | 0.00 | Affected | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||
| c.2541G>T | Q847H 2D  AIThe SynGAP1 missense variant Q847H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default—predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely pathogenic, and the Foldetta stability analysis is unavailable. Overall, the majority of predictions (seven pathogenic vs. three benign) and the SGM‑Consensus support a pathogenic interpretation. Thus, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.653063 | Disordered | 0.577677 | Binding | 0.282 | 0.818 | 0.500 | -4.208 | Likely Benign | 0.720 | Likely Pathogenic | Likely Benign | 0.204 | Likely Benign | 0.1429 | 0.3634 | -2.82 | Deleterious | 0.990 | Probably Damaging | 0.925 | Probably Damaging | 2.27 | Pathogenic | 0.00 | Affected | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||
| c.2545G>A | D849N 2D  AIThe SynGAP1 missense variant D849N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that D849N is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.554191 | Binding | 0.319 | 0.813 | 0.500 | -5.081 | Likely Benign | 0.149 | Likely Benign | Likely Benign | 0.063 | Likely Benign | 0.1723 | 0.8380 | -0.47 | Neutral | 0.002 | Benign | 0.003 | Benign | 4.22 | Benign | 0.00 | Affected | 2 | 1 | 0.0 | -0.98 | |||||||||||||||||||||||||||||||||||
| c.2545G>C | D849H 2D  AIThe SynGAP1 missense variant D849H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. AlphaMissense‑Default remains uncertain, and the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.554191 | Binding | 0.319 | 0.813 | 0.500 | -4.624 | Likely Benign | 0.345 | Ambiguous | Likely Benign | 0.149 | Likely Benign | 0.1977 | 0.8673 | -0.52 | Neutral | 0.918 | Possibly Damaging | 0.697 | Possibly Damaging | 4.14 | Benign | 0.00 | Affected | 1 | -1 | 0.3 | 22.05 | |||||||||||||||||||||||||||||||||||
| c.2545G>T | D849Y 2D  AIThe SynGAP1 missense variant D849Y has no ClinVar entry and is not reported in gnomAD. Consensus from multiple in‑silico predictors shows a split: benign calls come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic calls arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default remains uncertain. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” status. High‑accuracy tools further support a benign interpretation: AlphaMissense‑Optimized predicts benign, SGM‑Consensus is likely benign, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Taken together, the majority of reliable predictors and consensus analyses indicate a benign effect. This conclusion is consistent with the absence of a ClinVar pathogenic classification, so there is no contradiction with existing clinical evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.554191 | Binding | 0.319 | 0.813 | 0.500 | -6.200 | Likely Benign | 0.383 | Ambiguous | Likely Benign | 0.150 | Likely Benign | 0.0767 | 0.7690 | -1.92 | Neutral | 0.971 | Probably Damaging | 0.773 | Possibly Damaging | 4.13 | Benign | 0.00 | Affected | -4 | -3 | 2.2 | 48.09 | |||||||||||||||||||||||||||||||||||
| c.2546A>C | D849A 2D  AIThe SynGAP1 missense variant D849A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.554191 | Binding | 0.319 | 0.813 | 0.500 | -2.843 | Likely Benign | 0.193 | Likely Benign | Likely Benign | 0.163 | Likely Benign | 0.4618 | 0.7799 | -0.83 | Neutral | 0.611 | Possibly Damaging | 0.239 | Benign | 4.25 | Benign | 0.00 | Affected | 0 | -2 | 5.3 | -44.01 | |||||||||||||||||||||||||||||||||||
| c.2546A>G | D849G 2D  AISynGAP1 missense variant D849G is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) indicates likely benign. Foldetta results are unavailable. Overall, the consensus of available predictions indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.554191 | Binding | 0.319 | 0.813 | 0.500 | -2.124 | Likely Benign | 0.168 | Likely Benign | Likely Benign | 0.154 | Likely Benign | 0.4622 | 0.7587 | -0.55 | Neutral | 0.393 | Benign | 0.140 | Benign | 4.17 | Benign | 0.00 | Affected | 1 | -1 | 3.1 | -58.04 | |||||||||||||||||||||||||||||||||||
| c.2546A>T | D849V 2D  AIThe SynGAP1 missense variant D849V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of computational evidence points to a benign effect, and this is consistent with the lack of any ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.554191 | Binding | 0.319 | 0.813 | 0.500 | -3.819 | Likely Benign | 0.319 | Likely Benign | Likely Benign | 0.137 | Likely Benign | 0.1161 | 0.7963 | -2.10 | Neutral | 0.918 | Possibly Damaging | 0.481 | Possibly Damaging | 4.15 | Benign | 0.00 | Affected | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||
| c.2547T>A | D849E 2D  AIThe SynGAP1 missense variant D849E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.554191 | Binding | 0.319 | 0.813 | 0.500 | -2.911 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.087 | Likely Benign | 0.1955 | 0.7819 | -0.55 | Neutral | 0.393 | Benign | 0.187 | Benign | 4.25 | Benign | 0.00 | Affected | 3 | 2 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2547T>G | D849E 2D  AIThe SynGAP1 missense variant D849E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.554191 | Binding | 0.319 | 0.813 | 0.500 | -2.911 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.087 | Likely Benign | 0.1955 | 0.7819 | -0.55 | Neutral | 0.393 | Benign | 0.187 | Benign | 4.25 | Benign | 0.00 | Affected | 3 | 2 | 0.0 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2548G>T | G850W 2D  AIThe SynGAP1 missense variant G850W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. Two tools—ESM1b and AlphaMissense‑Default—return uncertain results. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (two benign, two uncertain), and Foldetta data are unavailable. Overall, the balance of evidence (four benign vs. three pathogenic predictions, with a benign high‑accuracy result and no contradictory ClinVar annotation) indicates that the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.648219 | Disordered | 0.540897 | Binding | 0.312 | 0.820 | 0.500 | -7.826 | In-Between | 0.351 | Ambiguous | Likely Benign | 0.170 | Likely Benign | 0.0734 | 0.4002 | -1.94 | Neutral | 0.996 | Probably Damaging | 0.933 | Probably Damaging | 4.16 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||||||
| c.254C>A | T85K 2D  AIThe SynGAP1 missense variant T85K is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, and FATHMM. Tools that agree on a pathogenic effect include polyPhen‑2 HumDiv, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments are mixed: AlphaMissense‑Optimized predicts pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields an equal split (2 pathogenic, 2 benign) and is therefore considered unavailable; Foldetta results are not provided and are likewise unavailable. Overall, the majority of available predictions (5 pathogenic vs. 4 benign) indicate that the variant is most likely pathogenic. This conclusion does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.680603 | Disordered | 0.542004 | Binding | 0.288 | 0.888 | 0.500 | -9.278 | Likely Pathogenic | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.127 | Likely Benign | 0.0823 | 0.2796 | -2.32 | Neutral | 0.588 | Possibly Damaging | 0.036 | Benign | 3.88 | Benign | 0.00 | Affected | 0 | -1 | -3.2 | 27.07 | ||||||||||||||||||||||||||||||||||||
| c.254C>G | T85R 2D  AIThe SynGAP1 missense variant T85R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Functional prediction tools show a split: benign calls come from REVEL, PROVEAN, polyPhen‑2 HumVar, and FATHMM, while pathogenic calls come from polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized; ESM1b is uncertain. High‑accuracy assessment gives AlphaMissense‑Optimized a pathogenic score, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default (pathogenic), ESM1b (uncertain), FATHMM (benign), and PROVEAN (benign)—leans toward benign. Foldetta, a protein‑folding stability predictor, has no available result for this variant. Overall, the balance of evidence tilts toward a benign interpretation, with no ClinVar entry to contradict this assessment. Thus, the variant is most likely benign, though the presence of pathogenic predictions from several tools indicates that further functional validation would be prudent. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.680603 | Disordered | 0.542004 | Binding | 0.288 | 0.888 | 0.500 | -7.936 | In-Between | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.116 | Likely Benign | 0.0754 | 0.2482 | -2.32 | Neutral | 0.813 | Possibly Damaging | 0.072 | Benign | 3.91 | Benign | 0.00 | Affected | -1 | -1 | -3.8 | 55.08 | ||||||||||||||||||||||||||||||||||||
| c.254C>T | T85I 2D  AIThe SynGAP1 missense variant T85I is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, and FATHMM, whereas a majority of tools (PROVEAN, polyPhen‑2 HumDiv, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized) predict a pathogenic impact. The high‑accuracy consensus, SGM‑Consensus, classifies the variant as Likely Pathogenic. AlphaMissense‑Optimized also predicts pathogenicity. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so its status is unavailable. Overall, the preponderance of evidence from multiple independent predictors indicates that T85I is most likely pathogenic, and this assessment does not contradict any ClinVar annotation (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.680603 | Disordered | 0.542004 | Binding | 0.288 | 0.888 | 0.500 | -9.310 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.113 | Likely Benign | 0.0634 | 0.5443 | -2.50 | Deleterious | 0.813 | Possibly Damaging | 0.072 | Benign | 3.82 | Benign | 0.00 | Affected | 0 | -1 | 5.2 | 12.05 | |||||||||||||||||||||||||||||||||||
| c.2557G>C | G853R 2D  AIThe SynGAP1 missense variant G853R is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. High‑accuracy predictions therefore point to a benign outcome: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, and no Foldetta data is available. Overall, the majority of evidence supports a benign classification, which does not contradict the current ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.496246 | Uncertain | 0.284 | 0.815 | 0.625 | Uncertain | 1 | -4.749 | Likely Benign | 0.366 | Ambiguous | Likely Benign | 0.091 | Likely Benign | 0.0918 | 0.4323 | -1.27 | Neutral | 0.846 | Possibly Damaging | 0.624 | Possibly Damaging | 4.18 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||
| c.2557G>T | G853C 2D  AIThe SynGAP1 G853C missense variant has no ClinVar record and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. ESM1b is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.496246 | Uncertain | 0.284 | 0.815 | 0.625 | -7.021 | In-Between | 0.096 | Likely Benign | Likely Benign | 0.120 | Likely Benign | 0.1292 | 0.4944 | -1.65 | Neutral | 0.992 | Probably Damaging | 0.873 | Possibly Damaging | 4.10 | Benign | 0.00 | Affected | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||
| c.2558G>A | G853D 2D  AIThe SynGAP1 missense variant G853D is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools (polyPhen‑2 HumDiv and SIFT) predict pathogenicity, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of predictors and the high‑accuracy tools points to a benign classification, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.496246 | Uncertain | 0.284 | 0.815 | 0.625 | -5.116 | Likely Benign | 0.220 | Likely Benign | Likely Benign | 0.156 | Likely Benign | 0.1745 | 0.1862 | -0.86 | Neutral | 0.611 | Possibly Damaging | 0.346 | Benign | 4.19 | Benign | 0.00 | Affected | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||
| c.256G>A | V86I 2D  AIThe SynGAP1 missense variant V86I is listed in ClinVar (ID 588267.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all classify the variant as benign or likely benign. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is “Likely Benign.” No Foldetta (FoldX‑MD/Rosetta stability) result is available, so it does not influence the assessment. Overall, the majority of predictions indicate that V86I is most likely benign, which is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.552911 | Binding | 0.295 | 0.887 | 0.500 | Uncertain | 1 | -4.726 | Likely Benign | 0.338 | Likely Benign | Likely Benign | 0.076 | Likely Benign | 0.0887 | 0.4417 | -0.31 | Neutral | 0.267 | Benign | 0.097 | Benign | 3.94 | Benign | 0.00 | Affected | 4.32 | 1 | 4 | 3 | 0.3 | 14.03 | |||||||||||||||||||||||||||||||
| c.256G>C | V86L 2D  AIThe SynGAP1 missense variant V86L is reported in ClinVar as “not listed” and is present in the gnomAD database (ID 6‑33425864‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as likely benign. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus remains benign; Foldetta stability analysis is unavailable. Overall, the balance of evidence favors a benign interpretation, and this conclusion does not contradict the ClinVar status, which currently contains no pathogenic assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.552911 | Binding | 0.295 | 0.887 | 0.500 | 6-33425864-G-C | 1 | 6.20e-7 | -3.658 | Likely Benign | 0.965 | Likely Pathogenic | Likely Pathogenic | 0.081 | Likely Benign | 0.1121 | 0.5386 | -0.84 | Neutral | 0.267 | Benign | 0.097 | Benign | 3.85 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 2 | -0.4 | 14.03 | ||||||||||||||||||||||||||||||
| c.256G>T | V86F 2D  AIThe SynGAP1 missense variant V86F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of predictions lean toward a benign impact, and this conclusion does not contradict ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.552911 | Binding | 0.295 | 0.887 | 0.500 | -5.011 | Likely Benign | 0.981 | Likely Pathogenic | Likely Pathogenic | 0.126 | Likely Benign | 0.0755 | 0.4078 | -1.54 | Neutral | 0.824 | Possibly Damaging | 0.403 | Benign | 3.75 | Benign | 0.00 | Affected | -1 | -1 | -1.4 | 48.04 | |||||||||||||||||||||||||||||||||||
| c.2578G>A | V860I 2D  AIThe SynGAP1 missense variant V860I is catalogued in ClinVar as a benign alteration (ClinVar ID 411591.0) and is present in the gnomAD database (gnomAD ID 6‑33443130‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized returns benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” No Foldetta stability prediction is available for this variant. Overall, the consensus of computational evidence strongly favors a benign impact, aligning with the ClinVar designation and showing no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.545602 | Disordered | 0.518121 | Binding | 0.269 | 0.803 | 0.250 | Benign | 1 | 6-33443130-G-A | 21 | 1.30e-5 | -4.516 | Likely Benign | 0.095 | Likely Benign | Likely Benign | 0.039 | Likely Benign | 0.0842 | 0.4477 | -0.42 | Neutral | 0.009 | Benign | 0.006 | Benign | 4.24 | Benign | 0.00 | Affected | 3.77 | 5 | 4 | 3 | 0.3 | 14.03 | ||||||||||||||||||||||||||||
| c.2578G>C | V860L 2D  AIThe SynGAP1 missense variant V860L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.545602 | Disordered | 0.518121 | Binding | 0.269 | 0.803 | 0.250 | -3.053 | Likely Benign | 0.123 | Likely Benign | Likely Benign | 0.028 | Likely Benign | 0.1052 | 0.5446 | -0.89 | Neutral | 0.124 | Benign | 0.037 | Benign | 4.17 | Benign | 0.00 | Affected | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2578G>T | V860F 2D  AIThe SynGAP1 missense variant V860F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy methods give a benign consensus: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.545602 | Disordered | 0.518121 | Binding | 0.269 | 0.803 | 0.250 | -4.576 | Likely Benign | 0.143 | Likely Benign | Likely Benign | 0.101 | Likely Benign | 0.0717 | 0.4138 | -2.25 | Neutral | 0.846 | Possibly Damaging | 0.522 | Possibly Damaging | 4.09 | Benign | 0.00 | Affected | -1 | -1 | -1.4 | 48.04 | |||||||||||||||||||||||||||||||||||
| c.2579T>A | V860D 2D  AIThe SynGAP1 missense variant V860D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.545602 | Disordered | 0.518121 | Binding | 0.269 | 0.803 | 0.250 | -4.310 | Likely Benign | 0.590 | Likely Pathogenic | Likely Benign | 0.164 | Likely Benign | 0.1382 | 0.1047 | -1.98 | Neutral | 0.971 | Probably Damaging | 0.690 | Possibly Damaging | 4.08 | Benign | 0.00 | Affected | -2 | -3 | -7.7 | 15.96 | |||||||||||||||||||||||||||||||||||
| c.2579T>C | V860A 2D  AIThe SynGAP1 missense variant V860A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while only SIFT predicts pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence supports a benign impact for V860A, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.545602 | Disordered | 0.518121 | Binding | 0.269 | 0.803 | 0.250 | -3.379 | Likely Benign | 0.161 | Likely Benign | Likely Benign | 0.223 | Likely Benign | 0.3215 | 0.2723 | -0.91 | Neutral | 0.393 | Benign | 0.185 | Benign | 4.20 | Benign | 0.00 | Affected | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||||||
| c.2579T>G | V860G 2D  AIThe SynGAP1 missense variant V860G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD. Prediction tools cluster into two groups: the benign group includes REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized; the pathogenic group contains only SIFT. High‑accuracy assessments reinforce the benign prediction: AlphaMissense‑Optimized is benign, the SGM Consensus (derived from the majority of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is benign, while Foldetta results are unavailable. Overall, the collective evidence indicates that V860G is most likely benign, and this conclusion does not contradict the ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.545602 | Disordered | 0.518121 | Binding | 0.269 | 0.803 | 0.250 | -3.471 | Likely Benign | 0.173 | Likely Benign | Likely Benign | 0.198 | Likely Benign | 0.2312 | 0.2547 | -1.54 | Neutral | 0.012 | Benign | 0.009 | Benign | 4.11 | Benign | 0.00 | Affected | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||
| c.257T>A | V86D 2D  AIThe SynGAP1 missense variant V86D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on benign impact include REVEL, PROVEAN, and FATHMM, whereas those that agree on pathogenic impact are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized; ESM1b is uncertain. High‑accuracy assessment shows AlphaMissense‑Optimized predicts pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) leans benign (two benign, one pathogenic, one uncertain). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of conventional tools predict pathogenicity, whereas the high‑accuracy consensus suggests benign. Based on the aggregate predictions, the variant is most likely pathogenic, and this assessment does not contradict ClinVar status, which currently has no entry for V86D. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.685117 | Disordered | 0.552911 | Binding | 0.295 | 0.887 | 0.500 | -7.141 | In-Between | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.147 | Likely Benign | 0.1590 | 0.1047 | -2.38 | Neutral | 0.824 | Possibly Damaging | 0.485 | Possibly Damaging | 3.73 | Benign | 0.00 | Affected | -2 | -3 | -7.7 | 15.96 | ||||||||||||||||||||||||||||||||||||
| c.257T>C | V86A 2D  AIThe SynGAP1 missense variant V86A has no ClinVar entry and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Pathogenic predictions arise from SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments further highlight a split: AlphaMissense‑Optimized classifies the variant as pathogenic, whereas the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates it is likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of tools (six benign vs. three pathogenic) suggest the variant is most likely benign, and this assessment does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.685117 | Disordered | 0.552911 | Binding | 0.295 | 0.887 | 0.500 | -3.022 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.059 | Likely Benign | 0.3205 | 0.2723 | -1.35 | Neutral | 0.267 | Benign | 0.153 | Benign | 3.80 | Benign | 0.00 | Affected | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||||||
| c.257T>G | V86G 2D  AIThe SynGAP1 missense variant V86G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, ESM1b, and FATHMM. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑to‑2 split. Foldetta, which evaluates protein‑folding stability via FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence (five pathogenic versus four benign predictions) indicates that V86G is most likely pathogenic, and this conclusion does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.685117 | Disordered | 0.552911 | Binding | 0.295 | 0.887 | 0.500 | -4.212 | Likely Benign | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.130 | Likely Benign | 0.2321 | 0.2547 | -2.50 | Deleterious | 0.307 | Benign | 0.824 | Possibly Damaging | 3.74 | Benign | 0.00 | Affected | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||
| c.259T>A | S87T 2D  AIThe SynGAP1 missense variant S87T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (majority vote) also indicates benign. No Foldetta stability prediction is available for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status, as none is assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.550904 | Binding | 0.302 | 0.878 | 0.500 | -6.706 | Likely Benign | 0.776 | Likely Pathogenic | Likely Benign | 0.032 | Likely Benign | 0.1001 | 0.4708 | -1.23 | Neutral | 0.140 | Benign | 0.153 | Benign | 3.80 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.259T>C | S87P 2D  AIThe SynGAP1 missense variant S87P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, and FATHMM. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Based on the available predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.550904 | Binding | 0.302 | 0.878 | 0.500 | -8.566 | Likely Pathogenic | 0.985 | Likely Pathogenic | Likely Pathogenic | 0.073 | Likely Benign | 0.1639 | 0.4260 | -2.04 | Neutral | 0.676 | Possibly Damaging | 0.307 | Benign | 3.75 | Benign | 0.00 | Affected | 1 | -1 | -0.8 | 10.04 | ||||||||||||||||||||||||||||||||||||
| c.259T>G | S87A 2D  AIThe SynGAP1 missense variant S87A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default, while ESM1b remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also favors benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the balance of evidence (six benign predictions versus two pathogenic) indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.550904 | Binding | 0.302 | 0.878 | 0.500 | -7.817 | In-Between | 0.676 | Likely Pathogenic | Likely Benign | 0.039 | Likely Benign | 0.4272 | 0.3540 | -1.24 | Neutral | 0.140 | Benign | 0.097 | Benign | 3.84 | Benign | 0.00 | Affected | 1 | 1 | 2.6 | -16.00 | ||||||||||||||||||||||||||||||||||||
| c.25C>A | H9N 2D  AIThe SynGAP1 missense variant H9N is reported in gnomAD (ID 6‑33420289‑C‑A) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote) is likely benign; Foldetta results are unavailable. Overall, the consensus of the available predictions points to a benign impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | 6-33420289-C-A | -3.789 | Likely Benign | 0.103 | Likely Benign | Likely Benign | 0.081 | Likely Benign | 0.2327 | 0.3823 | -0.36 | Neutral | 0.024 | Benign | 0.003 | Benign | 4.23 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 2 | -0.3 | -23.04 | ||||||||||||||||||||||||||||||||
| c.25C>G | H9D 2D  AIThe SynGAP1 missense variant H9D has no ClinVar entry and is not reported in gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign, while only SIFT predicts it to be pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, the SGM‑Consensus (majority of the high‑accuracy tools) is benign, and the Foldetta stability analysis is unavailable. Overall, the consensus of available predictions indicates that the variant is most likely benign, with no conflict with ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -2.912 | Likely Benign | 0.168 | Likely Benign | Likely Benign | 0.217 | Likely Benign | 0.2823 | 0.2893 | -0.11 | Neutral | 0.024 | Benign | 0.002 | Benign | 4.24 | Benign | 0.00 | Affected | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||
| c.25C>T | H9Y 2D  AIThe SynGAP1 missense variant H9Y is reported in gnomAD (ID 6‑33420289‑C‑T) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts it to be pathogenic, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus itself is likely benign. Foldetta results are not available, so they do not influence the assessment. Overall, the preponderance of evidence points to the variant being most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | 6-33420289-C-T | -3.833 | Likely Benign | 0.136 | Likely Benign | Likely Benign | 0.082 | Likely Benign | 0.1227 | 0.5024 | -0.14 | Neutral | 0.047 | Benign | 0.006 | Benign | 4.24 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 0 | 1.9 | 26.03 | ||||||||||||||||||||||||||||||||
| c.260C>A | S87Y 2D  AIThe SynGAP1 missense variant S87Y is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM. Tools that agree on a pathogenic effect include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic vs. two benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑accuracy predictors (six out of nine) indicate a pathogenic impact, whereas three predict benign. Therefore, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.550904 | Binding | 0.302 | 0.878 | 0.500 | -10.410 | Likely Pathogenic | 0.986 | Likely Pathogenic | Likely Pathogenic | 0.052 | Likely Benign | 0.0522 | 0.4798 | -2.20 | Neutral | 0.880 | Possibly Damaging | 0.608 | Possibly Damaging | 3.75 | Benign | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | ||||||||||||||||||||||||||||||||||||
| c.260C>G | S87C 2D  AIThe SynGAP1 missense variant S87C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM, while those that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy consensus methods give the following results: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is uncertain due to a 2‑to‑2 split; and Foldetta, which assesses protein‑folding stability, is unavailable for this variant. Overall, the majority of available predictions (five pathogenic versus three benign) indicate a likely pathogenic impact. This assessment does not contradict any ClinVar status, as no ClinVar entry exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.550904 | Binding | 0.302 | 0.878 | 0.500 | -8.769 | Likely Pathogenic | 0.915 | Likely Pathogenic | Ambiguous | 0.095 | Likely Benign | 0.0794 | 0.4849 | -2.14 | Neutral | 0.880 | Possibly Damaging | 0.700 | Possibly Damaging | 3.74 | Benign | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||
| c.260C>T | S87F 2D  AIThe SynGAP1 missense variant S87F is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM. Tools that agree on a pathogenic effect include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic vs. two benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑confidence predictors (six out of nine) indicate a pathogenic impact, whereas three predict benign. Therefore, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.690604 | Disordered | 0.550904 | Binding | 0.302 | 0.878 | 0.500 | -9.673 | Likely Pathogenic | 0.990 | Likely Pathogenic | Likely Pathogenic | 0.054 | Likely Benign | 0.0493 | 0.4937 | -2.34 | Neutral | 0.676 | Possibly Damaging | 0.485 | Possibly Damaging | 3.74 | Benign | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||||||||
| c.2620C>A | Q874K 2D  AIThe SynGAP1 missense variant Q874K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact for this variant. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.635258 | Binding | 0.289 | 0.873 | 0.250 | -6.379 | Likely Benign | 0.604 | Likely Pathogenic | Likely Benign | 0.169 | Likely Benign | 0.2008 | 0.4893 | -2.35 | Neutral | 0.963 | Probably Damaging | 0.973 | Probably Damaging | 2.73 | Benign | 0.00 | Affected | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||
| c.2620C>G | Q874E 2D  AIThe SynGAP1 missense variant Q874E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta’s protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign impact for Q874E, and this conclusion does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.635258 | Binding | 0.289 | 0.873 | 0.250 | -4.576 | Likely Benign | 0.197 | Likely Benign | Likely Benign | 0.193 | Likely Benign | 0.1489 | 0.3577 | -1.95 | Neutral | 0.963 | Probably Damaging | 0.973 | Probably Damaging | 2.73 | Benign | 0.00 | Affected | 2 | 2 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||
| c.2621A>C | Q874P 2D  AIThe SynGAP1 missense variant Q874P is reported in gnomAD (ID 6‑33443173‑A‑C) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar classification (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.635258 | Binding | 0.289 | 0.873 | 0.250 | 6-33443173-A-C | 9 | 5.58e-6 | -4.622 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.219 | Likely Benign | 0.2373 | 0.5911 | -2.89 | Deleterious | 0.996 | Probably Damaging | 0.992 | Probably Damaging | 2.77 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | 1.9 | -31.01 | ||||||||||||||||||||||||||||||
| c.2621A>G | Q874R 2D  AIThe SynGAP1 missense variant Q874R is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default remains uncertain. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized independently predicts a benign effect. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.490133 | Structured | 0.635258 | Binding | 0.289 | 0.873 | 0.250 | -4.834 | Likely Benign | 0.527 | Ambiguous | Likely Benign | 0.200 | Likely Benign | 0.1619 | 0.2924 | -2.33 | Neutral | 0.985 | Probably Damaging | 0.982 | Probably Damaging | 2.69 | Benign | 0.00 | Affected | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||||||
| c.2621A>T | Q874L 2D  AIThe SynGAP1 missense variant Q874L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 benign vs 2 pathogenic). Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs four benign) lean toward pathogenicity. Thus, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because the variant has not been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.490133 | Structured | 0.635258 | Binding | 0.289 | 0.873 | 0.250 | -6.084 | Likely Benign | 0.608 | Likely Pathogenic | Likely Benign | 0.199 | Likely Benign | 0.0828 | 0.6595 | -3.39 | Deleterious | 0.985 | Probably Damaging | 0.982 | Probably Damaging | 2.67 | Benign | 0.00 | Affected | -2 | -2 | 7.3 | -14.97 | ||||||||||||||||||||||||||||||||||||
| c.2622G>C | Q874H 2D  AIThe SynGAP1 missense variant Q874H is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; AlphaMissense‑Default is uncertain. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also predicts benign, while Foldetta results are unavailable. Overall, the balance of evidence from both general and high‑accuracy predictors indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.490133 | Structured | 0.635258 | Binding | 0.289 | 0.873 | 0.250 | -4.658 | Likely Benign | 0.543 | Ambiguous | Likely Benign | 0.236 | Likely Benign | 0.1588 | 0.5088 | -2.90 | Deleterious | 0.996 | Probably Damaging | 0.995 | Probably Damaging | 2.65 | Benign | 0.00 | Affected | 3 | 0 | 0.3 | 9.01 | ||||||||||||||||||||||||||||||||||||
| c.2622G>T | Q874H 2D  AIThe SynGAP1 missense variant Q874H has no ClinVar record and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized, while PROVEAN, polyPhen‑2 (HumDiv and HumVar), and SIFT all predict a pathogenic outcome; AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a benign majority, and Foldetta data are unavailable. Overall, the balance of evidence leans toward a benign impact, and this conclusion does not contradict the ClinVar status, which is currently unreported. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.490133 | Structured | 0.635258 | Binding | 0.289 | 0.873 | 0.250 | -4.658 | Likely Benign | 0.543 | Ambiguous | Likely Benign | 0.236 | Likely Benign | 0.1588 | 0.5088 | -2.90 | Deleterious | 0.996 | Probably Damaging | 0.995 | Probably Damaging | 2.65 | Benign | 0.00 | Affected | 3 | 0 | 0.3 | 9.01 | ||||||||||||||||||||||||||||||||||||
| c.262G>A | V88M 2D  AISynGAP1 missense variant V88M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD. Functional prediction tools show a split: benign predictions come from REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while pathogenic predictions arise from polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The high‑accuracy consensus, SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. AlphaMissense‑Optimized alone predicts Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points toward a benign effect, and this assessment does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | -5.851 | Likely Benign | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.103 | Likely Benign | 0.1293 | 0.4463 | -0.91 | Neutral | 0.975 | Probably Damaging | 0.171 | Benign | 3.67 | Benign | 0.00 | Affected | 2 | 1 | -2.3 | 32.06 | |||||||||||||||||||||||||||||||||||
| c.262G>C | V88L 2D  AIThe SynGAP1 V88L variant has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Those that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of tools suggest a benign impact, but the high‑accuracy predictions are conflicting. The variant is most likely benign based on the collective evidence, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | -5.808 | Likely Benign | 0.975 | Likely Pathogenic | Likely Pathogenic | 0.066 | Likely Benign | 0.1457 | 0.4562 | -0.79 | Neutral | 0.225 | Benign | 0.027 | Benign | 3.74 | Benign | 0.00 | Affected | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.262G>T | V88L 2D  AIThe SynGAP1 missense variant V88L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM, whereas SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM Consensus (a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign effect; a Foldetta stability analysis is unavailable. Overall, the majority of predictions lean toward a benign impact, and there is no ClinVar annotation to contradict this assessment. Thus, the variant is most likely benign based on the current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | -5.808 | Likely Benign | 0.975 | Likely Pathogenic | Likely Pathogenic | 0.066 | Likely Benign | 0.1457 | 0.4562 | -0.79 | Neutral | 0.225 | Benign | 0.027 | Benign | 3.74 | Benign | 0.00 | Affected | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2633C>A | T878K 2D  AIThe SynGAP1 missense variant T878K is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, SGM‑Consensus indicates likely benign, and Foldetta results are unavailable. Taken together, the majority of evidence points to a benign impact for T878K, and this conclusion does not contradict the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.628767 | Binding | 0.288 | 0.878 | 0.250 | -5.694 | Likely Benign | 0.592 | Likely Pathogenic | Likely Benign | 0.099 | Likely Benign | 0.1168 | 0.3300 | -1.51 | Neutral | 0.611 | Possibly Damaging | 0.196 | Benign | 2.66 | Benign | 0.00 | Affected | 0 | -1 | -3.2 | 27.07 | |||||||||||||||||||||||||||||||||||
| c.2633C>G | T878R 2D  AIThe SynGAP1 missense variant T878R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.628767 | Binding | 0.288 | 0.878 | 0.250 | -4.289 | Likely Benign | 0.496 | Ambiguous | Likely Benign | 0.119 | Likely Benign | 0.1006 | 0.2920 | -1.39 | Neutral | 0.611 | Possibly Damaging | 0.398 | Benign | 2.64 | Benign | 0.00 | Affected | -1 | -1 | -3.8 | 55.08 | |||||||||||||||||||||||||||||||||||
| c.2633C>T | T878I 2D  AIThe SynGAP1 missense variant T878I is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect. **Benign:** REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized. **Pathogenic:** polyPhen‑2 HumDiv, SIFT. AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus itself is benign; Foldetta results are unavailable. Overall, the computational evidence overwhelmingly points to a benign effect, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.628767 | Binding | 0.288 | 0.878 | 0.250 | -6.247 | Likely Benign | 0.459 | Ambiguous | Likely Benign | 0.055 | Likely Benign | 0.0980 | 0.6502 | -1.99 | Neutral | 0.761 | Possibly Damaging | 0.398 | Benign | 2.63 | Benign | 0.00 | Affected | 0 | -1 | 5.2 | 12.05 | |||||||||||||||||||||||||||||||||||
| c.263T>A | V88E 2D  AIThe SynGAP1 missense variant V88E is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) favors a benign outcome. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of predictions (five benign vs. three pathogenic) and the SGM Consensus support a benign classification, whereas AlphaMissense‑Optimized alone suggests pathogenicity. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | -7.978 | In-Between | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.095 | Likely Benign | 0.1444 | 0.1725 | -1.95 | Neutral | 0.001 | Benign | 0.000 | Benign | 3.68 | Benign | 0.00 | Affected | -2 | -2 | -7.7 | 29.98 | ||||||||||||||||||||||||||||||||||||
| c.263T>C | V88A 2D  AIThe SynGAP1 missense variant V88A is listed in ClinVar (ID 2656486.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are AlphaMissense‑Default, AlphaMissense‑Optimized, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote) is benign; Foldetta stability analysis is unavailable. Overall, the balance of evidence favors a benign interpretation, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | Uncertain | 1 | -5.860 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.050 | Likely Benign | 0.3563 | 0.2769 | -1.22 | Neutral | 0.053 | Benign | 0.008 | Benign | 3.75 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||
| c.263T>G | V88G 2D  AIThe SynGAP1 missense variant V88G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. Tools that predict a pathogenic effect are SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2) and Foldetta results are unavailable. Overall, the majority of predictions (5 benign vs. 4 pathogenic) and the lack of a ClinVar pathogenic classification suggest that V88G is most likely benign, with no contradiction to existing ClinVar data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.552910 | Binding | 0.323 | 0.870 | 0.500 | -8.588 | Likely Pathogenic | 0.989 | Likely Pathogenic | Likely Pathogenic | 0.084 | Likely Benign | 0.2608 | 0.2609 | -2.40 | Neutral | 0.024 | Benign | 0.208 | Benign | 3.68 | Benign | 0.00 | Affected | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||
| c.2642T>C | L881S 2D  AIThe SynGAP1 missense variant L881S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for the L881S variant, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.629350 | Binding | 0.299 | 0.874 | 0.250 | -3.063 | Likely Benign | 0.169 | Likely Benign | Likely Benign | 0.051 | Likely Benign | 0.3363 | 0.0850 | -0.49 | Neutral | 0.068 | Benign | 0.012 | Benign | 2.49 | Pathogenic | 0.00 | Affected | -3 | -2 | -4.6 | -26.08 | |||||||||||||||||||||||||||||||||||
| c.2642T>G | L881W 2D  AIThe SynGAP1 missense variant L881W is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for the variant, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.629350 | Binding | 0.299 | 0.874 | 0.250 | -6.646 | Likely Benign | 0.231 | Likely Benign | Likely Benign | 0.075 | Likely Benign | 0.0662 | 0.3288 | -0.96 | Neutral | 0.014 | Benign | 0.008 | Benign | 2.44 | Pathogenic | 0.00 | Affected | -2 | -2 | -4.7 | 73.05 | |||||||||||||||||||||||||||||||||||
| c.2644G>T | G882W 2D  AIThe SynGAP1 missense variant G882W has no ClinVar entry and is absent from gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further show AlphaMissense‑Optimized as benign, while the SGM‑Consensus remains pathogenic; Foldetta stability analysis is unavailable. Overall, the preponderance of evidence points to a pathogenic effect, and this conclusion does not conflict with the ClinVar status, which currently contains no classification for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.690604 | Disordered | 0.632888 | Binding | 0.306 | 0.878 | 0.250 | -8.637 | Likely Pathogenic | 0.606 | Likely Pathogenic | Likely Benign | 0.166 | Likely Benign | 0.0829 | 0.4616 | -2.35 | Neutral | 0.983 | Probably Damaging | 0.813 | Possibly Damaging | 2.01 | Pathogenic | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | |||||||||||||||||||||||||||||||||||
| c.2653C>A | P885T 2D  AIThe SynGAP1 missense variant P885T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -5.152 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.068 | Likely Benign | 0.1438 | 0.6484 | -1.44 | Neutral | 0.369 | Benign | 0.171 | Benign | 2.78 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.2653C>G | P885A 2D  AIThe SynGAP1 missense variant P885A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -3.908 | Likely Benign | 0.062 | Likely Benign | Likely Benign | 0.044 | Likely Benign | 0.3425 | 0.5481 | -1.29 | Neutral | 0.112 | Benign | 0.084 | Benign | 2.80 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.2653C>T | P885S 2D  AIThe SynGAP1 missense variant P885S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -4.089 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.037 | Likely Benign | 0.3363 | 0.5881 | -1.10 | Neutral | 0.369 | Benign | 0.222 | Benign | 2.87 | Benign | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||
| c.2654C>A | P885H 2D  AIThe SynGAP1 missense variant P885H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence, including the high‑accuracy tools, points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -5.239 | Likely Benign | 0.171 | Likely Benign | Likely Benign | 0.083 | Likely Benign | 0.1552 | 0.5280 | -1.68 | Neutral | 0.938 | Possibly Damaging | 0.749 | Possibly Damaging | 2.74 | Benign | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.2654C>G | P885R 2D  AIThe SynGAP1 missense variant P885R is reported in gnomAD (ID 6‑33443206‑C‑G) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. No Foldetta stability analysis is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | 6-33443206-C-G | 1 | 6.20e-7 | -4.166 | Likely Benign | 0.308 | Likely Benign | Likely Benign | 0.072 | Likely Benign | 0.1311 | 0.3505 | -1.99 | Neutral | 0.586 | Possibly Damaging | 0.377 | Benign | 2.84 | Benign | 0.00 | Affected | 4.32 | 4 | -2 | 0 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||
| c.2654C>T | P885L 2D  AIThe SynGAP1 missense variant P885L is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.690604 | Disordered | 0.636133 | Binding | 0.344 | 0.917 | 0.250 | -4.352 | Likely Benign | 0.150 | Likely Benign | Likely Benign | 0.089 | Likely Benign | 0.2138 | 0.6951 | -1.99 | Neutral | 0.000 | Benign | 0.001 | Benign | 2.75 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||
| c.2656G>A | A886T 2D  AIThe SynGAP1 missense variant A886T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while only SIFT and FATHMM predict pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -4.319 | Likely Benign | 0.075 | Likely Benign | Likely Benign | 0.044 | Likely Benign | 0.1581 | 0.6737 | -0.78 | Neutral | 0.369 | Benign | 0.237 | Benign | 2.21 | Pathogenic | 0.00 | Affected | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||||||
| c.2656G>C | A886P 2D  AIThe SynGAP1 missense variant A886P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) support a benign classification. This consensus does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -1.931 | Likely Benign | 0.091 | Likely Benign | Likely Benign | 0.073 | Likely Benign | 0.1985 | 0.4773 | -1.20 | Neutral | 0.812 | Possibly Damaging | 0.575 | Possibly Damaging | 2.15 | Pathogenic | 0.00 | Affected | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||
| c.2656G>T | A886S 2D  AIThe SynGAP1 missense variant A886S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -2.366 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.055 | Likely Benign | 0.2755 | 0.5639 | -0.50 | Neutral | 0.224 | Benign | 0.185 | Benign | 2.24 | Pathogenic | 0.00 | Affected | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||
| c.2657C>A | A886E 2D  AIThe SynGAP1 missense variant A886E is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors a benign outcome. Foldetta, which would provide a protein‑folding stability estimate, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact. This conclusion is not contradicted by ClinVar, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -3.747 | Likely Benign | 0.422 | Ambiguous | Likely Benign | 0.096 | Likely Benign | 0.1483 | 0.2008 | -1.31 | Neutral | 0.423 | Benign | 0.230 | Benign | 2.17 | Pathogenic | 0.00 | Affected | 0 | -1 | -5.3 | 58.04 | ||||||||||||||||||||||||||||||||||||
| c.2657C>G | A886G 2D  AIThe SynGAP1 missense variant A886G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for A886G, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | -1.993 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.074 | Likely Benign | 0.2173 | 0.3913 | -0.70 | Neutral | 0.597 | Possibly Damaging | 0.366 | Benign | 2.28 | Pathogenic | 0.00 | Affected | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2657C>T | A886V 2D  AIThe SynGAP1 missense variant A886V is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (gnomAD ID 6‑33443209‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus call (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.716283 | Disordered | 0.619166 | Binding | 0.359 | 0.922 | 0.500 | Uncertain | 1 | 6-33443209-C-T | 18 | 1.12e-5 | -4.478 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.061 | Likely Benign | 0.1131 | 0.5471 | -0.20 | Neutral | 0.888 | Possibly Damaging | 0.314 | Benign | 2.17 | Pathogenic | 0.00 | Affected | 4.32 | 4 | 0 | 0 | 2.4 | 28.05 | ||||||||||||||||||||||||||||
| c.265C>A | P89T 2D  AIThe SynGAP1 missense variant P89T has no ClinVar entry and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while pathogenic calls come from polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments further highlight this discordance: AlphaMissense‑Optimized predicts pathogenic, whereas the SGM‑Consensus (majority vote) predicts benign. Foldetta stability analysis is unavailable. Overall, the preponderance of evidence leans toward a benign effect, but the presence of a pathogenic prediction from AlphaMissense‑Optimized introduces uncertainty. This assessment does not contradict ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | -5.429 | Likely Benign | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.105 | Likely Benign | 0.1786 | 0.4702 | -2.49 | Neutral | 0.588 | Possibly Damaging | 0.036 | Benign | 3.74 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.265C>G | P89A 2D  AIThe SynGAP1 missense variant P89A is listed in ClinVar (ID 1031674.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a “Likely Benign” outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑confidence predictions indicate a benign impact, and this is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Thus, the variant is most likely benign based on current predictive evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | Uncertain | 2 | -5.778 | Likely Benign | 0.920 | Likely Pathogenic | Ambiguous | 0.095 | Likely Benign | 0.3407 | 0.3768 | -2.47 | Neutral | 0.225 | Benign | 0.020 | Benign | 3.77 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||
| c.265C>T | P89S 2D  AIThe SynGAP1 missense variant P89S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as likely benign. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM‑Consensus (majority vote) indicates likely benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of predictions lean toward a benign impact, and this assessment does not contradict the absence of a ClinVar classification. Thus, based on the available computational evidence, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | -5.198 | Likely Benign | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.099 | Likely Benign | 0.3459 | 0.4191 | -2.40 | Neutral | 0.225 | Benign | 0.020 | Benign | 3.76 | Benign | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||
| c.2662G>A | A888T 2D  AIThe SynGAP1 missense variant A888T is catalogued in gnomAD (6‑33443214‑G‑A) but has no ClinVar entry. In silico assessment shows a consensus of benign predictions: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate a neutral effect. Only SIFT predicts a deleterious outcome. High‑accuracy tools reinforce the benign consensus: AlphaMissense‑Optimized reports benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also classifies the variant as likely benign. Foldetta data are unavailable. Overall, the preponderance of evidence supports a benign classification, and this is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.575860 | Binding | 0.352 | 0.928 | 0.625 | 6-33443214-G-A | 1 | 6.20e-7 | -4.792 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.059 | Likely Benign | 0.1548 | 0.7115 | -1.11 | Neutral | 0.001 | Benign | 0.002 | Benign | 2.62 | Benign | 0.00 | Affected | 4.32 | 4 | 0 | 1 | -2.5 | 30.03 | ||||||||||||||||||||||||||||||
| c.2662G>C | A888P 2D  AIThe SynGAP1 missense variant A888P is reported in gnomAD (variant ID 6‑33443214‑G‑C) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence supports a benign classification for A888P, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.575860 | Binding | 0.352 | 0.928 | 0.625 | 6-33443214-G-C | 5 | 3.10e-6 | -2.368 | Likely Benign | 0.083 | Likely Benign | Likely Benign | 0.050 | Likely Benign | 0.1859 | 0.5764 | -0.02 | Neutral | 0.000 | Benign | 0.000 | Benign | 2.56 | Benign | 0.00 | Affected | 4.32 | 4 | -1 | 1 | -3.4 | 26.04 | ||||||||||||||||||||||||||||||
| c.2662G>T | A888S 2D  AIThe SynGAP1 missense variant A888S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.575860 | Binding | 0.352 | 0.928 | 0.625 | -3.580 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.034 | Likely Benign | 0.2661 | 0.5626 | -0.66 | Neutral | 0.017 | Benign | 0.025 | Benign | 2.64 | Benign | 0.00 | Affected | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||
| c.2663C>A | A888D 2D  AIThe SynGAP1 missense variant A888D is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all classify the change as benign, while SIFT uniquely predicts it as pathogenic. The consensus prediction from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized reports benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not conflict with the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.575860 | Binding | 0.352 | 0.928 | 0.625 | -3.870 | Likely Benign | 0.526 | Ambiguous | Likely Benign | 0.078 | Likely Benign | 0.1646 | 0.1386 | -1.52 | Neutral | 0.077 | Benign | 0.097 | Benign | 2.56 | Benign | 0.00 | Affected | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||
| c.2663C>G | A888G 2D  AIThe SynGAP1 missense variant A888G is reported in gnomAD (ID 6‑33443215‑C‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts it to be pathogenic, representing a single dissenting opinion. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.575860 | Binding | 0.352 | 0.928 | 0.625 | 6-33443215-C-G | 5 | 3.10e-6 | -2.523 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.047 | Likely Benign | 0.2312 | 0.5207 | -0.32 | Neutral | 0.033 | Benign | 0.036 | Benign | 2.69 | Benign | 0.00 | Affected | 4.32 | 4 | 0 | 1 | -2.2 | -14.03 | 10.1016/j.ajhg.2020.11.011 | |||||||||||||||||||||||||||||
| c.2663C>T | A888V 2D  AIThe SynGAP1 missense variant A888V is catalogued in gnomAD (ID 6‑33443215‑C‑T) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report benign or tolerated outcomes, while the single pathogenic signal comes from SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates a benign likelihood. Foldetta results are unavailable, so they do not influence the assessment. Overall, the preponderance of evidence points to a benign impact for A888V, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.784345 | Disordered | 0.575860 | Binding | 0.352 | 0.928 | 0.625 | 6-33443215-C-T | 1 | 6.20e-7 | -4.241 | Likely Benign | 0.106 | Likely Benign | Likely Benign | 0.034 | Likely Benign | 0.1285 | 0.7056 | -0.91 | Neutral | 0.000 | Benign | 0.001 | Benign | 2.78 | Benign | 0.00 | Affected | 4.32 | 4 | 0 | 0 | 2.4 | 28.05 | ||||||||||||||||||||||||||||||
| c.266C>A | P89Q 2D  AIThe SynGAP1 missense variant P89Q is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 pathogenic vs. 2 benign). Foldetta results are unavailable. Overall, the balance of evidence (five pathogenic vs. four benign predictions) indicates the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | -4.779 | Likely Benign | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.107 | Likely Benign | 0.1633 | 0.4310 | -2.63 | Deleterious | 0.642 | Possibly Damaging | 0.038 | Benign | 3.74 | Benign | 0.00 | Affected | 0 | -1 | -1.9 | 31.01 | ||||||||||||||||||||||||||||||||||||
| c.266C>G | P89R 2D  AIThe SynGAP1 missense variant P89R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 pathogenic vs. 2 benign). Foldetta results are unavailable. Overall, the balance of evidence (five pathogenic vs. four benign predictions) indicates the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | -3.636 | Likely Benign | 0.983 | Likely Pathogenic | Likely Pathogenic | 0.116 | Likely Benign | 0.1720 | 0.3362 | -3.00 | Deleterious | 0.642 | Possibly Damaging | 0.097 | Benign | 3.83 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||||
| c.266C>T | P89L 2D  AISynGAP1 missense variant P89L is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM, while pathogenic calls are made by PROVEAN, polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments indicate that AlphaMissense‑Optimized predicts pathogenicity, whereas the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑to‑2 split, and Foldetta results are unavailable. Overall, the majority of tools favor a pathogenic effect, but the evidence is not unanimous. Therefore, the variant is most likely pathogenic according to the current predictions, and this assessment does not contradict its ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.703578 | Disordered | 0.545797 | Binding | 0.316 | 0.865 | 0.500 | Uncertain | 2 | -6.775 | Likely Benign | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.119 | Likely Benign | 0.2399 | 0.5638 | -3.29 | Deleterious | 0.889 | Possibly Damaging | 0.058 | Benign | 3.73 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||
| c.2672T>A | L891H 2D  AIThe SynGAP1 missense variant L891H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.712013 | Disordered | 0.505861 | Binding | 0.305 | 0.923 | 0.750 | -4.604 | Likely Benign | 0.320 | Likely Benign | Likely Benign | 0.111 | Likely Benign | 0.1099 | 0.1189 | -1.79 | Neutral | 0.122 | Benign | 0.134 | Benign | 2.69 | Benign | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||
| c.2675C>A | S892Y 2D  AIThe SynGAP1 missense variant S892Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, the SGM‑Consensus (majority vote) also predicts benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.473390 | Uncertain | 0.319 | 0.926 | 0.875 | -4.696 | Likely Benign | 0.645 | Likely Pathogenic | Likely Benign | 0.113 | Likely Benign | 0.0814 | 0.5051 | -2.29 | Neutral | 0.998 | Probably Damaging | 0.959 | Probably Damaging | 2.55 | Benign | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||
| c.2675C>G | S892C 2D  AIThe SynGAP1 missense variant S892C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for S892C, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.473390 | Uncertain | 0.319 | 0.926 | 0.875 | -6.855 | Likely Benign | 0.280 | Likely Benign | Likely Benign | 0.113 | Likely Benign | 0.1189 | 0.5323 | -2.42 | Neutral | 0.999 | Probably Damaging | 0.969 | Probably Damaging | 2.54 | Benign | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2675C>T | S892F 2D  AIThe SynGAP1 missense variant S892F is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2). Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs four benign) lean toward pathogenicity. Thus, the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.754692 | Disordered | 0.473390 | Uncertain | 0.319 | 0.926 | 0.875 | -4.709 | Likely Benign | 0.605 | Likely Pathogenic | Likely Benign | 0.090 | Likely Benign | 0.0715 | 0.5124 | -3.07 | Deleterious | 0.998 | Probably Damaging | 0.959 | Probably Damaging | 2.55 | Benign | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||||||||
| c.2680G>T | G894W 2D  AIThe SynGAP1 missense variant G894W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (two pathogenic vs. two benign votes). Foldetta, which assesses protein‑folding stability, has no available output for this variant. Overall, the majority of evaluated predictors (five pathogenic vs. three benign) lean toward a pathogenic interpretation. Because there is no ClinVar annotation to contradict this assessment, the variant is most likely pathogenic based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.788093 | Disordered | 0.425700 | Uncertain | 0.310 | 0.925 | 0.750 | -6.927 | Likely Benign | 0.814 | Likely Pathogenic | Ambiguous | 0.177 | Likely Benign | 0.0698 | 0.4184 | -2.70 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 2.59 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||||||
| c.268G>A | V90M 2D  AIThe SynGAP1 missense variant V90M is listed in gnomAD (6‑33425876‑G‑A) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status, as none is reported. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | 6-33425876-G-A | 1 | 6.20e-7 | -5.017 | Likely Benign | 0.463 | Ambiguous | Likely Benign | 0.053 | Likely Benign | 0.1221 | 0.4393 | -0.15 | Neutral | 0.872 | Possibly Damaging | 0.162 | Benign | 3.98 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 2 | -2.3 | 32.06 | ||||||||||||||||||||||||||||||
| c.268G>C | V90L 2D  AIThe SynGAP1 missense variant V90L is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all classify the change as benign, while SIFT uniquely predicts it as pathogenic. The consensus from the SGM framework, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a Likely Benign verdict. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized reports a benign outcome, the SGM Consensus also indicates Likely Benign, and Foldetta data are unavailable. With the majority of evidence pointing to a benign effect and no conflicting ClinVar annotation, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | -3.676 | Likely Benign | 0.522 | Ambiguous | Likely Benign | 0.056 | Likely Benign | 0.1334 | 0.4487 | -0.24 | Neutral | 0.103 | Benign | 0.015 | Benign | 4.02 | Benign | 0.00 | Affected | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.268G>T | V90L 2D  AIThe SynGAP1 missense variant V90L is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus indicates Likely Benign, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect for V90L, and this conclusion does not contradict any ClinVar status, as none is assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | -3.676 | Likely Benign | 0.522 | Ambiguous | Likely Benign | 0.056 | Likely Benign | 0.1334 | 0.4487 | -0.24 | Neutral | 0.103 | Benign | 0.015 | Benign | 4.02 | Benign | 0.00 | Affected | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2693C>A | S898Y 2D  AISynGAP1 missense variant S898Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that classify the variant as benign include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Overall, the majority of tools and the consensus prediction indicate a pathogenic effect, and this conclusion does not contradict any ClinVar annotation because none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.690604 | Disordered | 0.426070 | Uncertain | 0.305 | 0.922 | 0.500 | -5.927 | Likely Benign | 0.712 | Likely Pathogenic | Likely Benign | 0.182 | Likely Benign | 0.1244 | 0.6838 | -2.69 | Deleterious | 0.998 | Probably Damaging | 0.959 | Probably Damaging | 2.44 | Pathogenic | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||
| c.2693C>T | S898F 2D  AIThe SynGAP1 missense variant S898F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as Likely Pathogenic, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) results are unavailable. Based on the overall distribution of predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.690604 | Disordered | 0.426070 | Uncertain | 0.305 | 0.922 | 0.500 | -5.242 | Likely Benign | 0.673 | Likely Pathogenic | Likely Benign | 0.181 | Likely Benign | 0.1183 | 0.6643 | -2.87 | Deleterious | 0.998 | Probably Damaging | 0.959 | Probably Damaging | 2.44 | Pathogenic | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||||||||
| c.2696T>A | I899N 2D  AIThe SynGAP1 missense variant I899N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect for I899N, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.443727 | Uncertain | 0.292 | 0.928 | 0.375 | -3.328 | Likely Benign | 0.469 | Ambiguous | Likely Benign | 0.058 | Likely Benign | 0.0933 | 0.0470 | -0.68 | Neutral | 0.611 | Possibly Damaging | 0.140 | Benign | 2.76 | Benign | 0.00 | Affected | -2 | -3 | -8.0 | 0.94 | |||||||||||||||||||||||||||||||||||
| c.2696T>G | I899S 2D  AIThe SynGAP1 missense variant I899S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic call comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign consensus. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not conflict with the ClinVar record, which contains no assertion for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.443727 | Uncertain | 0.292 | 0.928 | 0.375 | -0.857 | Likely Benign | 0.340 | Likely Benign | Likely Benign | 0.082 | Likely Benign | 0.2838 | 0.0840 | -0.38 | Neutral | 0.003 | Benign | 0.004 | Benign | 2.88 | Benign | 0.00 | Affected | -1 | -2 | -5.3 | -26.08 | |||||||||||||||||||||||||||||||||||
| c.269T>A | V90E 2D  AIThe SynGAP1 missense variant V90E is listed in ClinVar (ID 971665.0) with an uncertain significance status and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all predict benign. Only two tools—SIFT and AlphaMissense‑Default—suggest a pathogenic outcome. When the high‑accuracy consensus is considered, AlphaMissense‑Optimized remains benign, and the SGM Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign effect. No Foldetta stability assessment is available for this variant. Overall, the preponderance of evidence points to a benign impact, which does not contradict the ClinVar uncertain classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | Uncertain | 1 | -4.079 | Likely Benign | 0.703 | Likely Pathogenic | Likely Benign | 0.108 | Likely Benign | 0.1323 | 0.2233 | -0.38 | Neutral | 0.001 | Benign | 0.000 | Benign | 4.00 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -2 | -7.7 | 29.98 | |||||||||||||||||||||||||||||||
| c.269T>C | V90A 2D  AIThe SynGAP1 missense variant V90A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that V90A is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | -0.984 | Likely Benign | 0.288 | Likely Benign | Likely Benign | 0.055 | Likely Benign | 0.3129 | 0.3154 | 0.27 | Neutral | 0.053 | Benign | 0.008 | Benign | 4.11 | Benign | 0.00 | Affected | 0 | 0 | -2.4 | -28.05 | |||||||||||||||||||||||||||||||||||
| c.269T>G | V90G 2D  AIThe SynGAP1 missense variant V90G is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a benign verdict. Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.570702 | Disordered | 0.542047 | Binding | 0.343 | 0.873 | 0.500 | -2.617 | Likely Benign | 0.358 | Ambiguous | Likely Benign | 0.094 | Likely Benign | 0.2047 | 0.2936 | -0.55 | Neutral | 0.024 | Benign | 0.208 | Benign | 4.00 | Benign | 0.00 | Affected | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||
| c.26A>C | H9P 2D  AIThe SynGAP1 missense variant H9P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The prediction is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -2.838 | Likely Benign | 0.061 | Likely Benign | Likely Benign | 0.224 | Likely Benign | 0.2644 | 0.5169 | -0.16 | Neutral | 0.107 | Benign | 0.006 | Benign | 4.19 | Benign | 0.00 | Affected | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||
| c.26A>G | H9R 2D  AIThe SynGAP1 missense variant H9R is reported in gnomAD (variant ID 6‑33420290‑A‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that H9R is most likely benign, and this conclusion does not contradict any ClinVar status (none is provided). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | 6-33420290-A-G | -1.736 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.113 | Likely Benign | 0.2330 | 0.3266 | -0.04 | Neutral | 0.012 | Benign | 0.002 | Benign | 4.25 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 2 | -1.3 | 19.05 | ||||||||||||||||||||||||||||||||
| c.26A>T | H9L 2D  AIThe SynGAP1 missense variant H9L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign; Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -2.603 | Likely Benign | 0.135 | Likely Benign | Likely Benign | 0.151 | Likely Benign | 0.1255 | 0.6285 | 0.48 | Neutral | 0.024 | Benign | 0.002 | Benign | 4.25 | Benign | 0.00 | Affected | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||
| c.2705C>A | A902D 2D  AIThe SynGAP1 missense variant A902D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which collectively suggest a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default predict a pathogenic impact. The high‑accuracy AlphaMissense‑Optimized result is uncertain, and Foldetta stability analysis is unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this assessment does not contradict any ClinVar annotation because no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.517703 | Binding | 0.319 | 0.919 | 0.375 | -5.273 | Likely Benign | 0.823 | Likely Pathogenic | Ambiguous | 0.101 | Likely Benign | 0.1653 | 0.1609 | -1.21 | Neutral | 0.986 | Probably Damaging | 0.787 | Possibly Damaging | 2.59 | Benign | 0.00 | Affected | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||
| c.271G>A | E91K 2D  AISynGAP1 E91K is not reported in ClinVar and is absent from gnomAD. Computational predictors fall into two groups: benign calls (REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus “Likely Benign”) and pathogenic calls (polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, AlphaMissense‑Optimized). High‑accuracy tools give conflicting results: AlphaMissense‑Optimized predicts pathogenic, whereas the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) predicts benign; Foldetta data are unavailable. Consequently, the evidence is split, but the consensus of the most reliable predictors leans toward a benign effect. Thus, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -4.964 | Likely Benign | 0.962 | Likely Pathogenic | Likely Pathogenic | 0.146 | Likely Benign | 0.2693 | 0.7444 | -1.07 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 3.92 | Benign | 0.00 | Affected | 0 | 1 | -0.4 | -0.94 | |||||||||||||||||||||||||||||||||||
| c.271G>C | E91Q 2D  AIThe SynGAP1 missense variant E91Q has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic impact are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. AlphaMissense‑Optimized is uncertain, and no Foldetta stability assessment is available. Overall, the predictions are mixed, with an equal number of benign and pathogenic calls, but the consensus‑based SGM‑Consensus and the majority of individual benign predictions lean toward a benign interpretation. Thus, the variant is most likely benign based on current computational evidence, and this assessment does not contradict ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -4.053 | Likely Benign | 0.805 | Likely Pathogenic | Ambiguous | 0.103 | Likely Benign | 0.1487 | 0.7442 | -0.89 | Neutral | 0.947 | Possibly Damaging | 0.727 | Possibly Damaging | 3.89 | Benign | 0.00 | Affected | 2 | 2 | 0.0 | -0.98 | |||||||||||||||||||||||||||||||||||
| c.272A>C | E91A 2D  AIThe SynGAP1 missense variant E91A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. AlphaMissense‑Optimized is uncertain, and no Foldetta stability result is available. Overall, the majority of consensus‑based and individual predictors lean toward a benign classification, with several high‑confidence tools indicating pathogenicity, leaving the assessment inconclusive. Based on the available predictions, the variant is most likely benign, and this does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -3.302 | Likely Benign | 0.815 | Likely Pathogenic | Ambiguous | 0.094 | Likely Benign | 0.4511 | 0.7077 | -1.58 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 3.89 | Benign | 0.00 | Affected | 0 | -1 | 5.3 | -58.04 | |||||||||||||||||||||||||||||||||||
| c.272A>G | E91G 2D  AIThe SynGAP1 missense variant E91G is listed in ClinVar (ID 436922.0) as benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicating a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar designation and not contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | Likely Benign | 1 | -3.226 | Likely Benign | 0.783 | Likely Pathogenic | Likely Benign | 0.110 | Likely Benign | 0.3418 | 0.6030 | -2.18 | Neutral | 0.947 | Possibly Damaging | 0.727 | Possibly Damaging | 3.86 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -2 | 3.1 | -72.06 | |||||||||||||||||||||||||||||||
| c.272A>T | E91V 2D  AIThe SynGAP1 E91V missense variant has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic impact are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” AlphaMissense‑Optimized yields an uncertain result, and no Foldetta stability assessment is available. Overall, the evidence is mixed, but the consensus of several independent benign predictors and the SGM‑Consensus lean toward a benign interpretation. Thus, the variant is most likely benign based on current predictions, and this assessment does not contradict any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -3.697 | Likely Benign | 0.934 | Likely Pathogenic | Ambiguous | 0.124 | Likely Benign | 0.0940 | 0.7457 | -2.16 | Neutral | 0.947 | Possibly Damaging | 0.788 | Possibly Damaging | 3.84 | Benign | 0.00 | Affected | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||||||||
| c.2734A>C | T912P 2D  AIThe SynGAP1 missense variant T912P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | -2.955 | Likely Benign | 0.111 | Likely Benign | Likely Benign | 0.087 | Likely Benign | 0.1832 | 0.4608 | -0.78 | Neutral | 0.217 | Benign | 0.121 | Benign | 4.00 | Benign | 0.00 | Affected | 0 | -1 | -0.9 | -3.99 | |||||||||||||||||||||||||||||||||||
| c.2734A>G | T912A 2D  AIThe SynGAP1 missense variant T912A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | -3.084 | Likely Benign | 0.097 | Likely Benign | Likely Benign | 0.088 | Likely Benign | 0.3754 | 0.3841 | -1.50 | Neutral | 0.973 | Probably Damaging | 0.856 | Possibly Damaging | 4.03 | Benign | 0.00 | Affected | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||||||||||
| c.2734A>T | T912S 2D  AIThe SynGAP1 missense variant T912S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for T912S, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | -2.647 | Likely Benign | 0.127 | Likely Benign | Likely Benign | 0.074 | Likely Benign | 0.3078 | 0.3893 | -1.05 | Neutral | 0.994 | Probably Damaging | 0.856 | Possibly Damaging | 4.18 | Benign | 0.00 | Affected | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2735C>A | T912N 2D  AIThe SynGAP1 missense variant T912N is listed in ClinVar with an uncertain significance (ClinVar ID 2337624.0) and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as likely benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates likely benign. No Foldetta (FoldX‑MD/Rosetta) stability result is available, so it does not influence the overall assessment. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict the current ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | Uncertain | 1 | -4.260 | Likely Benign | 0.190 | Likely Benign | Likely Benign | 0.116 | Likely Benign | 0.1095 | 0.3750 | -1.15 | Neutral | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 3.96 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | 0 | -2.8 | 13.00 | |||||||||||||||||||||||||||||||
| c.2735C>G | T912S 2D  AIThe SynGAP1 missense variant T912S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for T912S, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | -2.647 | Likely Benign | 0.127 | Likely Benign | Likely Benign | 0.128 | Likely Benign | 0.3078 | 0.3893 | -1.05 | Neutral | 0.994 | Probably Damaging | 0.856 | Possibly Damaging | 4.18 | Benign | 0.00 | Affected | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2735C>T | T912I 2D  AIThe SynGAP1 missense variant T912I is listed in gnomAD (ID 6‑33443287‑C‑T) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and Foldetta (FoldX‑MD/Rosetta stability assessment) has no available result for this variant. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta data is missing. Overall, the majority of reliable predictors indicate a benign effect, and this is consistent with the absence of a ClinVar pathogenic classification. Thus, the variant is most likely benign, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.740671 | Binding | 0.285 | 0.909 | 0.250 | 6-33443287-C-T | 2 | 1.24e-6 | -4.461 | Likely Benign | 0.522 | Ambiguous | Likely Benign | 0.117 | Likely Benign | 0.0945 | 0.5615 | -1.27 | Neutral | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 3.95 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | 5.2 | 12.05 | ||||||||||||||||||||||||||||||
| c.273G>C | E91D 2D  AIThe SynGAP1 missense variant E91D is reported in gnomAD (variant ID 6‑33425881‑G‑C) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports it as likely benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; no Foldetta stability data are available. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar status (none is assigned). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | 6-33425881-G-C | 1 | 6.20e-7 | -3.160 | Likely Benign | 0.293 | Likely Benign | Likely Benign | 0.095 | Likely Benign | 0.2000 | 0.5219 | -0.84 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 3.98 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 3 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||
| c.273G>T | E91D 2D  AIThe SynGAP1 missense variant E91D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of any ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.533667 | Binding | 0.303 | 0.875 | 0.500 | -3.160 | Likely Benign | 0.293 | Likely Benign | Likely Benign | 0.095 | Likely Benign | 0.2000 | 0.5219 | -0.84 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 3.98 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 3 | 0.0 | -14.03 | |||||||||||||||||||||||||||||||||
| c.2743G>C | G915R 2D  AIThe SynGAP1 missense variant G915R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G915R, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.808641 | Binding | 0.302 | 0.880 | 0.375 | -4.528 | Likely Benign | 0.656 | Likely Pathogenic | Likely Benign | 0.104 | Likely Benign | 0.0805 | 0.4204 | -2.31 | Neutral | 0.999 | Probably Damaging | 0.969 | Probably Damaging | 2.69 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.2749C>A | P917T 2D  AIThe SynGAP1 missense variant P917T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | -4.012 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.044 | Likely Benign | 0.1445 | 0.5711 | -1.68 | Neutral | 0.139 | Benign | 0.088 | Benign | 2.69 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.2749C>G | P917A 2D  AIThe SynGAP1 missense variant P917A is not reported in ClinVar and is present in gnomAD (ID 6‑33443301‑C‑G). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) all classify the change as benign or likely benign. Only SIFT predicts a pathogenic effect. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized reports benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the collective predictions point to a benign impact, and this conclusion does not contradict the ClinVar status, which currently has no entry for P917A. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | 6-33443301-C-G | 1 | 6.20e-7 | -3.681 | Likely Benign | 0.062 | Likely Benign | Likely Benign | 0.053 | Likely Benign | 0.3458 | 0.4772 | -1.33 | Neutral | 0.065 | Benign | 0.037 | Benign | 2.72 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 1 | 3.4 | -26.04 | ||||||||||||||||||||||||||||||
| c.2749C>T | P917S 2D  AIThe SynGAP1 missense variant P917S is catalogued in gnomAD (ID 6‑33443301‑C‑T) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report benign. Only SIFT predicts pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, labels the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote) is benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no reported result for this variant, so it provides no additional evidence. Overall, the preponderance of predictions indicates that P917S is most likely benign, and this conclusion is not contradicted by any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | 6-33443301-C-T | 1 | 6.20e-7 | -3.562 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.056 | Likely Benign | 0.3478 | 0.5121 | -0.54 | Neutral | 0.003 | Benign | 0.002 | Benign | 2.70 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||
| c.274G>A | G92R 2D  AIThe SynGAP1 missense variant G92R is listed in gnomAD (6-33425882‑G‑A) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which is a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain,” SGM‑Consensus as “Likely Benign,” and Foldetta results are unavailable. Taken together, the preponderance of evidence from multiple independent predictors and the consensus score points to a benign classification. This conclusion is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | 6-33425882-G-A | 1 | 6.20e-7 | -2.909 | Likely Benign | 0.876 | Likely Pathogenic | Ambiguous | 0.139 | Likely Benign | 0.1041 | 0.4605 | -2.38 | Neutral | 0.999 | Probably Damaging | 0.979 | Probably Damaging | 4.01 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -3 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||
| c.274G>C | G92R 2D  AIThe SynGAP1 missense variant G92R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the majority of high‑confidence tools and the consensus prediction lean toward a benign interpretation, with no conflict with ClinVar status. Thus, the variant is most likely benign based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -2.909 | Likely Benign | 0.876 | Likely Pathogenic | Ambiguous | 0.139 | Likely Benign | 0.1041 | 0.4605 | -2.38 | Neutral | 0.999 | Probably Damaging | 0.979 | Probably Damaging | 4.01 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -3 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||
| c.274G>T | G92W 2D  AIThe SynGAP1 missense variant G92W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Tools that agree on a pathogenic effect include PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments are mixed: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of available predictions (five pathogenic vs. three benign) suggest a pathogenic impact. This conclusion is not contradicted by ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -6.502 | Likely Benign | 0.789 | Likely Pathogenic | Ambiguous | 0.224 | Likely Benign | 0.0640 | 0.4546 | -2.61 | Deleterious | 1.000 | Probably Damaging | 0.979 | Probably Damaging | 3.95 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||||||
| c.2750C>A | P917H 2D  AIThe SynGAP1 missense variant P917H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence—including the high‑accuracy tools—points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | -5.395 | Likely Benign | 0.182 | Likely Benign | Likely Benign | 0.122 | Likely Benign | 0.1584 | 0.4410 | -2.00 | Neutral | 0.975 | Probably Damaging | 0.766 | Possibly Damaging | 2.65 | Benign | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.2750C>G | P917R 2D  AIThe SynGAP1 missense variant P917R is listed in ClinVar with an “Uncertain” status and is present in gnomAD (gnomAD ID 6‑33443302‑C‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also as benign, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | Uncertain | 1 | 6-33443302-C-G | 5 | 3.10e-6 | -4.475 | Likely Benign | 0.363 | Ambiguous | Likely Benign | 0.142 | Likely Benign | 0.1270 | 0.3012 | -1.70 | Neutral | 0.642 | Possibly Damaging | 0.316 | Benign | 2.68 | Benign | 0.00 | Affected | 3.77 | 5 | -2 | 0 | -2.9 | 59.07 | ||||||||||||||||||||||||||||
| c.2750C>T | P917L 2D  AIThe SynGAP1 missense variant P917L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.599170 | Disordered | 0.863949 | Binding | 0.314 | 0.862 | 0.375 | -3.369 | Likely Benign | 0.172 | Likely Benign | Likely Benign | 0.075 | Likely Benign | 0.2001 | 0.6351 | -2.35 | Neutral | 0.425 | Benign | 0.233 | Benign | 2.75 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||
| c.2758C>A | Q920K 2D  AIThe SynGAP1 missense variant Q920K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for Q920K, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -4.234 | Likely Benign | 0.380 | Ambiguous | Likely Benign | 0.145 | Likely Benign | 0.2041 | 0.4331 | -1.72 | Neutral | 0.771 | Possibly Damaging | 0.412 | Benign | 2.67 | Benign | 0.00 | Affected | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||
| c.2758C>G | Q920E 2D  AIThe SynGAP1 missense variant Q920E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to the variant being most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -3.863 | Likely Benign | 0.174 | Likely Benign | Likely Benign | 0.125 | Likely Benign | 0.1443 | 0.2622 | -1.23 | Neutral | 0.771 | Possibly Damaging | 0.492 | Possibly Damaging | 2.67 | Benign | 0.00 | Affected | 2 | 2 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||
| c.2759A>C | Q920P 2D  AIThe SynGAP1 missense variant Q920P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of ClinVar annotation. Thus, the variant is most likely benign, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -2.127 | Likely Benign | 0.065 | Likely Benign | Likely Benign | 0.235 | Likely Benign | 0.2438 | 0.5318 | -1.68 | Neutral | 0.989 | Probably Damaging | 0.834 | Possibly Damaging | 2.58 | Benign | 0.00 | Affected | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||||||||
| c.2759A>G | Q920R 2D  AIThe SynGAP1 missense variant Q920R is not reported in ClinVar and is absent from gnomAD. Consensus from multiple in‑silico predictors shows a split: benign calls come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic calls arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy tools further support this view: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus confirms Likely Benign, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -3.170 | Likely Benign | 0.352 | Ambiguous | Likely Benign | 0.168 | Likely Benign | 0.1626 | 0.2369 | -1.41 | Neutral | 0.961 | Probably Damaging | 0.596 | Possibly Damaging | 2.63 | Benign | 0.00 | Affected | 1 | 1 | -1.0 | 28.06 | |||||||||||||||||||||||||||||||||||
| c.2759A>T | Q920L 2D  AIThe SynGAP1 missense variant Q920L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -4.048 | Likely Benign | 0.280 | Likely Benign | Likely Benign | 0.181 | Likely Benign | 0.0774 | 0.5995 | -2.36 | Neutral | 0.891 | Possibly Damaging | 0.596 | Possibly Damaging | 2.60 | Benign | 0.00 | Affected | -2 | -2 | 7.3 | -14.97 | |||||||||||||||||||||||||||||||||||
| c.275G>A | G92E 2D  AIThe SynGAP1 missense variant G92E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -3.240 | Likely Benign | 0.651 | Likely Pathogenic | Likely Benign | 0.156 | Likely Benign | 0.1521 | 0.4426 | -2.27 | Neutral | 0.999 | Probably Damaging | 0.972 | Probably Damaging | 4.07 | Benign | 0.00 | Affected | 0 | -2 | -3.1 | 72.06 | |||||||||||||||||||||||||||||||||||
| c.275G>C | G92A 2D  AIThe SynGAP1 missense variant G92A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized returns “Benign” and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates “Likely Benign.” No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for G92A, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -4.044 | Likely Benign | 0.219 | Likely Benign | Likely Benign | 0.080 | Likely Benign | 0.3922 | 0.4930 | -1.53 | Neutral | 0.996 | Probably Damaging | 0.910 | Probably Damaging | 4.08 | Benign | 0.00 | Affected | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.275G>T | G92V 2D  AIThe SynGAP1 missense variant G92V is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic predictions come from PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority vote) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of computational evidence points to a benign impact for G92V, and this conclusion does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.575842 | Disordered | 0.537848 | Binding | 0.337 | 0.874 | 0.625 | -4.627 | Likely Benign | 0.338 | Likely Benign | Likely Benign | 0.164 | Likely Benign | 0.1171 | 0.4363 | -2.62 | Deleterious | 0.999 | Probably Damaging | 0.972 | Probably Damaging | 4.00 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.2760A>C | Q920H 2D  AIThe SynGAP1 missense variant Q920H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -3.771 | Likely Benign | 0.254 | Likely Benign | Likely Benign | 0.079 | Likely Benign | 0.1419 | 0.4334 | -1.79 | Neutral | 0.989 | Probably Damaging | 0.883 | Possibly Damaging | 2.63 | Benign | 0.00 | Affected | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||
| c.2760A>T | Q920H 2D  AIThe SynGAP1 missense variant Q920H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.927260 | Binding | 0.306 | 0.845 | 0.250 | -3.771 | Likely Benign | 0.254 | Likely Benign | Likely Benign | 0.079 | Likely Benign | 0.1419 | 0.4334 | -1.79 | Neutral | 0.989 | Probably Damaging | 0.883 | Possibly Damaging | 2.63 | Benign | 0.00 | Affected | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||
| c.2762T>A | L921Q 2D  AIThe SynGAP1 missense variant L921Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.608892 | Disordered | 0.943282 | Binding | 0.311 | 0.845 | 0.375 | -2.389 | Likely Benign | 0.311 | Likely Benign | Likely Benign | 0.132 | Likely Benign | 0.1097 | 0.1119 | -0.62 | Neutral | 0.994 | Probably Damaging | 0.940 | Probably Damaging | 2.41 | Pathogenic | 0.00 | Affected | -2 | -2 | -7.3 | 14.97 | |||||||||||||||||||||||||||||||||||
| c.2762T>C | L921P 2D  AIThe SynGAP1 missense variant L921P is recorded in gnomAD (ID 6‑33443314‑T‑C) but has no ClinVar submission. Functional prediction tools cluster into two groups: benign calls from REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized; pathogenic calls from polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default is uncertain. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive because the votes are split (two benign, one pathogenic, one uncertain). Foldetta, a protein‑folding stability predictor that combines FoldX‑MD and Rosetta outputs, has no reported result for this variant. Consequently, the evidence is evenly divided, with high‑accuracy tools not providing a decisive verdict. The variant is therefore inconclusive; it does not contradict ClinVar status, which currently has no entry. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.608892 | Disordered | 0.943282 | Binding | 0.311 | 0.845 | 0.375 | 6-33443314-T-C | 1 | 6.20e-7 | -2.087 | Likely Benign | 0.393 | Ambiguous | Likely Benign | 0.215 | Likely Benign | 0.3581 | 0.1343 | -1.83 | Neutral | 0.998 | Probably Damaging | 0.958 | Probably Damaging | 2.39 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||
| c.2762T>G | L921R 2D  AIThe SynGAP1 missense variant L921R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also predicts benign, and Foldetta results are unavailable. Overall, the majority of high‑confidence tools predict a benign impact, and there is no conflict with ClinVar status. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.608892 | Disordered | 0.943282 | Binding | 0.311 | 0.845 | 0.375 | -2.205 | Likely Benign | 0.563 | Ambiguous | Likely Benign | 0.217 | Likely Benign | 0.1306 | 0.0761 | -1.19 | Neutral | 0.994 | Probably Damaging | 0.912 | Probably Damaging | 2.41 | Pathogenic | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | ||||||||||||||||||||||||||||||||||||
| c.2770C>A | P924T 2D  AIThe SynGAP1 missense variant P924T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence indicates that P924T is most likely pathogenic, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -6.360 | Likely Benign | 0.961 | Likely Pathogenic | Likely Pathogenic | 0.400 | Likely Benign | 0.1190 | 0.4009 | -6.03 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.67 | Pathogenic | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.2770C>G | P924A 2D  AIThe SynGAP1 missense variant P924A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all classify the variant as damaging. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments are mixed: AlphaMissense‑Optimized yields an Uncertain result, SGM‑Consensus indicates Likely Pathogenic, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the preponderance of evidence from multiple in silico predictors points to a pathogenic effect, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -5.995 | Likely Benign | 0.854 | Likely Pathogenic | Ambiguous | 0.383 | Likely Benign | 0.2904 | 0.3423 | -5.90 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 0.68 | Pathogenic | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.2770C>T | P924S 2D  AIThe SynGAP1 missense variant P924S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessment shows AlphaMissense‑Optimized as uncertain, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—labels the variant as Likely Pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this residue. Overall, the preponderance of evidence points to a pathogenic effect for P924S, and this conclusion does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -4.686 | Likely Benign | 0.920 | Likely Pathogenic | Ambiguous | 0.388 | Likely Benign | 0.2952 | 0.3772 | -5.86 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.68 | Pathogenic | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||
| c.2771C>A | P924H 2D  AIThe SynGAP1 missense variant P924H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. The Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the preponderance of computational evidence indicates that P924H is most likely pathogenic, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -6.236 | Likely Benign | 0.957 | Likely Pathogenic | Likely Pathogenic | 0.457 | Likely Benign | 0.1317 | 0.3281 | -5.87 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 0.65 | Pathogenic | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.2771C>G | P924R 2D  AIThe SynGAP1 missense variant P924R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split: benign calls from REVEL and ESM1b, whereas the remaining seven tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) predict pathogenicity. Grouping by consensus, the pathogenic group outweighs the benign group. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized scores the variant as pathogenic, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. Foldetta data are unavailable. Overall, the preponderance of evidence indicates that P924R is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -6.024 | Likely Benign | 0.965 | Likely Pathogenic | Likely Pathogenic | 0.420 | Likely Benign | 0.1314 | 0.2083 | -6.20 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.66 | Pathogenic | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.2771C>T | P924L 2D  AIThe SynGAP1 missense variant P924L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence indicates that P924L is most likely pathogenic, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.521092 | Disordered | 0.971858 | Binding | 0.293 | 0.846 | 0.250 | -6.361 | Likely Benign | 0.971 | Likely Pathogenic | Likely Pathogenic | 0.422 | Likely Benign | 0.1771 | 0.4941 | -6.89 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.66 | Pathogenic | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||
| c.2773C>A | L925I 2D  AIThe SynGAP1 missense variant L925I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of consensus tools (five pathogenic vs. three benign) lean toward a pathogenic interpretation. This prediction does not contradict ClinVar status, as the variant is not yet catalogued there. Thus, based on current computational evidence, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -5.266 | Likely Benign | 0.828 | Likely Pathogenic | Ambiguous | 0.205 | Likely Benign | 0.1192 | 0.3405 | -1.38 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 1.37 | Pathogenic | 0.00 | Affected | 2 | 2 | 0.7 | 0.00 | ||||||||||||||||||||||||||||||||||||
| c.2773C>G | L925V 2D  AIThe SynGAP1 missense variant L925V is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of consensus tools predict a pathogenic impact, and this prediction does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely pathogenic based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -3.977 | Likely Benign | 0.831 | Likely Pathogenic | Ambiguous | 0.244 | Likely Benign | 0.1848 | 0.3230 | -2.09 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 1.36 | Pathogenic | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | ||||||||||||||||||||||||||||||||||||
| c.2773C>T | L925F 2D  AIThe SynGAP1 missense variant L925F is not reported in ClinVar and is absent from gnomAD. Prediction tools that classify it as benign include REVEL and ESM1b, whereas the majority of tools predict it to be pathogenic: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of computational evidence points to a pathogenic impact for the L925F substitution, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -4.147 | Likely Benign | 0.966 | Likely Pathogenic | Likely Pathogenic | 0.242 | Likely Benign | 0.0897 | 0.2881 | -3.04 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.32 | Pathogenic | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.2774T>A | L925H 2D  AIThe SynGAP1 missense variant L925H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict a pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Pathogenic.” No Foldetta stability result is available. Overall, the majority of evidence points to a pathogenic effect for L925H, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -3.369 | Likely Benign | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.475 | Likely Benign | 0.1219 | 0.1494 | -5.24 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.28 | Pathogenic | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||
| c.2774T>C | L925P 2D  AIThe SynGAP1 missense variant L925P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only ESM1b, whereas the remaining tools—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus—predict a pathogenic or likely pathogenic impact. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as likely pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of evidence from multiple in silico predictors indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -2.733 | Likely Benign | 0.985 | Likely Pathogenic | Likely Pathogenic | 0.501 | Likely Pathogenic | 0.3083 | 0.1996 | -5.12 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.28 | Pathogenic | 0.00 | Affected | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||||||
| c.2774T>G | L925R 2D  AIThe SynGAP1 missense variant L925R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only ESM1b, whereas the remaining tools—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—all predict a pathogenic or likely pathogenic impact. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized reports a pathogenic effect, and the SGM‑Consensus indicates a likely pathogenic classification. Foldetta results are not available for this variant. Based on the preponderance of pathogenic predictions and the lack of benign consensus, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.483068 | Structured | 0.977963 | Binding | 0.290 | 0.852 | 0.125 | -2.340 | Likely Benign | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.519 | Likely Pathogenic | 0.1278 | 0.1294 | -4.54 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.28 | Pathogenic | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||
| c.2776T>A | S926T 2D  AIThe SynGAP1 missense variant S926T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus (SGM Consensus) is inconclusive, with two pathogenic and two benign votes. AlphaMissense‑Optimized, a high‑accuracy model, predicts benign, while Foldetta stability analysis is unavailable. Overall, the majority of standard tools favor a pathogenic effect, whereas the single high‑accuracy model suggests benign. Thus, the variant is most likely pathogenic based on the collective predictions, and this assessment does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -2.917 | Likely Benign | 0.651 | Likely Pathogenic | Likely Benign | 0.226 | Likely Benign | 0.1174 | 0.5663 | -2.34 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.55 | Pathogenic | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||||||||
| c.2776T>C | S926P 2D  AIThe SynGAP1 missense variant S926P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic outcome: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The high‑accuracy consensus, SGM‑Consensus, is derived from a majority vote of AlphaMissense‑Default (pathogenic), ESM1b (benign), FATHMM (pathogenic), and PROVEAN (pathogenic), yielding a Likely Pathogenic classification. AlphaMissense‑Optimized is uncertain, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a pathogenic effect for S926P. This conclusion is not contradicted by ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -3.873 | Likely Benign | 0.916 | Likely Pathogenic | Ambiguous | 0.424 | Likely Benign | 0.1738 | 0.5058 | -3.79 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.51 | Pathogenic | 0.00 | Affected | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||
| c.2776T>G | S926A 2D  AIThe SynGAP1 missense variant S926A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of reliable predictions indicate a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -3.042 | Likely Benign | 0.463 | Ambiguous | Likely Benign | 0.186 | Likely Benign | 0.4657 | 0.4488 | -2.13 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.59 | Pathogenic | 0.00 | Affected | 1 | 1 | 2.6 | -16.00 | ||||||||||||||||||||||||||||||||||||
| c.2777C>A | S926Y 2D  AIThe SynGAP1 missense variant S926Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—predict a pathogenic outcome. High‑accuracy assessments reinforce this trend: AlphaMissense‑Optimized classifies the variant as pathogenic; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports it as Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect for S926Y, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -6.602 | Likely Benign | 0.968 | Likely Pathogenic | Likely Pathogenic | 0.444 | Likely Benign | 0.0602 | 0.4932 | -4.53 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||
| c.2777C>G | S926C 2D  AIThe SynGAP1 missense variant S926C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is unavailable for this variant. Overall, the majority of predictions lean toward pathogenicity, with the high‑accuracy AlphaMissense‑Optimized result providing a conflicting benign signal. Thus, the variant is most likely pathogenic based on the collective evidence, and this assessment does not contradict any ClinVar status because none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -6.546 | Likely Benign | 0.680 | Likely Pathogenic | Likely Benign | 0.414 | Likely Benign | 0.0815 | 0.5487 | -3.81 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2777C>T | S926F 2D  AIThe SynGAP1 missense variant S926F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is labeled Likely Pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that S926F is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.461924 | Structured | 0.981753 | Binding | 0.295 | 0.854 | 0.250 | -6.157 | Likely Benign | 0.980 | Likely Pathogenic | Likely Pathogenic | 0.458 | Likely Benign | 0.0585 | 0.5220 | -4.57 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 1.50 | Pathogenic | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||||||||
| c.2779T>A | F927I 2D  AIThe SynGAP1 missense variant F927I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence from multiple in silico tools indicates that F927I is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.793 | Likely Benign | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.370 | Likely Benign | 0.2255 | 0.1238 | -4.50 | Deleterious | 0.997 | Probably Damaging | 0.994 | Probably Damaging | 1.34 | Pathogenic | 0.00 | Affected | 1 | 0 | 1.7 | -34.02 | |||||||||||||||||||||||||||||||||||
| c.2779T>C | F927L 2D  AIThe SynGAP1 missense variant F927L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is labeled “Likely Pathogenic”). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely pathogenicity. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that F927L is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.227 | Likely Benign | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.317 | Likely Benign | 0.2214 | 0.2209 | -4.48 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.39 | Pathogenic | 0.00 | Affected | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||
| c.2779T>G | F927V 2D  AIThe SynGAP1 missense variant F927V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable and therefore not considered. Overall, the preponderance of evidence from multiple in silico predictors and high‑accuracy tools indicates that the variant is most likely pathogenic, with no ClinVar annotation to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.919 | Likely Benign | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.470 | Likely Benign | 0.2207 | 0.1206 | -5.21 | Deleterious | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 1.34 | Pathogenic | 0.00 | Affected | -1 | -1 | 1.4 | -48.04 | |||||||||||||||||||||||||||||||||||
| c.277C>G | R93G 2D  AIThe SynGAP1 missense variant R93G is listed in ClinVar (ID 2504251.0) with an “Uncertain” clinical significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus (derived from the same set of predictors) labels it “Likely Benign”; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that R93G is most likely benign, which does not contradict the current ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | Uncertain | 1 | -2.674 | Likely Benign | 0.400 | Ambiguous | Likely Benign | 0.093 | Likely Benign | 0.3809 | 0.3814 | -1.69 | Neutral | 0.103 | Benign | 0.019 | Benign | 3.99 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -3 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||
| c.277C>T | R93W 2D  AIThe SynGAP1 missense variant R93W is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for R93W, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | -5.652 | Likely Benign | 0.514 | Ambiguous | Likely Benign | 0.094 | Likely Benign | 0.1413 | 0.3835 | -2.22 | Neutral | 0.981 | Probably Damaging | 0.257 | Benign | 3.96 | Benign | 0.00 | Affected | 2 | -3 | 3.6 | 30.03 | |||||||||||||||||||||||||||||||||||
| c.2780T>A | F927Y 2D  AIThe SynGAP1 missense variant F927Y is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas tools that agree on a pathogenic effect include polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (5 pathogenic vs. 3 benign) lean toward a pathogenic interpretation. Thus, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -5.244 | Likely Benign | 0.954 | Likely Pathogenic | Ambiguous | 0.291 | Likely Benign | 0.1551 | 0.1518 | -2.29 | Neutral | 0.997 | Probably Damaging | 0.987 | Probably Damaging | 1.40 | Pathogenic | 0.00 | Affected | 7 | 3 | -4.1 | 16.00 | ||||||||||||||||||||||||||||||||||||
| c.2780T>C | F927S 2D  AIThe SynGAP1 missense variant F927S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The high‑accuracy consensus, SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely pathogenic outcome (3 pathogenic vs. 1 benign). AlphaMissense‑Optimized alone predicts pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Based on the preponderance of pathogenic predictions—including the high‑accuracy consensus—the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -5.480 | Likely Benign | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.481 | Likely Benign | 0.4748 | 0.0591 | -5.68 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.32 | Pathogenic | 0.00 | Affected | -3 | -2 | -3.6 | -60.10 | |||||||||||||||||||||||||||||||||||
| c.2780T>G | F927C 2D  AIThe SynGAP1 missense variant F927C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Functional prediction tools uniformly indicate a deleterious effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as pathogenic. No tool in the dataset predicts a benign outcome. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as Likely Pathogenic. Foldetta results are not available. Based on the consensus of all available predictions, the variant is most likely pathogenic, and this conclusion is consistent with the absence of a ClinVar entry. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -8.298 | Likely Pathogenic | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.523 | Likely Pathogenic | 0.2921 | 0.1291 | -6.02 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.31 | Pathogenic | 0.00 | Affected | -4 | -2 | -0.3 | -44.04 | |||||||||||||||||||||||||||||||||||
| c.2781C>A | F927L 2D  AIThe SynGAP1 missense variant F927L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is labeled “Likely Pathogenic”). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely pathogenicity. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that F927L is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.227 | Likely Benign | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.296 | Likely Benign | 0.2214 | 0.2209 | -4.48 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.39 | Pathogenic | 0.00 | Affected | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||
| c.2781C>G | F927L 2D  AIThe SynGAP1 missense variant F927L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is labeled Likely Pathogenic. Foldetta results are not available. Based on the preponderance of pathogenic predictions and the high‑accuracy tools, the variant is most likely pathogenic; this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.529623 | Disordered | 0.985043 | Binding | 0.324 | 0.854 | 0.250 | -4.227 | Likely Benign | 1.000 | Likely Pathogenic | Likely Pathogenic | 0.296 | Likely Benign | 0.2214 | 0.2209 | -4.48 | Deleterious | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 1.39 | Pathogenic | 0.00 | Affected | 2 | 0 | 1.0 | -34.02 | |||||||||||||||||||||||||||||||||||
| c.2782C>A | Q928K 2D  AIThe SynGAP1 missense variant Q928K has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). AlphaMissense‑Optimized yields an uncertain result, and no Foldetta stability assessment is available. High‑accuracy evidence therefore consists of an uncertain AlphaMissense‑Optimized score, a Likely Pathogenic SGM‑Consensus, and an unavailable Foldetta prediction. Overall, the majority of tools predict pathogenicity, and there is no ClinVar status to contradict this assessment. Thus, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -4.941 | Likely Benign | 0.897 | Likely Pathogenic | Ambiguous | 0.259 | Likely Benign | 0.1728 | 0.5306 | -2.80 | Deleterious | 0.985 | Probably Damaging | 0.981 | Probably Damaging | 1.60 | Pathogenic | 0.00 | Affected | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||||||||||
| c.2782C>G | Q928E 2D  AIThe SynGAP1 missense variant Q928E is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized predicting benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2) and Foldetta results are unavailable. Based on the overall distribution of predictions, the variant is most likely pathogenic; this assessment does not contradict the ClinVar status, which currently contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -5.168 | Likely Benign | 0.590 | Likely Pathogenic | Likely Benign | 0.283 | Likely Benign | 0.1368 | 0.3477 | -2.06 | Neutral | 0.985 | Probably Damaging | 0.981 | Probably Damaging | 1.58 | Pathogenic | 0.00 | Affected | 2 | 2 | 0.0 | 0.98 | ||||||||||||||||||||||||||||||||||||
| c.2783A>C | Q928P 2D  AIThe SynGAP1 missense variant Q928P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. AlphaMissense‑Optimized returns an uncertain result, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available prediction for this variant. Based on the preponderance of pathogenic predictions and the SGM‑Consensus designation, the variant is most likely pathogenic; this assessment does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -6.275 | Likely Benign | 0.832 | Likely Pathogenic | Ambiguous | 0.441 | Likely Benign | 0.2016 | 0.5768 | -4.28 | Deleterious | 0.998 | Probably Damaging | 0.995 | Probably Damaging | 1.54 | Pathogenic | 0.00 | Affected | 0 | -1 | 1.9 | -31.01 | |||||||||||||||||||||||||||||||||||
| c.2783A>G | Q928R 2D  AIThe SynGAP1 missense variant Q928R is listed in gnomAD (ID 6‑33443335‑A‑G) but has no ClinVar entry. Functional prediction tools fall into two consensus groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). AlphaMissense‑Optimized returns an uncertain result, and no Foldetta (FoldX‑MD/Rosetta stability) data are available. High‑accuracy assessments therefore indicate a likely pathogenic outcome (SGM‑Consensus) with no contradictory evidence from ClinVar. Based on the preponderance of pathogenic predictions, the variant is most likely pathogenic, and this assessment does not conflict with the current ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | 6-33443335-A-G | 1 | 6.20e-7 | -4.089 | Likely Benign | 0.826 | Likely Pathogenic | Ambiguous | 0.290 | Likely Benign | 0.1357 | 0.3453 | -2.91 | Deleterious | 0.994 | Probably Damaging | 0.988 | Probably Damaging | 1.58 | Pathogenic | 0.00 | Affected | 4.32 | 4 | 1 | 1 | -1.0 | 28.06 | ||||||||||||||||||||||||||||||
| c.2783A>T | Q928L 2D  AIThe SynGAP1 missense variant Q928L has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of other in‑silico predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) and the SGM‑Consensus score (Likely Pathogenic) all indicate a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Based on the preponderance of pathogenic predictions and the SGM‑Consensus outcome, the variant is most likely pathogenic. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -6.237 | Likely Benign | 0.919 | Likely Pathogenic | Ambiguous | 0.373 | Likely Benign | 0.0757 | 0.6091 | -4.57 | Deleterious | 0.994 | Probably Damaging | 0.988 | Probably Damaging | 1.56 | Pathogenic | 0.00 | Affected | -2 | -2 | 7.3 | -14.97 | |||||||||||||||||||||||||||||||||||
| c.2784G>C | Q928H 2D  AIThe SynGAP1 missense variant Q928H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that classify the variant as benign include REVEL and ESM1b, whereas the majority of tools predict it to be pathogenic: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further support a pathogenic interpretation: AlphaMissense‑Optimized is uncertain, but the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—agrees on a pathogenic outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -4.358 | Likely Benign | 0.929 | Likely Pathogenic | Ambiguous | 0.329 | Likely Benign | 0.1389 | 0.4811 | -3.65 | Deleterious | 0.998 | Probably Damaging | 0.996 | Probably Damaging | 1.54 | Pathogenic | 0.00 | Affected | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||
| c.2784G>T | Q928H 2D  AIThe SynGAP1 missense variant Q928H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that classify the variant as benign include REVEL and ESM1b, whereas the majority of tools predict it to be pathogenic: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further support a pathogenic interpretation: AlphaMissense‑Optimized is uncertain, but the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—agrees on a pathogenic outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.497853 | Structured | 0.986260 | Binding | 0.324 | 0.852 | 0.250 | -4.358 | Likely Benign | 0.929 | Likely Pathogenic | Ambiguous | 0.330 | Likely Benign | 0.1389 | 0.4811 | -3.65 | Deleterious | 0.998 | Probably Damaging | 0.996 | Probably Damaging | 1.54 | Pathogenic | 0.00 | Affected | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||||||||||
| c.2785A>C | N929H 2D  AIThe SynGAP1 missense variant N929H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. ESM1b is uncertain, and Foldetta results are unavailable. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Pathogenic. With no conflicting ClinVar annotation, the collective evidence indicates that the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -7.070 | In-Between | 0.967 | Likely Pathogenic | Likely Pathogenic | 0.342 | Likely Benign | 0.1507 | 0.7659 | -3.52 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.45 | Pathogenic | 0.00 | Affected | 2 | 1 | 0.3 | 23.04 | |||||||||||||||||||||||||||||||||||
| c.2785A>G | N929D 2D  AIThe SynGAP1 missense variant N929D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which reports “Likely Pathogenic”). High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely pathogenicity. Foldetta results are unavailable. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -6.856 | Likely Benign | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.365 | Likely Benign | 0.1991 | 0.4605 | -3.86 | Deleterious | 0.999 | Probably Damaging | 0.995 | Probably Damaging | 1.49 | Pathogenic | 0.00 | Affected | 2 | 1 | 0.0 | 0.98 | |||||||||||||||||||||||||||||||||||
| c.2785A>T | N929Y 2D  AIThe SynGAP1 missense variant N929Y is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL predicts benign, whereas the remaining eleven predictors—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized—predict pathogenicity. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as Likely Pathogenic. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus result is consistent with a likely pathogenic classification. Foldetta predictions are unavailable. Taken together, the preponderance of evidence indicates that the variant is most likely pathogenic, and this assessment does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -10.327 | Likely Pathogenic | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.391 | Likely Benign | 0.0656 | 0.6489 | -6.02 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.45 | Pathogenic | 0.00 | Affected | -2 | -2 | 2.2 | 49.07 | |||||||||||||||||||||||||||||||||||
| c.2786A>C | N929T 2D  AIThe SynGAP1 missense variant N929T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining 11 predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) all indicate pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts a pathogenic change, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability analysis is available. Taken together, the overwhelming majority of evidence supports a pathogenic classification for N929T, and this conclusion is consistent with the absence of a ClinVar entry (i.e., there is no conflicting ClinVar status). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -9.245 | Likely Pathogenic | 0.987 | Likely Pathogenic | Likely Pathogenic | 0.257 | Likely Benign | 0.1469 | 0.8140 | -4.68 | Deleterious | 0.999 | Probably Damaging | 0.995 | Probably Damaging | 1.47 | Pathogenic | 0.00 | Affected | 0 | 0 | 2.8 | -13.00 | |||||||||||||||||||||||||||||||||||
| c.2786A>G | N929S 2D  AIThe SynGAP1 missense variant N929S is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Functional prediction tools show a consensus toward pathogenicity: SIFT, polyPhen‑2 (HumDiv and HumVar), PROVEAN, FATHMM, and AlphaMissense‑Default all predict a deleterious effect, while REVEL uniquely predicts a benign outcome. The remaining tools, ESM1b and AlphaMissense‑Optimized, return uncertain results. High‑accuracy assessments further support a damaging interpretation: the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic, and AlphaMissense‑Optimized remains uncertain; Foldetta data are unavailable. Taken together, the preponderance of evidence points to a pathogenic effect for N929S. This conclusion does not conflict with ClinVar, which currently contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -7.100 | In-Between | 0.948 | Likely Pathogenic | Ambiguous | 0.249 | Likely Benign | 0.4117 | 0.7639 | -3.89 | Deleterious | 0.999 | Probably Damaging | 0.992 | Probably Damaging | 1.48 | Pathogenic | 0.00 | Affected | 1 | 1 | 2.7 | -27.03 | |||||||||||||||||||||||||||||||||||
| c.2786A>T | N929I 2D  AIThe SynGAP1 missense variant N929I is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining eight tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) predict pathogenicity. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as Likely Pathogenic. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus result is consistent. Foldetta, a protein‑folding stability method that combines FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, and this conclusion does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -11.799 | Likely Pathogenic | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.297 | Likely Benign | 0.0780 | 0.6486 | -6.82 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 1.45 | Pathogenic | 0.00 | Affected | -2 | -3 | 8.0 | -0.94 | |||||||||||||||||||||||||||||||||||
| c.2787C>A | N929K 2D  AIThe SynGAP1 missense variant N929K is not reported in ClinVar and is absent from gnomAD. Prediction tools cluster into two groups: benign—REVEL—and pathogenic—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely pathogenic outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus confirms a likely pathogenic status; Foldetta results are not available. Taken together, the preponderance of evidence supports a pathogenic interpretation for N929K, and this conclusion is consistent with the absence of a ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -10.041 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.182 | Likely Benign | 0.2098 | 0.6046 | -4.35 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.48 | Pathogenic | 0.00 | Affected | 1 | 0 | -0.4 | 14.07 | |||||||||||||||||||||||||||||||||||
| c.2787C>G | N929K 2D  AIThe SynGAP1 missense variant N929K is not reported in ClinVar and is absent from gnomAD. Prediction tools cluster into two groups: benign—REVEL—and pathogenic—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely pathogenic outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus confirms a likely pathogenic status; Foldetta results are not available. Taken together, the preponderance of evidence supports a pathogenic interpretation for N929K, and this conclusion is consistent with the absence of a ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.557691 | Disordered | 0.986867 | Binding | 0.321 | 0.851 | 0.375 | -10.041 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.182 | Likely Benign | 0.2098 | 0.6046 | -4.35 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 1.48 | Pathogenic | 0.00 | Affected | 1 | 0 | -0.4 | 14.07 | |||||||||||||||||||||||||||||||||||
| c.2788C>A | P930T 2D  AIThe SynGAP1 missense variant P930T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: REVEL classifies it as benign, whereas the remaining 11 predictors (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) all indicate pathogenicity. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts a pathogenic change, and the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. No Foldetta stability analysis is available. Overall, the consensus of the majority of tools supports a pathogenic classification, and this is consistent with the absence of any ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -9.558 | Likely Pathogenic | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.454 | Likely Benign | 0.1666 | 0.5593 | -5.97 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.67 | Pathogenic | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.2788C>G | P930A 2D  AIThe SynGAP1 missense variant P930A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. Foldetta results are not available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that P930A is most likely pathogenic, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -8.938 | Likely Pathogenic | 0.963 | Likely Pathogenic | Likely Pathogenic | 0.392 | Likely Benign | 0.3376 | 0.4983 | -6.03 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 0.68 | Pathogenic | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.2788C>T | P930S 2D  AIThe SynGAP1 missense variant P930S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are limited to REVEL, which scores the variant as benign. In contrast, the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus is Likely Pathogenic; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that P930S is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -8.439 | Likely Pathogenic | 0.984 | Likely Pathogenic | Likely Pathogenic | 0.379 | Likely Benign | 0.3301 | 0.5457 | -5.84 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.68 | Pathogenic | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||
| c.2789C>A | P930H 2D  AIThe SynGAP1 missense variant P930H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. All other evaluated predictors—PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—classify the variant as pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from multiple independent predictors and high‑accuracy tools indicates that P930H is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -9.824 | Likely Pathogenic | 0.983 | Likely Pathogenic | Likely Pathogenic | 0.497 | Likely Benign | 0.1942 | 0.4392 | -6.44 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | 0.65 | Pathogenic | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.2789C>G | P930R 2D  AIThe SynGAP1 missense variant P930R is not reported in ClinVar and is absent from gnomAD. Prediction tools that assess pathogenicity all converge on a deleterious effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict pathogenicity. No tool in the dataset predicts a benign outcome. High‑accuracy assessments further support this view: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the consensus of available predictions indicates that P930R is most likely pathogenic, and this conclusion is consistent with the absence of a ClinVar entry (i.e., no contradictory status). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -9.474 | Likely Pathogenic | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.505 | Likely Pathogenic | 0.1460 | 0.3515 | -6.67 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.66 | Pathogenic | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.2789C>T | P930L 2D  AIThe SynGAP1 missense variant P930L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts Pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from multiple in silico predictors points to a pathogenic effect for P930L, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.538167 | Disordered | 0.988036 | Binding | 0.304 | 0.855 | 0.375 | -10.690 | Likely Pathogenic | 0.988 | Likely Pathogenic | Likely Pathogenic | 0.440 | Likely Benign | 0.2275 | 0.6113 | -7.25 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 0.66 | Pathogenic | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||
| c.278G>A | R93Q 2D  AIThe SynGAP1 missense variant R93Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect; there is no conflict with ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | -3.938 | Likely Benign | 0.167 | Likely Benign | Likely Benign | 0.074 | Likely Benign | 0.3568 | 0.2669 | -0.38 | Neutral | 0.203 | Benign | 0.006 | Benign | 4.14 | Benign | 0.00 | Affected | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||||||||||
| c.278G>C | R93P 2D  AIThe SynGAP1 missense variant R93P is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus indicates Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | -3.164 | Likely Benign | 0.473 | Ambiguous | Likely Benign | 0.121 | Likely Benign | 0.2200 | 0.4844 | -0.32 | Neutral | 0.361 | Benign | 0.038 | Benign | 3.99 | Benign | 0.00 | Affected | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||||
| c.278G>T | R93L 2D  AIThe SynGAP1 R93L missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus indicates Likely Benign, and Foldetta results are unavailable. Overall, the preponderance of evidence points to the variant being most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.549151 | Binding | 0.290 | 0.874 | 0.625 | -2.850 | Likely Benign | 0.425 | Ambiguous | Likely Benign | 0.064 | Likely Benign | 0.2197 | 0.4861 | -1.72 | Neutral | 0.103 | Benign | 0.019 | Benign | 4.00 | Benign | 0.00 | Affected | -3 | -2 | 8.3 | -43.03 | |||||||||||||||||||||||||||||||||||
| c.2792T>A | L931H 2D  AIThe SynGAP1 missense variant L931H has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include only REVEL, whereas the majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic impact. ESM1b and AlphaMissense‑Optimized are uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Based on the preponderance of pathogenic predictions and the SGM‑Consensus outcome, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.549308 | Disordered | 0.989212 | Binding | 0.335 | 0.856 | 0.375 | -7.495 | In-Between | 0.954 | Likely Pathogenic | Ambiguous | 0.301 | Likely Benign | 0.1198 | 0.1205 | -3.76 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.38 | Pathogenic | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||
| c.2795T>C | F932S 2D  AIThe SynGAP1 missense variant F932S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include only REVEL, which scores the variant as benign. In contrast, the majority of tools predict a pathogenic effect: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as pathogenic. ESM1b is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from multiple prediction algorithms and high‑accuracy tools indicates that F932S is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -7.196 | In-Between | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.260 | Likely Benign | 0.4257 | 0.0584 | -4.45 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.31 | Pathogenic | 0.00 | Affected | -3 | -2 | -3.6 | -60.10 | |||||||||||||||||||||||||||||||||||
| c.2795T>G | F932C 2D  AIThe SynGAP1 missense variant F932C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include only REVEL, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized predicts Pathogenic, and the SGM‑Consensus also indicates Likely Pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence from multiple in silico predictors and high‑accuracy tools indicates that the F932C variant is most likely pathogenic, and this conclusion does not contradict any existing ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.675549 | Disordered | 0.989197 | Binding | 0.293 | 0.858 | 0.500 | -8.601 | Likely Pathogenic | 0.982 | Likely Pathogenic | Likely Pathogenic | 0.309 | Likely Benign | 0.2446 | 0.1506 | -4.62 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.30 | Pathogenic | 0.00 | Affected | -4 | -2 | -0.3 | -44.04 | |||||||||||||||||||||||||||||||||||
| c.27T>A | H9Q 2D  AIThe SynGAP1 missense variant H9Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus, SGM‑Consensus, classifies the variant as Likely Benign, and AlphaMissense‑Optimized also reports a benign prediction. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -3.477 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.069 | Likely Benign | 0.2074 | 0.4297 | -0.05 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.24 | Benign | 0.00 | Affected | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||
| c.27T>G | H9Q 2D  AIThe SynGAP1 missense variant H9Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus, SGM‑Consensus, classifies the variant as Likely Benign, and AlphaMissense‑Optimized also reports a benign prediction. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.486429 | Structured | 0.528099 | Binding | 0.394 | 0.916 | 0.750 | -3.477 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.071 | Likely Benign | 0.2074 | 0.4297 | -0.05 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.24 | Benign | 0.00 | Affected | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||
| c.2803G>A | A935T 2D  AIThe SynGAP1 missense variant A935T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are not available. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -4.247 | Likely Benign | 0.228 | Likely Benign | Likely Benign | 0.204 | Likely Benign | 0.1670 | 0.6599 | -1.27 | Neutral | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.33 | Pathogenic | 0.00 | Affected | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||||||
| c.2803G>C | A935P 2D  AIThe SynGAP1 missense variant A935P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also yields a benign prediction, and Foldetta results are unavailable. Overall, the majority of high‑confidence tools predict a benign impact, and there is no conflict with ClinVar status. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -3.239 | Likely Benign | 0.371 | Ambiguous | Likely Benign | 0.269 | Likely Benign | 0.2062 | 0.5466 | -2.08 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.29 | Pathogenic | 0.00 | Affected | 1 | -1 | -3.4 | 26.04 | ||||||||||||||||||||||||||||||||||||
| c.2803G>T | A935S 2D  AIThe SynGAP1 missense variant A935S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the overall assessment. Overall, the majority of evidence points to a benign effect for A935S, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -3.361 | Likely Benign | 0.139 | Likely Benign | Likely Benign | 0.167 | Likely Benign | 0.2719 | 0.5496 | -0.60 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.53 | Benign | 0.00 | Affected | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||
| c.2804C>A | A935D 2D  AIThe SynGAP1 missense variant A935D is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is uncertain, but the SGM‑Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—classifies the variant as likely pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the preponderance of evidence points to a pathogenic impact for A935D, and this conclusion does not conflict with the current ClinVar status, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -4.089 | Likely Benign | 0.817 | Likely Pathogenic | Ambiguous | 0.247 | Likely Benign | 0.1832 | 0.1860 | -2.69 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.31 | Pathogenic | 0.00 | Affected | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||
| c.2804C>G | A935G 2D  AIThe SynGAP1 missense variant A935G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -2.868 | Likely Benign | 0.179 | Likely Benign | Likely Benign | 0.152 | Likely Benign | 0.2318 | 0.4679 | -1.97 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.32 | Pathogenic | 0.00 | Affected | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2804C>T | A935V 2D  AIThe SynGAP1 missense variant A935V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also leans benign (2 benign vs. 1 pathogenic votes). Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for A935V, and this conclusion does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.736850 | Disordered | 0.980490 | Binding | 0.286 | 0.865 | 0.625 | -4.750 | Likely Benign | 0.397 | Ambiguous | Likely Benign | 0.194 | Likely Benign | 0.1340 | 0.5941 | -2.06 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.30 | Pathogenic | 0.00 | Affected | 0 | 0 | 2.4 | 28.05 | ||||||||||||||||||||||||||||||||||||
| c.280C>A | P94T 2D  AIThe SynGAP1 missense variant P94T is reported in gnomAD (variant ID 6‑33425888‑C‑A) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign status. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Consequently, the collective evidence indicates that P94T is most likely benign, and this assessment does not contradict any ClinVar classification because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | 6-33425888-C-A | 1 | 6.20e-7 | -4.254 | Likely Benign | 0.085 | Likely Benign | Likely Benign | 0.088 | Likely Benign | 0.1326 | 0.4789 | -2.35 | Neutral | 0.198 | Benign | 0.015 | Benign | 4.12 | Benign | 0.00 | Affected | 4.32 | 1 | -1 | 0 | 0.9 | 3.99 | ||||||||||||||||||||||||||||||
| c.280C>G | P94A 2D  AIThe SynGAP1 missense variant P94A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and there is no conflict with ClinVar status, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | -3.450 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.085 | Likely Benign | 0.2825 | 0.4043 | -2.13 | Neutral | 0.092 | Benign | 0.008 | Benign | 4.14 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.280C>T | P94S 2D  AIThe SynGAP1 missense variant P94S is listed in ClinVar as a benign variant (ClinVar ID 650740.0) and is present in the gnomAD database (gnomAD ID 6‑33425888‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (derived from the same four high‑accuracy tools) also as benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions, including the high‑accuracy tools, indicate a benign effect, which aligns with the ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | Benign | 1 | 6-33425888-C-T | 5 | 3.10e-6 | -3.151 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.093 | Likely Benign | 0.2823 | 0.4388 | -2.36 | Neutral | 0.092 | Benign | 0.008 | Benign | 4.13 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | -1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||
| c.2815C>A | P939T 2D  AIThe SynGAP1 missense variant P939T is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy predictions show AlphaMissense‑Optimized as benign, while the SGM Consensus remains inconclusive and Foldetta is unavailable. Overall, more tools (five) predict pathogenicity than benign (four), suggesting the variant is most likely pathogenic. This assessment does not contradict ClinVar status, as the variant has not yet been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -5.739 | Likely Benign | 0.099 | Likely Benign | Likely Benign | 0.139 | Likely Benign | 0.1788 | 0.5310 | -3.34 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.22 | Pathogenic | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | ||||||||||||||||||||||||||||||||||||
| c.2815C>G | P939A 2D  AIThe SynGAP1 missense variant P939A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign versus two pathogenic votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy tools therefore provide a benign prediction from AlphaMissense‑Optimized, an inconclusive SGM Consensus, and no Foldetta data. Overall, the majority of individual predictors (five pathogenic versus four benign) lean toward a pathogenic interpretation, and this does not contradict the lack of ClinVar annotation. **Thus, the variant is most likely pathogenic based on the current computational predictions.** Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -4.614 | Likely Benign | 0.076 | Likely Benign | Likely Benign | 0.111 | Likely Benign | 0.3565 | 0.4336 | -3.57 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.40 | Pathogenic | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | ||||||||||||||||||||||||||||||||||||
| c.2815C>T | P939S 2D  AIThe SynGAP1 missense variant P939S is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs. 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy tools specifically show AlphaMissense‑Optimized as benign, while SGM Consensus and Foldetta remain unavailable. Overall, the majority of predictions (5 pathogenic vs. 4 benign) indicate that P939S is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -4.331 | Likely Benign | 0.098 | Likely Benign | Likely Benign | 0.101 | Likely Benign | 0.3372 | 0.4517 | -3.44 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.24 | Pathogenic | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||||||||
| c.2816C>A | P939Q 2D  AIThe SynGAP1 missense variant P939Q is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign vs. two pathogenic votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus remains inconclusive and Foldetta is unavailable. Overall, five of the nine evaluated tools predict pathogenicity versus four predicting benignity, giving a slight tilt toward a pathogenic interpretation. This prediction does not contradict ClinVar status, as no ClinVar entry exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -4.950 | Likely Benign | 0.131 | Likely Benign | Likely Benign | 0.156 | Likely Benign | 0.1551 | 0.4342 | -3.45 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.18 | Pathogenic | 0.00 | Affected | 0 | -1 | -1.9 | 31.01 | ||||||||||||||||||||||||||||||||||||
| c.2816C>G | P939R 2D  AIThe SynGAP1 missense variant P939R is reported in gnomAD (ID 6‑33443368‑C‑G) but has no ClinVar entry. Functional prediction tools show mixed results: benign calls come from REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. Grouping by consensus, the benign‑predicted tools outnumbered the pathogenic ones by one, but the overall tally favors pathogenicity (5 pathogenic vs 4 benign). High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is benign, but the SGM Consensus (a 2‑vs‑2 majority among AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields no clear verdict, and Foldetta data are unavailable. Consequently, the variant is most likely pathogenic according to the available predictions, and this assessment does not contradict any ClinVar status because none is reported. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | 6-33443368-C-G | 1 | 6.20e-7 | -4.863 | Likely Benign | 0.260 | Likely Benign | Likely Benign | 0.199 | Likely Benign | 0.1360 | 0.3336 | -3.79 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.18 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -2 | 0 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||
| c.2816C>T | P939L 2D  AIThe SynGAP1 missense variant P939L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. The SGM Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. High‑accuracy predictions show AlphaMissense‑Optimized as benign, while SGM Consensus and Foldetta are unavailable. Overall, five tools predict pathogenicity versus four predicting benign, so the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.935841 | Binding | 0.397 | 0.897 | 0.625 | -4.191 | Likely Benign | 0.138 | Likely Benign | Likely Benign | 0.159 | Likely Benign | 0.2113 | 0.5142 | -3.69 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.18 | Pathogenic | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||
| c.281C>A | P94H 2D  AIThe SynGAP1 missense variant P94H is reported in gnomAD (variant ID 6‑33425889‑C‑A) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. In contrast, polyPhen‑2 HumDiv and SIFT predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. No Foldetta stability analysis is available, so it does not influence the assessment. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that P94H is most likely benign, and this conclusion is not contradicted by any ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | 6-33425889-C-A | 1 | 6.20e-7 | -3.708 | Likely Benign | 0.106 | Likely Benign | Likely Benign | 0.077 | Likely Benign | 0.1486 | 0.3913 | -2.31 | Neutral | 0.637 | Possibly Damaging | 0.102 | Benign | 4.11 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | 0 | -1.6 | 40.02 | ||||||||||||||||||||||||||||||
| c.281C>G | P94R 2D  AIThe SynGAP1 missense variant P94R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | -3.272 | Likely Benign | 0.281 | Likely Benign | Likely Benign | 0.104 | Likely Benign | 0.1445 | 0.2780 | -2.31 | Neutral | 0.110 | Benign | 0.012 | Benign | 4.11 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.281C>T | P94L 2D  AIThe SynGAP1 missense variant P94L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.671169 | Disordered | 0.570978 | Binding | 0.350 | 0.869 | 0.625 | -2.721 | Likely Benign | 0.111 | Likely Benign | Likely Benign | 0.074 | Likely Benign | 0.2125 | 0.5862 | -2.27 | Neutral | 0.198 | Benign | 0.017 | Benign | 4.13 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||
| c.2822C>A | P941H 2D  AIThe SynGAP1 missense variant P941H is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. In contrast, two tools—polyPhen‑2 HumDiv and SIFT—classify the change as pathogenic. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; no Foldetta stability data are available. Overall, the majority of evidence points to a benign effect, and this assessment does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.874069 | Disordered | 0.900790 | Binding | 0.403 | 0.906 | 0.625 | -4.439 | Likely Benign | 0.098 | Likely Benign | Likely Benign | 0.058 | Likely Benign | 0.1621 | 0.4826 | -0.09 | Neutral | 0.589 | Possibly Damaging | 0.309 | Benign | 2.71 | Benign | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.2824C>A | P942T 2D  AIThe SynGAP1 missense variant P942T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for P942T, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | -5.745 | Likely Benign | 0.067 | Likely Benign | Likely Benign | 0.027 | Likely Benign | 0.1683 | 0.5599 | -1.23 | Neutral | 0.224 | Benign | 0.096 | Benign | 2.49 | Pathogenic | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.2824C>G | P942A 2D  AIThe SynGAP1 missense variant P942A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | -4.404 | Likely Benign | 0.047 | Likely Benign | Likely Benign | 0.023 | Likely Benign | 0.3362 | 0.4621 | -0.90 | Neutral | 0.059 | Benign | 0.061 | Benign | 2.52 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.2824C>T | P942S 2D  AIThe SynGAP1 missense variant P942S is catalogued in gnomAD (ID 6‑33443376‑C‑T) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | 6-33443376-C-T | 1 | 6.20e-7 | -3.919 | Likely Benign | 0.062 | Likely Benign | Likely Benign | 0.036 | Likely Benign | 0.3226 | 0.4806 | -0.80 | Neutral | 0.011 | Benign | 0.015 | Benign | 2.43 | Pathogenic | 0.00 | Affected | 4.32 | 4 | -1 | 1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||
| c.2825C>A | P942Q 2D  AIThe SynGAP1 missense variant P942Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for P942Q, and this conclusion does not contradict any ClinVar annotation (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | -6.094 | Likely Benign | 0.080 | Likely Benign | Likely Benign | 0.028 | Likely Benign | 0.1508 | 0.4703 | -1.12 | Neutral | 0.026 | Benign | 0.015 | Benign | 2.36 | Pathogenic | 0.00 | Affected | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||||||||||
| c.2825C>G | P942R 2D  AIThe SynGAP1 missense variant P942R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | -5.405 | Likely Benign | 0.159 | Likely Benign | Likely Benign | 0.045 | Likely Benign | 0.1307 | 0.3693 | -0.78 | Neutral | 0.411 | Benign | 0.139 | Benign | 2.36 | Pathogenic | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.2825C>T | P942L 2D  AIThe SynGAP1 missense variant P942L is listed in ClinVar (ID 2851884.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33443377‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.878102 | Binding | 0.365 | 0.915 | 0.625 | Uncertain | 1 | 6-33443377-C-T | 4 | 2.48e-6 | -5.063 | Likely Benign | 0.086 | Likely Benign | Likely Benign | 0.048 | Likely Benign | 0.2094 | 0.5507 | -2.00 | Neutral | 0.411 | Benign | 0.239 | Benign | 2.37 | Pathogenic | 0.00 | Affected | 4.32 | 4 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||
| c.2830G>A | G944S 2D  AIThe SynGAP1 missense variant G944S is listed in ClinVar as a benign alteration (ClinVar ID 833552.0) and is present in the gnomAD database (gnomAD ID 6‑33443382‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available for this variant. Overall, the majority of computational evidence supports a benign classification, which aligns with the ClinVar status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | Benign | 1 | 6-33443382-G-A | 13 | 8.05e-6 | -5.303 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.223 | Likely Benign | 0.2471 | 0.5502 | -0.75 | Neutral | 0.007 | Benign | 0.004 | Benign | 3.77 | Benign | 0.00 | Affected | 4.32 | 4 | 1 | 0 | -0.4 | 30.03 | ||||||||||||||||||||||||||||
| c.2830G>C | G944R 2D  AIThe SynGAP1 missense variant G944R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G944R, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | -6.577 | Likely Benign | 0.359 | Ambiguous | Likely Benign | 0.459 | Likely Benign | 0.1030 | 0.4733 | -1.82 | Neutral | 0.639 | Possibly Damaging | 0.299 | Benign | 3.73 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.2830G>T | G944C 2D  AIThe SynGAP1 missense variant G944C is listed in ClinVar with no submitted interpretation and is present in gnomAD (variant ID 6‑33443382‑G‑T). Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 (HumDiv and HumVar), SIFT, and ESM1b. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments reinforce this: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority vote of the four contributing tools) also indicates Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar status, which remains unclassified. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | 6-33443382-G-T | 1 | 6.20e-7 | -9.121 | Likely Pathogenic | 0.093 | Likely Benign | Likely Benign | 0.426 | Likely Benign | 0.1399 | 0.4439 | -1.79 | Neutral | 0.975 | Probably Damaging | 0.848 | Possibly Damaging | 3.70 | Benign | 0.00 | Affected | 4.32 | 4 | -3 | -3 | 2.9 | 46.09 | ||||||||||||||||||||||||||||||
| c.2831G>A | G944D 2D  AIThe SynGAP1 missense variant G944D is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools largely support a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the majority‑vote SGM‑Consensus also indicates a likely benign outcome. Only SIFT predicts a pathogenic effect, and ESM1b remains uncertain. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports a likely benign classification. Foldetta stability analysis is not available for this residue. Overall, the preponderance of evidence points to a benign impact, and this assessment does not conflict with the absence of a ClinVar claim. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | -7.684 | In-Between | 0.296 | Likely Benign | Likely Benign | 0.421 | Likely Benign | 0.1833 | 0.2826 | -1.42 | Neutral | 0.224 | Benign | 0.131 | Benign | 3.70 | Benign | 0.00 | Affected | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||
| c.2831G>C | G944A 2D  AIThe SynGAP1 missense variant G944A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | -6.397 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.314 | Likely Benign | 0.3352 | 0.4957 | -0.61 | Neutral | 0.059 | Benign | 0.042 | Benign | 3.81 | Benign | 0.00 | Affected | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2831G>T | G944V 2D  AIThe SynGAP1 missense variant G944V is catalogued in gnomAD (ID 6‑33443383‑G‑T) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all classify the substitution as benign or likely benign. Only SIFT predicts a pathogenic outcome, while ESM1b remains uncertain. High‑accuracy assessments confirm the benign consensus: AlphaMissense‑Optimized reports a benign effect, and the SGM‑Consensus likewise indicates a likely benign status. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the collective evidence points to a benign variant, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Thus, the variant is most likely benign, and this assessment does not contradict its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.977651 | Disordered | 0.852408 | Binding | 0.360 | 0.923 | 0.750 | 6-33443383-G-T | 1 | 6.20e-7 | -7.536 | In-Between | 0.090 | Likely Benign | Likely Benign | 0.446 | Likely Benign | 0.1455 | 0.3507 | -1.41 | Neutral | 0.126 | Benign | 0.096 | Benign | 3.70 | Benign | 0.00 | Affected | 4.32 | 4 | -3 | -1 | 4.6 | 42.08 | ||||||||||||||||||||||||||||||
| c.2836G>A | G946R 2D  AIThe SynGAP1 missense variant G946R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while ESM1b is uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus itself is Likely Benign. Foldetta results are unavailable. Overall, the majority of computational evidence indicates that G946R is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | -7.127 | In-Between | 0.308 | Likely Benign | Likely Benign | 0.296 | Likely Benign | 0.1157 | 0.5133 | -0.69 | Neutral | 0.818 | Possibly Damaging | 0.435 | Benign | 4.65 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.2836G>C | G946R 2D  AIThe SynGAP1 missense variant G946R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while ESM1b is uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus itself is Likely Benign. Foldetta results are unavailable. Overall, the majority of computational evidence indicates that G946R is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | -7.127 | In-Between | 0.308 | Likely Benign | Likely Benign | 0.296 | Likely Benign | 0.1157 | 0.5133 | -0.69 | Neutral | 0.818 | Possibly Damaging | 0.435 | Benign | 4.65 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.2837G>A | G946E 2D  AIThe SynGAP1 missense variant G946E is listed in ClinVar (ID 1299783.0) as benign and is present in gnomAD (6‑33443389‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while polyPhen‑2 HumDiv, SIFT, and ESM1b predict a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar designation and showing no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | Benign | 3 | 6-33443389-G-A | 13 | 8.05e-6 | -8.793 | Likely Pathogenic | 0.257 | Likely Benign | Likely Benign | 0.341 | Likely Benign | 0.1691 | 0.4859 | -0.51 | Neutral | 0.818 | Possibly Damaging | 0.355 | Benign | 4.58 | Benign | 0.00 | Affected | 4.32 | 4 | 0 | -2 | -3.1 | 72.06 | ||||||||||||||||||||||||||||
| c.2837G>C | G946A 2D  AIThe SynGAP1 missense variant G946A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G946A, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | -7.004 | In-Between | 0.079 | Likely Benign | Likely Benign | 0.191 | Likely Benign | 0.3378 | 0.4957 | 0.33 | Neutral | 0.649 | Possibly Damaging | 0.209 | Benign | 4.77 | Benign | 0.00 | Affected | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2837G>T | G946V 2D  AIThe SynGAP1 missense variant G946V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. ESM1b is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G946V, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.985417 | Disordered | 0.845792 | Binding | 0.357 | 0.920 | 0.750 | -7.528 | In-Between | 0.109 | Likely Benign | Likely Benign | 0.368 | Likely Benign | 0.1524 | 0.3707 | -0.30 | Neutral | 0.901 | Possibly Damaging | 0.516 | Possibly Damaging | 4.57 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.283C>A | H95N 2D  AIThe SynGAP1 missense variant H95N is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the only pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) indicates likely benign. Foldetta results are unavailable, so they do not influence the assessment. Overall, the computational evidence overwhelmingly supports a benign classification for H95N, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -3.454 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.089 | Likely Benign | 0.1821 | 0.2491 | -1.12 | Neutral | 0.219 | Benign | 0.009 | Benign | 4.20 | Benign | 0.00 | Affected | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||
| c.283C>G | H95D 2D  AIThe SynGAP1 missense variant H95D is not listed in ClinVar and is present in gnomAD (variant ID 6‑33425891‑C‑G). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all indicate benign or likely benign. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized scores benign, and the SGM‑Consensus also reports likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the computational evidence overwhelmingly supports a benign classification, and this is consistent with the absence of a ClinVar pathogenic report. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | 6-33425891-C-G | 3 | 1.86e-6 | -2.387 | Likely Benign | 0.188 | Likely Benign | Likely Benign | 0.092 | Likely Benign | 0.2759 | 0.1801 | -0.81 | Neutral | 0.084 | Benign | 0.009 | Benign | 4.22 | Benign | 0.00 | Affected | 4.32 | 1 | -1 | 1 | -0.3 | -22.05 | ||||||||||||||||||||||||||||||
| c.283C>T | H95Y 2D  AIThe SynGAP1 missense variant H95Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence indicates that H95Y is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -3.610 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.090 | Likely Benign | 0.0880 | 0.4122 | -1.45 | Neutral | 0.219 | Benign | 0.014 | Benign | 4.14 | Benign | 0.00 | Affected | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||
| c.2845G>A | G949S 2D  AIThe SynGAP1 missense variant G949S is listed in ClinVar as a benign alteration (ClinVar ID 212352.0) and is present in the gnomAD database (6‑33443397‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar classification and indicating no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.988861 | Disordered | 0.874971 | Binding | 0.365 | 0.923 | 0.750 | Benign/Likely benign | 4 | 6-33443397-G-A | 122 | 7.56e-5 | -5.693 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.321 | Likely Benign | 0.2528 | 0.5159 | 0.30 | Neutral | 0.611 | Possibly Damaging | 0.102 | Benign | 2.23 | Pathogenic | 0.00 | Affected | 4.32 | 4 | 1 | 0 | -0.4 | 30.03 | 10.1016/j.ajhg.2020.11.011 | |||||||||||||||||||||||||||
| c.284A>C | H95P 2D  AIThe SynGAP1 missense variant H95P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign, while the single pathogenic call comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) confirms a benign outcome. Foldetta results are unavailable, so they do not influence the assessment. Overall, the majority of evidence indicates that H95P is most likely benign, and this conclusion is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -1.611 | Likely Benign | 0.045 | Likely Benign | Likely Benign | 0.164 | Likely Benign | 0.2244 | 0.3704 | -1.66 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.19 | Benign | 0.00 | Affected | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||
| c.284A>G | H95R 2D  AIThe SynGAP1 missense variant H95R is reported in gnomAD (variant ID 6‑33425892‑A‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that H95R is most likely benign, and this conclusion does not contradict any ClinVar status (none is provided). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | 6-33425892-A-G | 1 | 6.20e-7 | -2.789 | Likely Benign | 0.153 | Likely Benign | Likely Benign | 0.043 | Likely Benign | 0.1907 | 0.2087 | -1.31 | Neutral | 0.084 | Benign | 0.009 | Benign | 4.20 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 2 | -1.3 | 19.05 | ||||||||||||||||||||||||||||||
| c.284A>T | H95L 2D  AIThe SynGAP1 missense variant H95L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -1.967 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.085 | Likely Benign | 0.1017 | 0.5074 | -2.31 | Neutral | 0.084 | Benign | 0.007 | Benign | 4.17 | Benign | 0.00 | Affected | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||
| c.285C>A | H95Q 2D  AIThe SynGAP1 missense variant H95Q is reported in gnomAD (ID 6‑33425893‑C‑A) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. In contrast, polyPhen‑2 HumDiv and SIFT predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates benign. No Foldetta stability analysis is available, so it does not influence the overall assessment. Overall, the preponderance of predictions indicates that H95Q is most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | 6-33425893-C-A | 1 | 6.20e-7 | -3.355 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.070 | Likely Benign | 0.1551 | 0.3375 | -0.97 | Neutral | 0.633 | Possibly Damaging | 0.017 | Benign | 4.21 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 3 | -0.3 | -9.01 | ||||||||||||||||||||||||||||||
| c.285C>G | H95Q 2D  AIThe SynGAP1 missense variant H95Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 HumDiv and SIFT predict pathogenicity, but these two tools are in minority. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized scores benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.661982 | Disordered | 0.590542 | Binding | 0.335 | 0.875 | 0.625 | -3.355 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.070 | Likely Benign | 0.1551 | 0.3375 | -0.97 | Neutral | 0.633 | Possibly Damaging | 0.017 | Benign | 4.21 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 3 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||
| c.2863T>A | S955T 2D  AIThe SynGAP1 missense variant S955T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for S955T, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | -4.717 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.070 | Likely Benign | 0.2184 | 0.5927 | -0.96 | Neutral | 0.451 | Benign | 0.265 | Benign | 2.38 | Pathogenic | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2863T>C | S955P 2D  AIThe SynGAP1 missense variant S955P is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443415‑T‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are SIFT and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | Uncertain | 1 | 6-33443415-T-C | 3 | 1.86e-6 | -2.584 | Likely Benign | 0.073 | Likely Benign | Likely Benign | 0.098 | Likely Benign | 0.2629 | 0.5537 | -0.75 | Neutral | 0.001 | Benign | 0.004 | Benign | 2.33 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 1 | -1 | -0.8 | 10.04 | ||||||||||||||||||||||||||||
| c.2863T>G | S955A 2D  AIThe SynGAP1 missense variant S955A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | -4.258 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.091 | Likely Benign | 0.4262 | 0.5038 | -0.71 | Neutral | 0.004 | Benign | 0.006 | Benign | 2.41 | Pathogenic | 0.00 | Affected | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||
| c.2864C>A | S955Y 2D  AIThe SynGAP1 missense variant S955Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) support a benign classification. There is no ClinVar entry to contradict this assessment, so the variant is most likely benign based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | -6.212 | Likely Benign | 0.216 | Likely Benign | Likely Benign | 0.077 | Likely Benign | 0.1321 | 0.4932 | -1.62 | Neutral | 0.977 | Probably Damaging | 0.721 | Possibly Damaging | 2.32 | Pathogenic | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||
| c.2864C>G | S955C 2D  AIThe SynGAP1 missense variant S955C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 benign vs. 2 pathogenic). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (5 pathogenic vs. 4 benign) indicate a likely pathogenic impact. This conclusion is not contradicted by ClinVar status, as the variant has no existing ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | -8.675 | Likely Pathogenic | 0.117 | Likely Benign | Likely Benign | 0.064 | Likely Benign | 0.1741 | 0.5470 | -1.48 | Neutral | 0.977 | Probably Damaging | 0.796 | Possibly Damaging | 2.32 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||
| c.2864C>T | S955F 2D  AISynGAP1 missense variant S955F is listed in ClinVar as uncertain and is present in gnomAD (ID 6‑33443416‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, AlphaMissense‑Default, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM; ESM1b is inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also returns benign, and Foldetta results are unavailable. Overall, the majority of high‑confidence predictions favor a benign impact, and this does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.984871 | Disordered | 0.945325 | Binding | 0.350 | 0.924 | 0.750 | Conflicting | 4 | 6-33443416-C-T | 95 | 5.89e-5 | -7.374 | In-Between | 0.176 | Likely Benign | Likely Benign | 0.093 | Likely Benign | 0.1246 | 0.4943 | -1.73 | Neutral | 0.977 | Probably Damaging | 0.721 | Possibly Damaging | 2.32 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||
| c.286G>A | G96S 2D  AIThe SynGAP1 missense variant G96S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33425894‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | Uncertain | 1 | 6-33425894-G-A | 5 | 3.10e-6 | -3.049 | Likely Benign | 0.065 | Likely Benign | Likely Benign | 0.071 | Likely Benign | 0.3001 | 0.5336 | -0.76 | Neutral | 0.364 | Benign | 0.008 | Benign | 4.25 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 0 | -0.4 | 30.03 | ||||||||||||||||||||||||||||
| c.286G>C | G96R 2D  AIThe SynGAP1 missense variant G96R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for G96R, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -3.872 | Likely Benign | 0.349 | Ambiguous | Likely Benign | 0.059 | Likely Benign | 0.1300 | 0.5031 | -0.91 | Neutral | 0.687 | Possibly Damaging | 0.062 | Benign | 4.19 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.286G>T | G96C 2D  AIThe SynGAP1 missense variant G96C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The high‑accuracy consensus methods give a benign verdict: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign.” No result is available from Foldetta, so its folding‑stability assessment is not considered. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of any ClinVar classification. Thus, the variant is most likely benign and does not contradict ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -5.140 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.112 | Likely Benign | 0.1645 | 0.4142 | -1.87 | Neutral | 0.981 | Probably Damaging | 0.216 | Benign | 4.12 | Benign | 0.00 | Affected | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||
| c.287G>A | G96D 2D  AIThe SynGAP1 missense variant G96D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this assessment does not contradict any ClinVar status, as no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -3.699 | Likely Benign | 0.249 | Likely Benign | Likely Benign | 0.099 | Likely Benign | 0.2283 | 0.3150 | -0.95 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.18 | Benign | 0.00 | Affected | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||
| c.287G>C | G96A 2D  AIThe SynGAP1 missense variant G96A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -3.669 | Likely Benign | 0.066 | Likely Benign | Likely Benign | 0.064 | Likely Benign | 0.4098 | 0.4212 | -1.00 | Neutral | 0.092 | Benign | 0.007 | Benign | 4.23 | Benign | 0.00 | Affected | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.287G>T | G96V 2D  AIThe SynGAP1 missense variant G96V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.657645 | Disordered | 0.599491 | Binding | 0.335 | 0.871 | 0.625 | -4.266 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.109 | Likely Benign | 0.1575 | 0.3833 | -1.43 | Neutral | 0.334 | Benign | 0.029 | Benign | 4.18 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.289G>A | E97K 2D  AIThe SynGAP1 missense variant E97K is catalogued in gnomAD (ID 6‑33425897‑G‑A) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors points to a benign effect. This conclusion is not contradicted by ClinVar, as no ClinVar classification exists for E97K. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.609018 | Binding | 0.340 | 0.867 | 0.625 | 6-33425897-G-A | 1 | 6.20e-7 | -4.972 | Likely Benign | 0.643 | Likely Pathogenic | Likely Benign | 0.139 | Likely Benign | 0.2709 | 0.7908 | -0.30 | Neutral | 0.976 | Probably Damaging | 0.651 | Possibly Damaging | 4.16 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 0 | -0.4 | -0.94 | ||||||||||||||||||||||||||||||
| c.289G>C | E97Q 2D  AIThe SynGAP1 missense variant E97Q is reported in gnomAD (ID 6‑33425897‑G‑C) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus itself is Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence from both consensus and high‑accuracy predictors indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.609018 | Binding | 0.340 | 0.867 | 0.625 | 6-33425897-G-C | 2 | 1.24e-6 | -3.917 | Likely Benign | 0.300 | Likely Benign | Likely Benign | 0.113 | Likely Benign | 0.1507 | 0.7874 | -0.32 | Neutral | 0.978 | Probably Damaging | 0.832 | Possibly Damaging | 4.13 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 2 | 0.0 | -0.98 | ||||||||||||||||||||||||||||||
| c.28C>G | R10G 2D  AIThe SynGAP1 missense variant R10G is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized scores the variant as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.534167 | Disordered | 0.513657 | Binding | 0.330 | 0.915 | 0.625 | -3.931 | Likely Benign | 0.124 | Likely Benign | Likely Benign | 0.175 | Likely Benign | 0.3796 | 0.4015 | 0.48 | Neutral | 0.058 | Benign | 0.009 | Benign | 4.15 | Benign | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||
| c.28C>T | R10W 2D  AIThe SynGAP1 R10W missense variant is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33420292‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (which itself is “Likely Benign”). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.534167 | Disordered | 0.513657 | Binding | 0.330 | 0.915 | 0.625 | Uncertain | 1 | 6-33420292-C-T | 2 | 1.30e-6 | -5.707 | Likely Benign | 0.503 | Ambiguous | Likely Benign | 0.236 | Likely Benign | 0.1461 | 0.4542 | -0.31 | Neutral | 0.964 | Probably Damaging | 0.190 | Benign | 4.10 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | -3 | 3.6 | 30.03 | ||||||||||||||||||||||||||||
| c.290A>C | E97A 2D  AIThe SynGAP1 missense variant E97A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain. High‑accuracy assessments reinforce the benign prediction: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is also likely benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for E97A, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.609018 | Binding | 0.340 | 0.867 | 0.625 | -3.091 | Likely Benign | 0.374 | Ambiguous | Likely Benign | 0.098 | Likely Benign | 0.4505 | 0.7728 | -0.58 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 4.16 | Benign | 0.00 | Affected | 0 | -1 | 5.3 | -58.04 | |||||||||||||||||||||||||||||||||||
| c.290A>G | E97G 2D  AIThe SynGAP1 missense variant E97G is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.609018 | Binding | 0.340 | 0.867 | 0.625 | -2.752 | Likely Benign | 0.282 | Likely Benign | Likely Benign | 0.079 | Likely Benign | 0.3286 | 0.6569 | -1.03 | Neutral | 0.947 | Possibly Damaging | 0.727 | Possibly Damaging | 4.07 | Benign | 0.00 | Affected | 0 | -2 | 3.1 | -72.06 | |||||||||||||||||||||||||||||||||||
| c.290A>T | E97V 2D  AIThe SynGAP1 missense variant E97V is listed in gnomAD (ID 6‑33425898‑A‑T) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and Foldetta (FoldX‑MD/Rosetta stability assessment) has no available result. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta data is missing. Overall, the majority of evidence points to a benign effect. This conclusion is consistent with the absence of a ClinVar pathogenic classification, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.609018 | Binding | 0.340 | 0.867 | 0.625 | 6-33425898-A-T | 1 | 6.20e-7 | -3.743 | Likely Benign | 0.514 | Ambiguous | Likely Benign | 0.124 | Likely Benign | 0.1015 | 0.8155 | -1.17 | Neutral | 0.947 | Possibly Damaging | 0.788 | Possibly Damaging | 4.07 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -2 | 7.7 | -29.98 | ||||||||||||||||||||||||||||||
| c.2911C>A | P971T 2D  AIThe SynGAP1 missense variant P971T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.951523 | Binding | 0.545 | 0.905 | 0.625 | -5.627 | Likely Benign | 0.053 | Likely Benign | Likely Benign | 0.053 | Likely Benign | 0.1388 | 0.5888 | -0.95 | Neutral | 0.001 | Benign | 0.003 | Benign | 3.96 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.2911C>G | P971A 2D  AIThe SynGAP1 missense variant P971A is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.951523 | Binding | 0.545 | 0.905 | 0.625 | -4.244 | Likely Benign | 0.049 | Likely Benign | Likely Benign | 0.060 | Likely Benign | 0.3100 | 0.5282 | -0.51 | Neutral | 0.000 | Benign | 0.002 | Benign | 4.05 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.2911C>T | P971S 2D  AIThe SynGAP1 missense variant P971S is catalogued in gnomAD (ID 6‑33443463‑C‑T) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) all indicate a benign or likely benign outcome. Only SIFT classifies the change as pathogenic, representing a minority opinion. High‑accuracy assessments further support benignity: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise reports likely benign. No Foldetta stability analysis is available, so it does not influence the overall assessment. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion is not contradicted by any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.951523 | Binding | 0.545 | 0.905 | 0.625 | 6-33443463-C-T | 1 | 6.20e-7 | -4.188 | Likely Benign | 0.061 | Likely Benign | Likely Benign | 0.058 | Likely Benign | 0.3009 | 0.5667 | -0.51 | Neutral | 0.002 | Benign | 0.003 | Benign | 3.99 | Benign | 0.00 | Affected | 4.32 | 2 | -1 | 1 | 0.8 | -10.04 | ||||||||||||||||||||||||||||||
| c.2912C>A | P971H 2D  AIThe SynGAP1 missense variant P971H is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443464‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; a Foldetta stability prediction is not available. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.951523 | Binding | 0.545 | 0.905 | 0.625 | Uncertain | 1 | 6-33443464-C-A | 1 | 6.20e-7 | -5.243 | Likely Benign | 0.086 | Likely Benign | Likely Benign | 0.039 | Likely Benign | 0.1584 | 0.4858 | -1.11 | Neutral | 0.898 | Possibly Damaging | 0.477 | Possibly Damaging | 3.89 | Benign | 0.00 | Affected | 4.32 | 2 | -2 | 0 | -1.6 | 40.02 | ||||||||||||||||||||||||||||
| c.2912C>G | P971R 2D  AIThe SynGAP1 missense variant P971R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.951523 | Binding | 0.545 | 0.905 | 0.625 | -4.407 | Likely Benign | 0.149 | Likely Benign | Likely Benign | 0.042 | Likely Benign | 0.1306 | 0.3818 | -1.01 | Neutral | 0.453 | Possibly Damaging | 0.078 | Benign | 3.91 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.2912C>T | P971L 2D  AIThe SynGAP1 missense variant P971L is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.950334 | Disordered | 0.951523 | Binding | 0.545 | 0.905 | 0.625 | -4.892 | Likely Benign | 0.070 | Likely Benign | Likely Benign | 0.030 | Likely Benign | 0.2046 | 0.5985 | -1.57 | Neutral | 0.144 | Benign | 0.026 | Benign | 3.93 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||
| c.2917G>T | G973W 2D  AIThe SynGAP1 missense variant G973W is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized reports Benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for G973W, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.887230 | Disordered | 0.959498 | Binding | 0.390 | 0.897 | 0.625 | -6.896 | Likely Benign | 0.329 | Likely Benign | Likely Benign | 0.105 | Likely Benign | 0.0846 | 0.4046 | -1.53 | Neutral | 0.983 | Probably Damaging | 0.813 | Possibly Damaging | 4.10 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | |||||||||||||||||||||||||||||||||||
| c.291G>C | E97D 2D  AIThe SynGAP1 missense variant E97D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available, so it does not influence the overall assessment. Overall, the majority of evidence points to the variant being most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.609018 | Binding | 0.340 | 0.867 | 0.625 | -3.239 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.081 | Likely Benign | 0.1987 | 0.5559 | -0.49 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 4.12 | Benign | 0.00 | Affected | 4.32 | 1 | 3 | 2 | 0.0 | -14.03 | |||||||||||||||||||||||||||||||||
| c.291G>T | E97D 2D  AIThe SynGAP1 missense variant E97D is listed in ClinVar (ID 1313570.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33425899‑G‑T). Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect. This consensus does not contradict the ClinVar “Uncertain” status, which remains unresolved. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.724957 | Disordered | 0.609018 | Binding | 0.340 | 0.867 | 0.625 | Uncertain | 3 | 6-33425899-G-T | -3.239 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.081 | Likely Benign | 0.1987 | 0.5559 | -0.49 | Neutral | 0.880 | Possibly Damaging | 0.636 | Possibly Damaging | 4.12 | Benign | 0.00 | Affected | 4.32 | 1 | 3 | 2 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||
| c.292C>A | H98N 2D  AIThe SynGAP1 missense variant H98N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.733139 | Disordered | 0.631713 | Binding | 0.348 | 0.872 | 0.625 | -3.855 | Likely Benign | 0.070 | Likely Benign | Likely Benign | 0.103 | Likely Benign | 0.2003 | 0.3759 | -0.35 | Neutral | 0.115 | Benign | 0.012 | Benign | 4.24 | Benign | 0.00 | Affected | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||
| c.292C>G | H98D 2D  AIThe SynGAP1 missense variant H98D is reported in gnomAD (variant ID 6‑33425900‑C‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) all classify the change as benign. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is “Likely Benign.” No Foldetta stability result is available, so it does not influence the assessment. Overall, the consensus of the available predictions indicates that the variant is most likely benign, and this conclusion is not contradicted by any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.733139 | Disordered | 0.631713 | Binding | 0.348 | 0.872 | 0.625 | 6-33425900-C-G | 1 | 6.20e-7 | -1.739 | Likely Benign | 0.167 | Likely Benign | Likely Benign | 0.140 | Likely Benign | 0.2732 | 0.3018 | -0.42 | Neutral | 0.115 | Benign | 0.012 | Benign | 4.24 | Benign | 0.00 | Affected | 4.32 | 1 | -1 | 1 | -0.3 | -22.05 | ||||||||||||||||||||||||||||||
| c.292C>T | H98Y 2D  AIThe SynGAP1 missense variant H98Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.733139 | Disordered | 0.631713 | Binding | 0.348 | 0.872 | 0.625 | -4.050 | Likely Benign | 0.135 | Likely Benign | Likely Benign | 0.112 | Likely Benign | 0.0950 | 0.5024 | -0.86 | Neutral | 0.444 | Benign | 0.024 | Benign | 4.19 | Benign | 0.00 | Affected | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||
| c.2936T>G | F979C 2D  AIThe SynGAP1 missense variant F979C is not reported in ClinVar and has no gnomAD entry. Consensus from high‑accuracy predictors is benign: AlphaMissense‑Optimized scores it benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates likely benign. Other tools that agree with benign include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Pathogenic predictions come from polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, did not return a result for this variant, so its stability impact is unavailable. Overall, the majority of evidence points to a benign effect, and this is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.816150 | Disordered | 0.977500 | Binding | 0.274 | 0.889 | 0.625 | -6.395 | Likely Benign | 0.589 | Likely Pathogenic | Likely Benign | 0.160 | Likely Benign | 0.2646 | 0.2179 | -0.94 | Neutral | 0.994 | Probably Damaging | 0.888 | Possibly Damaging | 4.15 | Benign | 0.00 | Affected | -4 | -2 | -0.3 | -44.04 | |||||||||||||||||||||||||||||||||||
| c.2938C>A | H980N 2D  AIThe SynGAP1 missense variant H980N is not reported in ClinVar or gnomAD. Functional prediction tools largely agree on a benign effect. Benign calls come from REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign classification. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus confirms a benign result; Foldetta data are unavailable, so no additional stability evidence is considered. Overall, the computational evidence indicates that H980N is most likely benign, and this conclusion does not conflict with the absence of a ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.974598 | Binding | 0.309 | 0.892 | 0.625 | -4.728 | Likely Benign | 0.291 | Likely Benign | Likely Benign | 0.070 | Likely Benign | 0.2346 | 0.3638 | -1.07 | Neutral | 0.451 | Benign | 0.209 | Benign | 4.17 | Benign | 0.00 | Affected | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||
| c.2938C>G | H980D 2D  AIThe SynGAP1 missense variant H980D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. The Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of computational evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.974598 | Binding | 0.309 | 0.892 | 0.625 | -5.489 | Likely Benign | 0.729 | Likely Pathogenic | Likely Benign | 0.117 | Likely Benign | 0.2608 | 0.3066 | -1.38 | Neutral | 0.451 | Benign | 0.265 | Benign | 4.18 | Benign | 0.00 | Affected | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||
| c.2938C>T | H980Y 2D  AIThe SynGAP1 missense variant H980Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for H980Y, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.974598 | Binding | 0.309 | 0.892 | 0.625 | -4.104 | Likely Benign | 0.299 | Likely Benign | Likely Benign | 0.083 | Likely Benign | 0.1524 | 0.4706 | -1.12 | Neutral | 0.925 | Possibly Damaging | 0.529 | Possibly Damaging | 4.13 | Benign | 0.00 | Affected | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||
| c.2939A>C | H980P 2D  AIThe SynGAP1 missense variant H980P is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools largely favor a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. In contrast, two tools—polyPhen‑2 HumDiv and SIFT—classify the change as pathogenic. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign and the SGM‑Consensus (majority vote) is likely benign; no Foldetta stability data are available. Overall, the preponderance of evidence points to a benign effect for H980P, and this conclusion is not in conflict with the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.974598 | Binding | 0.309 | 0.892 | 0.625 | -3.071 | Likely Benign | 0.151 | Likely Benign | Likely Benign | 0.136 | Likely Benign | 0.2274 | 0.4301 | -1.70 | Neutral | 0.802 | Possibly Damaging | 0.432 | Benign | 4.15 | Benign | 0.00 | Affected | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||
| c.2939A>G | H980R 2D  AIThe SynGAP1 missense variant H980R is listed in gnomAD (ID 6‑33443491‑A‑G) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the absence of a ClinVar pathogenic classification. Thus, the variant is most likely benign and does not contradict ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.974598 | Binding | 0.309 | 0.892 | 0.625 | 6-33443491-A-G | 1 | 6.20e-7 | -2.736 | Likely Benign | 0.409 | Ambiguous | Likely Benign | 0.095 | Likely Benign | 0.2439 | 0.3810 | -1.44 | Neutral | 0.802 | Possibly Damaging | 0.354 | Benign | 4.17 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 2 | -1.3 | 19.05 | ||||||||||||||||||||||||||||||
| c.2939A>T | H980L 2D  AIThe SynGAP1 missense variant H980L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. The variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.974598 | Binding | 0.309 | 0.892 | 0.625 | -1.984 | Likely Benign | 0.293 | Likely Benign | Likely Benign | 0.187 | Likely Benign | 0.1488 | 0.5538 | -1.98 | Neutral | 0.625 | Possibly Damaging | 0.265 | Benign | 4.16 | Benign | 0.00 | Affected | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||
| c.293A>C | H98P 2D  AIThe SynGAP1 missense variant H98P is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar classification to contradict this conclusion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.733139 | Disordered | 0.631713 | Binding | 0.348 | 0.872 | 0.625 | -1.460 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.275 | Likely Benign | 0.2062 | 0.4669 | -0.83 | Neutral | 0.659 | Possibly Damaging | 0.024 | Benign | 4.20 | Benign | 0.00 | Affected | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||
| c.293A>G | H98R 2D  AIThe SynGAP1 missense variant H98R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.733139 | Disordered | 0.631713 | Binding | 0.348 | 0.872 | 0.625 | -2.772 | Likely Benign | 0.180 | Likely Benign | Likely Benign | 0.116 | Likely Benign | 0.2271 | 0.3022 | -0.39 | Neutral | 0.115 | Benign | 0.006 | Benign | 4.27 | Benign | 0.00 | Affected | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||
| c.293A>T | H98L 2D  AIThe SynGAP1 H98L missense variant is reported in gnomAD (ID 6‑33425901‑A‑T) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments further support this: AlphaMissense‑Optimized predicts benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for H98L, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.733139 | Disordered | 0.631713 | Binding | 0.348 | 0.872 | 0.625 | 6-33425901-A-T | 1 | 6.32e-7 | -1.804 | Likely Benign | 0.113 | Likely Benign | Likely Benign | 0.194 | Likely Benign | 0.1125 | 0.6291 | -0.51 | Neutral | 0.115 | Benign | 0.012 | Benign | 4.24 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -2 | 7.0 | -23.98 | ||||||||||||||||||||||||||||||
| c.2940T>A | H980Q 2D  AIThe SynGAP1 missense variant H980Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for H980Q, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.974598 | Binding | 0.309 | 0.892 | 0.625 | -4.014 | Likely Benign | 0.385 | Ambiguous | Likely Benign | 0.090 | Likely Benign | 0.2236 | 0.3936 | -1.35 | Neutral | 0.802 | Possibly Damaging | 0.432 | Benign | 4.18 | Benign | 0.00 | Affected | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||
| c.2940T>G | H980Q 2D  AIThe SynGAP1 missense variant H980Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for H980Q, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.974598 | Binding | 0.309 | 0.892 | 0.625 | -4.014 | Likely Benign | 0.385 | Ambiguous | Likely Benign | 0.090 | Likely Benign | 0.2236 | 0.3936 | -1.35 | Neutral | 0.802 | Possibly Damaging | 0.432 | Benign | 4.18 | Benign | 0.00 | Affected | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||
| c.2941G>A | G981S 2D  AIThe SynGAP1 missense variant G981S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; the Foldetta stability analysis is not available for this variant. Overall, the majority of evidence—including the consensus and high‑accuracy tools—points to a benign effect. This conclusion is not contradicted by ClinVar, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.798249 | Disordered | 0.970320 | Binding | 0.275 | 0.897 | 0.625 | -2.749 | Likely Benign | 0.274 | Likely Benign | Likely Benign | 0.130 | Likely Benign | 0.2566 | 0.5470 | -0.57 | Neutral | 0.979 | Probably Damaging | 0.907 | Possibly Damaging | 3.87 | Benign | 0.00 | Affected | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||||
| c.2941G>C | G981R 2D  AIThe SynGAP1 missense variant G981R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) is not available for this variant. Overall, the majority of evidence points toward a benign impact, and this conclusion does not contradict any ClinVar annotation because none exists. Thus, the variant is most likely benign based on current predictive tools. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.798249 | Disordered | 0.970320 | Binding | 0.275 | 0.897 | 0.625 | -1.264 | Likely Benign | 0.953 | Likely Pathogenic | Ambiguous | 0.207 | Likely Benign | 0.0990 | 0.4776 | -2.11 | Neutral | 0.999 | Probably Damaging | 0.985 | Probably Damaging | 3.74 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.2941G>T | G981C 2D  AIThe SynGAP1 missense variant G981C is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact for G981C, and this conclusion does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.798249 | Disordered | 0.970320 | Binding | 0.275 | 0.897 | 0.625 | -5.646 | Likely Benign | 0.593 | Likely Pathogenic | Likely Benign | 0.228 | Likely Benign | 0.1398 | 0.4593 | -1.81 | Neutral | 1.000 | Probably Damaging | 0.992 | Probably Damaging | 3.72 | Benign | 0.00 | Affected | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||
| c.2942G>A | G981D 2D  AIThe SynGAP1 missense variant G981D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Taken together, the balance of evidence leans toward a benign interpretation; there is no ClinVar entry to contradict this assessment. Thus, the variant is most likely benign based on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.798249 | Disordered | 0.970320 | Binding | 0.275 | 0.897 | 0.625 | -5.280 | Likely Benign | 0.945 | Likely Pathogenic | Ambiguous | 0.159 | Likely Benign | 0.1961 | 0.2868 | -1.64 | Neutral | 1.000 | Probably Damaging | 0.985 | Probably Damaging | 3.75 | Benign | 0.00 | Affected | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||
| c.2942G>C | G981A 2D  AIThe SynGAP1 missense variant G981A is not reported in ClinVar and has no entry in gnomAD, indicating it is not catalogued in these databases. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.798249 | Disordered | 0.970320 | Binding | 0.275 | 0.897 | 0.625 | -3.374 | Likely Benign | 0.368 | Ambiguous | Likely Benign | 0.064 | Likely Benign | 0.3512 | 0.4925 | -0.95 | Neutral | 0.561 | Possibly Damaging | 0.376 | Benign | 3.95 | Benign | 0.00 | Affected | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2942G>T | G981V 2D  AIThe SynGAP1 missense variant G981V is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign impact for G981V, and this conclusion does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.798249 | Disordered | 0.970320 | Binding | 0.275 | 0.897 | 0.625 | -3.873 | Likely Benign | 0.714 | Likely Pathogenic | Likely Benign | 0.156 | Likely Benign | 0.1322 | 0.3861 | -2.10 | Neutral | 0.997 | Probably Damaging | 0.958 | Probably Damaging | 3.75 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.2944T>A | Y982N 2D  AIThe SynGAP1 Y982N variant has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic impact are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments are mixed: AlphaMissense‑Optimized is uncertain, SGM‑Consensus is benign, and Foldetta results are unavailable. Overall, the majority of consensus‑based and high‑accuracy tools lean toward a benign interpretation. Thus, the variant is most likely benign, and this assessment does not contradict ClinVar status, which currently has no entry for Y982N. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.707965 | Disordered | 0.966717 | Binding | 0.272 | 0.895 | 0.625 | -4.536 | Likely Benign | 0.881 | Likely Pathogenic | Ambiguous | 0.175 | Likely Benign | 0.2090 | 0.0545 | -1.14 | Neutral | 0.990 | Probably Damaging | 0.900 | Possibly Damaging | 3.88 | Benign | 0.00 | Affected | -2 | -2 | -2.2 | -49.07 | |||||||||||||||||||||||||||||||||||
| c.2944T>C | Y982H 2D  AIThe SynGAP1 missense variant Y982H has no ClinVar record and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, and the SGM‑Consensus result is benign; Foldetta predictions are unavailable. Overall, the evidence is mixed, but the majority of consensus‑based and high‑accuracy tools lean toward a benign interpretation. Thus, the variant is most likely benign, and this assessment does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.707965 | Disordered | 0.966717 | Binding | 0.272 | 0.895 | 0.625 | -2.675 | Likely Benign | 0.893 | Likely Pathogenic | Ambiguous | 0.093 | Likely Benign | 0.2352 | 0.0545 | -0.63 | Neutral | 0.990 | Probably Damaging | 0.900 | Possibly Damaging | 3.92 | Benign | 0.00 | Affected | 0 | 2 | -1.9 | -26.03 | |||||||||||||||||||||||||||||||||||
| c.2944T>G | Y982D 2D  AIThe SynGAP1 missense variant Y982D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which collectively suggest a likely benign outcome. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and Foldetta (combining FoldX‑MD and Rosetta) has no available result for this variant. Overall, the balance of evidence leans toward a benign interpretation, and this assessment does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.707965 | Disordered | 0.966717 | Binding | 0.272 | 0.895 | 0.625 | -5.021 | Likely Benign | 0.952 | Likely Pathogenic | Ambiguous | 0.194 | Likely Benign | 0.3959 | 0.0545 | -1.29 | Neutral | 0.990 | Probably Damaging | 0.856 | Possibly Damaging | 3.87 | Benign | 0.00 | Affected | -4 | -3 | -2.2 | -48.09 | |||||||||||||||||||||||||||||||||||
| c.2945A>C | Y982S 2D  AIThe SynGAP1 missense variant Y982S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which collectively suggest a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default predict a pathogenic impact. High‑accuracy assessments show the SGM‑Consensus as Likely Benign, AlphaMissense‑Optimized as Uncertain, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.707965 | Disordered | 0.966717 | Binding | 0.272 | 0.895 | 0.625 | -2.919 | Likely Benign | 0.841 | Likely Pathogenic | Ambiguous | 0.131 | Likely Benign | 0.4556 | 0.1883 | -1.04 | Neutral | 0.965 | Probably Damaging | 0.783 | Possibly Damaging | 3.89 | Benign | 0.00 | Affected | -3 | -2 | 0.5 | -76.10 | |||||||||||||||||||||||||||||||||||
| c.2945A>G | Y982C 2D  AIThe SynGAP1 missense variant Y982C is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443497‑A‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.707965 | Disordered | 0.966717 | Binding | 0.272 | 0.895 | 0.625 | Conflicting | 2 | 6-33443497-A-G | 2 | 1.24e-6 | -6.256 | Likely Benign | 0.746 | Likely Pathogenic | Likely Benign | 0.195 | Likely Benign | 0.2988 | 0.2345 | -1.67 | Neutral | 0.997 | Probably Damaging | 0.923 | Probably Damaging | 3.87 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -2 | 3.8 | -60.04 | ||||||||||||||||||||||||||||
| c.2945A>T | Y982F 2D  AIThe SynGAP1 missense variant Y982F is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.707965 | Disordered | 0.966717 | Binding | 0.272 | 0.895 | 0.625 | -3.431 | Likely Benign | 0.208 | Likely Benign | Likely Benign | 0.154 | Likely Benign | 0.2351 | 0.2980 | -0.36 | Neutral | 0.006 | Benign | 0.009 | Benign | 4.63 | Benign | 0.00 | Affected | 7 | 3 | 4.1 | -16.00 | |||||||||||||||||||||||||||||||||||
| c.2947A>C | S983R 2D  AIThe SynGAP1 missense variant S983R is reported in ClinVar as “Not submitted” and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The high‑accuracy consensus, SGM‑Consensus, is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN and also indicates a likely pathogenic outcome. AlphaMissense‑Optimized independently predicts pathogenicity. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from multiple in silico predictors points to a pathogenic effect for S983R, and this conclusion does not contradict any ClinVar annotation, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.707965 | Disordered | 0.960212 | Binding | 0.277 | 0.889 | 0.625 | -4.733 | Likely Benign | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.156 | Likely Benign | 0.1163 | 0.3740 | -2.66 | Deleterious | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.03 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2947A>G | S983G 2D  AIThe SynGAP1 missense variant S983G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 vs 2). High‑accuracy methods show AlphaMissense‑Optimized as benign; the SGM Consensus is unavailable, and Foldetta results are not provided, so no folding‑stability evidence is available. Overall, the balance of evidence (five pathogenic vs four benign predictions) suggests the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because the variant has not yet been classified in that database. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.707965 | Disordered | 0.960212 | Binding | 0.277 | 0.889 | 0.625 | -5.206 | Likely Benign | 0.638 | Likely Pathogenic | Likely Benign | 0.109 | Likely Benign | 0.2521 | 0.4480 | -2.22 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.17 | Pathogenic | 0.00 | Affected | 1 | 0 | 0.4 | -30.03 | ||||||||||||||||||||||||||||||||||||
| c.2947A>T | S983C 2D  AIThe SynGAP1 missense variant S983C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Functional prediction tools cluster into two groups: benign predictions come from REVEL and AlphaMissense‑Optimized, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, whereas the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely pathogenic effect. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points toward a pathogenic impact. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.707965 | Disordered | 0.960212 | Binding | 0.277 | 0.889 | 0.625 | -7.083 | In-Between | 0.741 | Likely Pathogenic | Likely Benign | 0.162 | Likely Benign | 0.1657 | 0.5298 | -2.64 | Deleterious | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.02 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2948G>A | S983N 2D  AISynGAP1 missense variant S983N is listed as Benign in ClinVar (ID 469153) and is present in gnomAD (6‑33443500‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive; Foldetta results are not available. Overall, the majority of available predictions (five pathogenic vs. three benign) suggest a pathogenic impact, which contradicts the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.707965 | Disordered | 0.960212 | Binding | 0.277 | 0.889 | 0.625 | Likely Benign | 1 | 6-33443500-G-A | 6 | 3.72e-6 | -5.604 | Likely Benign | 0.909 | Likely Pathogenic | Ambiguous | 0.136 | Likely Benign | 0.1933 | 0.4069 | -1.78 | Neutral | 0.991 | Probably Damaging | 0.988 | Probably Damaging | 2.04 | Pathogenic | 0.00 | Affected | 4.32 | 1 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||
| c.2948G>C | S983T 2D  AIThe SynGAP1 missense variant S983T is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (2 pathogenic vs. 2 benign). High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus and Foldetta (which would combine FoldX‑MD and Rosetta outputs) are unavailable. Overall, the balance of evidence leans toward pathogenicity, with five tools supporting a deleterious effect versus four supporting benign. This prediction does not contradict any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.707965 | Disordered | 0.960212 | Binding | 0.277 | 0.889 | 0.625 | -4.493 | Likely Benign | 0.588 | Likely Pathogenic | Likely Benign | 0.172 | Likely Benign | 0.2042 | 0.5517 | -1.65 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.08 | Pathogenic | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||||||||
| c.2948G>T | S983I 2D  AIThe SynGAP1 missense variant S983I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). In silico predictors that agree on a benign effect are REVEL and ESM1b, whereas the majority of tools predict a pathogenic outcome: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus (3 pathogenic vs. 1 benign) is likely pathogenic. Foldetta results are unavailable. Overall, the preponderance of evidence indicates that S983I is most likely pathogenic, and this conclusion is not contradicted by the current ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.707965 | Disordered | 0.960212 | Binding | 0.277 | 0.889 | 0.625 | -6.259 | Likely Benign | 0.968 | Likely Pathogenic | Likely Pathogenic | 0.190 | Likely Benign | 0.1380 | 0.4625 | -2.67 | Deleterious | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 2.02 | Pathogenic | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||
| c.2949C>A | S983R 2D  AIThe SynGAP1 missense variant S983R is reported in ClinVar as “Not submitted” and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus (3 pathogenic vs. 1 benign) is likely pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors indicates that S983R is most likely pathogenic, and this conclusion does not contradict the ClinVar status, which currently lacks a classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.707965 | Disordered | 0.960212 | Binding | 0.277 | 0.889 | 0.625 | -4.733 | Likely Benign | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.190 | Likely Benign | 0.1163 | 0.3740 | -2.66 | Deleterious | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.03 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2949C>G | S983R 2D  AIThe SynGAP1 missense variant S983R is reported in ClinVar as “Not submitted” and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM‑Consensus (3 pathogenic vs. 1 benign) is likely pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that S983R is most likely pathogenic, and this conclusion does not contradict the ClinVar status, which currently lacks a pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.707965 | Disordered | 0.960212 | Binding | 0.277 | 0.889 | 0.625 | -4.733 | Likely Benign | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.190 | Likely Benign | 0.1163 | 0.3740 | -2.66 | Deleterious | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.03 | Pathogenic | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.294T>A | H98Q 2D  AIThe SynGAP1 missense variant H98Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.733139 | Disordered | 0.631713 | Binding | 0.348 | 0.872 | 0.625 | -2.749 | Likely Benign | 0.104 | Likely Benign | Likely Benign | 0.088 | Likely Benign | 0.1831 | 0.4347 | -0.47 | Neutral | 0.002 | Benign | 0.000 | Benign | 4.26 | Benign | 0.00 | Affected | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||
| c.294T>G | H98Q 2D  AIThe SynGAP1 missense variant H98Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.733139 | Disordered | 0.631713 | Binding | 0.348 | 0.872 | 0.625 | -2.749 | Likely Benign | 0.104 | Likely Benign | Likely Benign | 0.088 | Likely Benign | 0.1831 | 0.4347 | -0.47 | Neutral | 0.002 | Benign | 0.000 | Benign | 4.26 | Benign | 0.00 | Affected | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||||
| c.2950A>C | K984Q 2D  AIThe SynGAP1 missense variant K984Q is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.951648 | Binding | 0.288 | 0.895 | 0.750 | -2.932 | Likely Benign | 0.612 | Likely Pathogenic | Likely Benign | 0.083 | Likely Benign | 0.5054 | 0.1240 | Weaken | -0.67 | Neutral | 0.905 | Possibly Damaging | 0.637 | Possibly Damaging | 2.66 | Benign | 0.00 | Affected | 1 | 1 | 0.4 | -0.04 | ||||||||||||||||||||||||||||||||||
| c.2950A>G | K984E 2D  AIThe SynGAP1 missense variant K984E has no ClinVar record and is not reported in gnomAD. Prediction tools that classify the variant as benign include REVEL, PROVEAN, ESM1b, and FATHMM, while polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default predict it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” AlphaMissense‑Optimized is uncertain, and no Foldetta stability assessment is available. Overall, the majority of individual predictors lean toward benign, and the consensus score explicitly labels it benign, whereas a comparable number of tools predict pathogenicity. Based on the available evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.951648 | Binding | 0.288 | 0.895 | 0.750 | -4.909 | Likely Benign | 0.932 | Likely Pathogenic | Ambiguous | 0.086 | Likely Benign | 0.4353 | 0.1200 | -0.88 | Neutral | 0.798 | Possibly Damaging | 0.535 | Possibly Damaging | 2.71 | Benign | 0.00 | Affected | 0 | 1 | 0.4 | 0.94 | |||||||||||||||||||||||||||||||||||
| c.2951A>C | K984T 2D  AIThe SynGAP1 missense variant K984T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the predictions are mixed; however, the SGM‑Consensus and the majority of benign‑predicting tools lean toward a benign interpretation. This assessment does not contradict ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.951648 | Binding | 0.288 | 0.895 | 0.750 | -3.429 | Likely Benign | 0.854 | Likely Pathogenic | Ambiguous | 0.087 | Likely Benign | 0.2441 | 0.3463 | -1.10 | Neutral | 0.951 | Possibly Damaging | 0.708 | Possibly Damaging | 2.71 | Benign | 0.00 | Affected | 0 | -1 | 3.2 | -27.07 | |||||||||||||||||||||||||||||||||||
| c.2951A>G | K984R 2D  AIThe SynGAP1 missense variant K984R is reported in gnomAD (ID 6‑33443503‑A‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the consensus of the majority of tools indicates that the variant is most likely benign, and this conclusion is not contradicted by any ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.951648 | Binding | 0.288 | 0.895 | 0.750 | 6-33443503-A-G | 2 | 1.24e-6 | -2.044 | Likely Benign | 0.104 | Likely Benign | Likely Benign | 0.082 | Likely Benign | 0.5098 | 0.1558 | Weaken | -0.41 | Neutral | 0.012 | Benign | 0.012 | Benign | 2.67 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 3 | -0.6 | 28.01 | |||||||||||||||||||||||||||||
| c.2951A>T | K984M 2D  AIThe SynGAP1 missense variant K984M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain” and the SGM‑Consensus as “Likely Benign”; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar status, which currently contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.951648 | Binding | 0.288 | 0.895 | 0.750 | -4.761 | Likely Benign | 0.934 | Likely Pathogenic | Ambiguous | 0.141 | Likely Benign | 0.1576 | 0.4168 | -1.82 | Neutral | 0.995 | Probably Damaging | 0.944 | Probably Damaging | 2.60 | Benign | 0.00 | Affected | 0 | -1 | 5.8 | 3.02 | |||||||||||||||||||||||||||||||||||
| c.2952G>C | K984N 2D  AIThe SynGAP1 missense variant K984N is catalogued in gnomAD (6‑33443504‑G‑C) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, and FATHMM, while pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. High‑accuracy assessments are mixed: AlphaMissense‑Optimized rates the variant as uncertain, SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) labels it “Likely Benign,” and Foldetta (which integrates FoldX‑MD and Rosetta outputs) has no available result for this residue. Overall, the balance of evidence leans toward a benign effect, with no pathogenic ClinVar classification to contradict this inference. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.951648 | Binding | 0.288 | 0.895 | 0.750 | 6-33443504-G-C | 1 | 6.20e-7 | -3.020 | Likely Benign | 0.955 | Likely Pathogenic | Ambiguous | 0.091 | Likely Benign | 0.4138 | 0.1677 | 0.52 | Neutral | 0.951 | Possibly Damaging | 0.637 | Possibly Damaging | 2.89 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 1 | 0.4 | -14.07 | ||||||||||||||||||||||||||||||
| c.2952G>T | K984N 2D  AISynGAP1 missense variant K984N has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict pathogenicity are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments are mixed: AlphaMissense‑Optimized is Uncertain, SGM‑Consensus is Likely Benign, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a benign interpretation, and this assessment does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.703578 | Disordered | 0.951648 | Binding | 0.288 | 0.895 | 0.750 | -3.020 | Likely Benign | 0.955 | Likely Pathogenic | Ambiguous | 0.091 | Likely Benign | 0.4138 | 0.1677 | 0.52 | Neutral | 0.951 | Possibly Damaging | 0.637 | Possibly Damaging | 2.89 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 1 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||
| c.2953A>C | S985R 2D  AIThe SynGAP1 missense variant S985R has no ClinVar entry and is not reported in gnomAD. Prediction tools that classify it as benign include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Those that predict pathogenicity are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus remains benign; Foldetta, a protein‑folding stability method, has no available result for this variant. Consequently, the evidence is split evenly between benign and pathogenic predictions, with no contradiction to ClinVar status. The variant’s impact remains uncertain, and no definitive benign or pathogenic classification can be assigned based solely on current computational predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.941547 | Binding | 0.302 | 0.896 | 0.750 | -6.148 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.174 | Likely Benign | 0.1213 | 0.3905 | -2.40 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.52 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2953A>G | S985G 2D  AIThe SynGAP1 missense variant S985G is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in‑silico tools cluster into two groups: benign (REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized) and pathogenic (polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT). The AlphaMissense‑Default score is uncertain, and Foldetta stability analysis is not available. High‑accuracy assessments show AlphaMissense‑Optimized predicting a benign effect, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. Foldetta, which integrates FoldX‑MD and Rosetta outputs, has no reported result for this variant. Overall, the majority of reliable predictors and the SGM‑Consensus support a benign classification. The variant is most likely benign, and this assessment does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.941547 | Binding | 0.302 | 0.896 | 0.750 | -6.151 | Likely Benign | 0.546 | Ambiguous | Likely Benign | 0.132 | Likely Benign | 0.2684 | 0.4540 | -2.29 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.51 | Benign | 0.00 | Affected | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||||||
| c.2953A>T | S985C 2D  AIThe SynGAP1 missense variant S985C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and PROVEAN, whereas the majority of tools predict a pathogenic impact: polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default all classify the variant as pathogenic. High‑accuracy assessments further support a deleterious outcome: AlphaMissense‑Optimized is uncertain, but the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is classified as Likely Pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from multiple in silico predictors indicates that S985C is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.720929 | Disordered | 0.941547 | Binding | 0.302 | 0.896 | 0.750 | -8.918 | Likely Pathogenic | 0.860 | Likely Pathogenic | Ambiguous | 0.147 | Likely Benign | 0.1531 | 0.5395 | -2.49 | Neutral | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2954G>A | S985N 2D  AIThe SynGAP1 missense variant S985N is listed in ClinVar (ID 2087879.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. Separately, the high‑accuracy AlphaMissense‑Optimized result is “Uncertain,” and the Foldetta protein‑folding stability assessment is unavailable. Based on the overall distribution of predictions, the variant is most likely benign; this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.941547 | Binding | 0.302 | 0.896 | 0.750 | Uncertain | 1 | -6.979 | Likely Benign | 0.845 | Likely Pathogenic | Ambiguous | 0.088 | Likely Benign | 0.1812 | 0.4822 | -1.68 | Neutral | 0.991 | Probably Damaging | 0.988 | Probably Damaging | 2.65 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||
| c.2954G>C | S985T 2D  AIThe SynGAP1 missense variant S985T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for S985T, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.941547 | Binding | 0.302 | 0.896 | 0.750 | -5.658 | Likely Benign | 0.475 | Ambiguous | Likely Benign | 0.153 | Likely Benign | 0.1889 | 0.5844 | -1.62 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 2.54 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2954G>T | S985I 2D  AIThe SynGAP1 missense variant S985I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect are REVEL and FATHMM, whereas the remaining tools—PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently predict a pathogenic impact. ESM1b remains uncertain. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a pathogenic verdict (2 pathogenic vs. 1 benign). Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the preponderance of evidence indicates that S985I is most likely pathogenic, and this conclusion does not contradict any ClinVar annotation because no ClinVar status exists for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.720929 | Disordered | 0.941547 | Binding | 0.302 | 0.896 | 0.750 | -7.858 | In-Between | 0.971 | Likely Pathogenic | Likely Pathogenic | 0.131 | Likely Benign | 0.1367 | 0.5206 | -2.78 | Deleterious | 0.997 | Probably Damaging | 0.996 | Probably Damaging | 2.50 | Benign | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | ||||||||||||||||||||||||||||||||||||
| c.2955T>A | S985R 2D  AIThe SynGAP1 missense variant S985R has no ClinVar entry and is not reported in gnomAD. Consensus predictions from multiple in‑silico tools are split: benign calls come from REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) which reports a likely benign outcome. Pathogenic calls arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments are mixed: AlphaMissense‑Optimized predicts pathogenic, whereas the SGM‑Consensus (a majority‑vote aggregator) predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the predictions are evenly divided, leaving the variant’s clinical significance uncertain; it does not contradict any ClinVar status because none is assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.941547 | Binding | 0.302 | 0.896 | 0.750 | -6.148 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.203 | Likely Benign | 0.1213 | 0.3905 | -2.40 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.52 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2955T>G | S985R 2D  AISynGAP1 missense variant S985R has no ClinVar record and is not present in gnomAD. Computational predictions are mixed: benign calls come from REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Pathogenic calls come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy tools give a split result: AlphaMissense‑Optimized predicts pathogenic, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) predicts likely benign; Foldetta data are unavailable. Consequently, the variant’s predicted impact is ambiguous, with an equal number of benign and pathogenic scores. No ClinVar evidence contradicts these predictions. Thus, the variant is best classified as of uncertain significance, with no clear bias toward benign or pathogenic status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.720929 | Disordered | 0.941547 | Binding | 0.302 | 0.896 | 0.750 | -6.148 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.203 | Likely Benign | 0.1213 | 0.3905 | -2.40 | Neutral | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.52 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.2956G>A | E986K 2D  AIThe SynGAP1 missense variant E986K is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect are REVEL and PROVEAN, while those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The remaining tools, ESM1b and AlphaMissense‑Optimized, return uncertain results and are not considered evidence for either side. The SGM Consensus, which takes a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a pathogenic verdict (2 pathogenic vs. 1 benign, with one uncertain). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of high‑accuracy predictors classify the variant as pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.750527 | Disordered | 0.929726 | Binding | 0.349 | 0.902 | 0.750 | -7.174 | In-Between | 0.950 | Likely Pathogenic | Ambiguous | 0.164 | Likely Benign | 0.2760 | 0.7973 | -2.19 | Neutral | 0.924 | Possibly Damaging | 0.722 | Possibly Damaging | 2.15 | Pathogenic | 0.00 | Affected | 0 | 1 | -0.4 | -0.94 | ||||||||||||||||||||||||||||||||||||
| c.2956G>C | E986Q 2D  AIThe SynGAP1 missense variant E986Q is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (five pathogenic vs. three benign) lean toward a pathogenic interpretation. This assessment does not contradict ClinVar status, as the variant is not yet catalogued there. Thus, based on current computational evidence, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.750527 | Disordered | 0.929726 | Binding | 0.349 | 0.902 | 0.750 | -4.471 | Likely Benign | 0.839 | Likely Pathogenic | Ambiguous | 0.160 | Likely Benign | 0.1700 | 0.7589 | -1.66 | Neutral | 0.974 | Probably Damaging | 0.842 | Possibly Damaging | 2.14 | Pathogenic | 0.00 | Affected | 2 | 2 | 0.0 | -0.98 | ||||||||||||||||||||||||||||||||||||
| c.2957A>C | E986A 2D  AIThe SynGAP1 missense variant E986A is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, and ESM1b, whereas tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta stability analysis is unavailable. Overall, the evidence is evenly divided, with no clear majority. Based on the current predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.750527 | Disordered | 0.929726 | Binding | 0.349 | 0.902 | 0.750 | -4.653 | Likely Benign | 0.895 | Likely Pathogenic | Ambiguous | 0.160 | Likely Benign | 0.4207 | 0.7733 | -2.32 | Neutral | 0.552 | Possibly Damaging | 0.388 | Benign | 2.14 | Pathogenic | 0.00 | Affected | 0 | -1 | 5.3 | -58.04 | ||||||||||||||||||||||||||||||||||||
| c.2957A>G | E986G 2D  AIThe SynGAP1 missense variant E986G is not reported in ClinVar and has no entries in gnomAD. Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas seven tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus) predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, while the SGM‑Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—classifies the variant as likely pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a pathogenic effect, and this assessment does not conflict with the ClinVar status, which currently contains no classification for E986G. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.750527 | Disordered | 0.929726 | Binding | 0.349 | 0.902 | 0.750 | -5.584 | Likely Benign | 0.834 | Likely Pathogenic | Ambiguous | 0.219 | Likely Benign | 0.2866 | 0.6225 | -3.14 | Deleterious | 0.924 | Possibly Damaging | 0.784 | Possibly Damaging | 2.13 | Pathogenic | 0.00 | Affected | 0 | -2 | 3.1 | -72.06 | |||||||||||||||||||||||||||||||||||
| c.2957A>T | E986V 2D  AIThe SynGAP1 E986V missense variant is not reported in ClinVar and has no gnomAD entry. Functional prediction tools cluster into two groups: benign predictions come from REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and ESM1b, whereas pathogenic predictions arise from PROVEAN, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments reinforce the pathogenic signal: AlphaMissense‑Optimized predicts pathogenic, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default (pathogenic), ESM1b (benign), FATHMM (pathogenic), and PROVEAN (pathogenic)—also indicates pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods points to a pathogenic effect for E986V, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.750527 | Disordered | 0.929726 | Binding | 0.349 | 0.902 | 0.750 | -4.511 | Likely Benign | 0.965 | Likely Pathogenic | Likely Pathogenic | 0.220 | Likely Benign | 0.1146 | 0.7960 | -3.48 | Deleterious | 0.018 | Benign | 0.028 | Benign | 2.10 | Pathogenic | 0.00 | Affected | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||||||||
| c.2958G>C | E986D 2D  AIThe SynGAP1 missense variant E986D is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (two pathogenic vs two benign votes) and Foldetta results are unavailable. Overall, the majority of conventional tools lean toward pathogenicity, but the single high‑accuracy benign prediction and the inconclusive consensus leave the variant’s impact uncertain. There is no contradiction with ClinVar status, as the variant has not been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.750527 | Disordered | 0.929726 | Binding | 0.349 | 0.902 | 0.750 | -4.001 | Likely Benign | 0.628 | Likely Pathogenic | Likely Benign | 0.129 | Likely Benign | 0.2081 | 0.5364 | -1.22 | Neutral | 0.974 | Probably Damaging | 0.715 | Possibly Damaging | 2.32 | Pathogenic | 0.00 | Affected | 3 | 2 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||||||||
| c.2958G>T | E986D 2D  AIThe SynGAP1 missense variant E986D is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (two pathogenic vs two benign votes) and Foldetta results are unavailable. Overall, the majority of conventional tools lean toward pathogenicity, but the single high‑accuracy benign prediction and the inconclusive consensus leave the variant’s impact uncertain. There is no contradiction with ClinVar status, as the variant has not been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.750527 | Disordered | 0.929726 | Binding | 0.349 | 0.902 | 0.750 | -4.001 | Likely Benign | 0.628 | Likely Pathogenic | Likely Benign | 0.129 | Likely Benign | 0.2081 | 0.5364 | -1.22 | Neutral | 0.974 | Probably Damaging | 0.715 | Possibly Damaging | 2.32 | Pathogenic | 0.00 | Affected | 3 | 2 | 0.0 | -14.03 | ||||||||||||||||||||||||||||||||||||
| c.295G>A | E99K 2D  AIThe SynGAP1 E99K missense variant is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) also as benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar reporting, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.645246 | Binding | 0.325 | 0.874 | 0.500 | -4.746 | Likely Benign | 0.678 | Likely Pathogenic | Likely Benign | 0.071 | Likely Benign | 0.2752 | 0.8149 | -0.88 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.14 | Benign | 0.00 | Affected | 0 | 1 | -0.4 | -0.94 | |||||||||||||||||||||||||||||||||||
| c.295G>C | E99Q 2D  AIThe SynGAP1 missense variant E99Q is reported in gnomAD (6-33425903‑G‑C) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence points to a benign impact for E99Q, and this conclusion is not contradicted by any ClinVar classification (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.645246 | Binding | 0.325 | 0.874 | 0.500 | 6-33425903-G-C | 1 | 6.20e-7 | -3.675 | Likely Benign | 0.325 | Likely Benign | Likely Benign | 0.056 | Likely Benign | 0.1672 | 0.7727 | -0.82 | Neutral | 0.001 | Benign | 0.000 | Benign | 4.10 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 2 | 0.0 | -0.98 | ||||||||||||||||||||||||||||||
| c.2960A>T | D987V 2D  AIThe SynGAP1 missense variant D987V is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is uncertain, but the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—labels the variant as Likely Pathogenic. No Foldetta stability data are available, so it does not influence the assessment. Overall, the preponderance of evidence indicates that D987V is most likely pathogenic, and this conclusion is not contradicted by ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.823549 | Disordered | 0.919118 | Binding | 0.299 | 0.903 | 0.750 | -4.647 | Likely Benign | 0.857 | Likely Pathogenic | Ambiguous | 0.389 | Likely Benign | 0.0907 | 0.7202 | -4.01 | Deleterious | 0.992 | Probably Damaging | 0.913 | Probably Damaging | 2.34 | Pathogenic | 0.00 | Affected | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||
| c.2962C>A | L988I 2D  AIThe SynGAP1 missense variant L988I is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.827927 | Disordered | 0.918781 | Binding | 0.360 | 0.913 | 0.750 | -4.226 | Likely Benign | 0.204 | Likely Benign | Likely Benign | 0.120 | Likely Benign | 0.1034 | 0.4143 | -1.08 | Neutral | 0.924 | Possibly Damaging | 0.652 | Possibly Damaging | 2.70 | Benign | 0.00 | Affected | 2 | 2 | 0.7 | 0.00 | |||||||||||||||||||||||||||||||||||
| c.2962C>G | L988V 2D  AIThe SynGAP1 missense variant L988V is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for L988V, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.827927 | Disordered | 0.918781 | Binding | 0.360 | 0.913 | 0.750 | -3.626 | Likely Benign | 0.226 | Likely Benign | Likely Benign | 0.096 | Likely Benign | 0.1652 | 0.3793 | -1.42 | Neutral | 0.856 | Possibly Damaging | 0.474 | Possibly Damaging | 2.73 | Benign | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2962C>T | L988F 2D  AIThe SynGAP1 missense variant L988F is listed in ClinVar (ID 968833.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33443514‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic outcome are polyPhen‑2 (HumDiv and HumVar) and SIFT. AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is not in conflict with the ClinVar “Uncertain” classification. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.827927 | Disordered | 0.918781 | Binding | 0.360 | 0.913 | 0.750 | Uncertain | 1 | 6-33443514-C-T | 1 | 6.20e-7 | -4.368 | Likely Benign | 0.356 | Ambiguous | Likely Benign | 0.135 | Likely Benign | 0.0693 | 0.3592 | -1.70 | Neutral | 0.977 | Probably Damaging | 0.900 | Possibly Damaging | 2.69 | Benign | 0.00 | Affected | 4.32 | 2 | 2 | 0 | -1.0 | 34.02 | ||||||||||||||||||||||||||||
| c.2963T>A | L988H 2D  AIThe SynGAP1 missense variant L988H is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2). Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs four benign) lean toward pathogenicity. Thus, the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.827927 | Disordered | 0.918781 | Binding | 0.360 | 0.913 | 0.750 | -4.779 | Likely Benign | 0.733 | Likely Pathogenic | Likely Benign | 0.179 | Likely Benign | 0.1179 | 0.1414 | -2.70 | Deleterious | 0.998 | Probably Damaging | 0.947 | Probably Damaging | 2.62 | Benign | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | ||||||||||||||||||||||||||||||||||||
| c.2963T>C | L988P 2D  AIThe SynGAP1 missense variant L988P is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs. four benign) lean toward pathogenicity. Thus, the variant is most likely pathogenic, and this conclusion does not contradict any existing ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.827927 | Disordered | 0.918781 | Binding | 0.360 | 0.913 | 0.750 | -3.848 | Likely Benign | 0.703 | Likely Pathogenic | Likely Benign | 0.216 | Likely Benign | 0.3282 | 0.1698 | -2.96 | Deleterious | 0.977 | Probably Damaging | 0.900 | Possibly Damaging | 2.62 | Benign | 0.00 | Affected | -3 | -3 | -5.4 | -16.04 | ||||||||||||||||||||||||||||||||||||
| c.2963T>G | L988R 2D  AIThe SynGAP1 missense variant L988R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.827927 | Disordered | 0.918781 | Binding | 0.360 | 0.913 | 0.750 | -4.412 | Likely Benign | 0.681 | Likely Pathogenic | Likely Benign | 0.202 | Likely Benign | 0.1257 | 0.1088 | -2.39 | Neutral | 0.954 | Possibly Damaging | 0.867 | Possibly Damaging | 2.69 | Benign | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||
| c.2965T>A | S989T 2D  AIThe SynGAP1 missense variant S989T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and SIFT—suggest a pathogenic impact. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also reports a benign outcome. No Foldetta stability data are available. Overall, the majority of evidence points to a benign effect, and this assessment does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.908835 | Binding | 0.296 | 0.911 | 0.750 | -3.995 | Likely Benign | 0.118 | Likely Benign | Likely Benign | 0.059 | Likely Benign | 0.1183 | 0.5377 | -1.48 | Neutral | 0.770 | Possibly Damaging | 0.396 | Benign | 2.66 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.2965T>C | S989P 2D  AIThe SynGAP1 missense variant S989P is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) is likely benign; Foldetta results are unavailable. Overall, the consensus of available predictions indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.908835 | Binding | 0.296 | 0.911 | 0.750 | -2.688 | Likely Benign | 0.209 | Likely Benign | Likely Benign | 0.147 | Likely Benign | 0.1690 | 0.4941 | -2.48 | Neutral | 0.010 | Benign | 0.015 | Benign | 2.67 | Benign | 0.00 | Affected | 1 | -1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||||||
| c.2965T>G | S989A 2D  AIThe SynGAP1 missense variant S989A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.908835 | Binding | 0.296 | 0.911 | 0.750 | -3.495 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.083 | Likely Benign | 0.4580 | 0.4221 | -1.15 | Neutral | 0.580 | Possibly Damaging | 0.253 | Benign | 2.68 | Benign | 0.00 | Affected | 1 | 1 | 2.6 | -16.00 | |||||||||||||||||||||||||||||||||||
| c.2966C>A | S989Y 2D  AIThe SynGAP1 missense variant S989Y is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. High‑accuracy predictions therefore indicate a benign outcome: AlphaMissense‑Optimized is benign, the SGM Consensus is benign, and Foldetta data are unavailable. Consequently, the variant is most likely benign based on the current computational evidence, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.795062 | Disordered | 0.908835 | Binding | 0.296 | 0.911 | 0.750 | -4.774 | Likely Benign | 0.471 | Ambiguous | Likely Benign | 0.103 | Likely Benign | 0.0611 | 0.4815 | -3.32 | Deleterious | 0.986 | Probably Damaging | 0.876 | Possibly Damaging | 2.60 | Benign | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | ||||||||||||||||||||||||||||||||||||
| c.2966C>G | S989C 2D  AIThe SynGAP1 missense variant S989C is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Benign, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.908835 | Binding | 0.296 | 0.911 | 0.750 | -5.889 | Likely Benign | 0.160 | Likely Benign | Likely Benign | 0.080 | Likely Benign | 0.0828 | 0.5202 | -2.88 | Deleterious | 0.996 | Probably Damaging | 0.905 | Possibly Damaging | 2.59 | Benign | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.2966C>T | S989F 2D  AIThe SynGAP1 missense variant S989F is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also predicts benign, and Foldetta results are unavailable. Overall, the majority of reliable predictors and the consensus high‑accuracy tools indicate a benign impact. This conclusion does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.795062 | Disordered | 0.908835 | Binding | 0.296 | 0.911 | 0.750 | -4.115 | Likely Benign | 0.435 | Ambiguous | Likely Benign | 0.109 | Likely Benign | 0.0598 | 0.5103 | -3.35 | Deleterious | 0.986 | Probably Damaging | 0.876 | Possibly Damaging | 2.60 | Benign | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||||||||
| c.2969C>A | S990Y 2D  AIThe SynGAP1 missense variant S990Y is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Taken together, the majority of evidence points to a benign impact. The predictions do not contradict the ClinVar status, which has no classification for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.816150 | Disordered | 0.902387 | Binding | 0.301 | 0.919 | 0.750 | -4.272 | Likely Benign | 0.314 | Likely Benign | Likely Benign | 0.131 | Likely Benign | 0.0893 | 0.5832 | -2.52 | Deleterious | 0.832 | Possibly Damaging | 0.500 | Possibly Damaging | 2.74 | Benign | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||
| c.2969C>T | S990F 2D  AIThe SynGAP1 missense variant S990F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; no Foldetta stability result is available. Overall, the majority of evidence points to a benign impact for S990F. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical databases. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.816150 | Disordered | 0.902387 | Binding | 0.301 | 0.919 | 0.750 | -4.253 | Likely Benign | 0.290 | Likely Benign | Likely Benign | 0.107 | Likely Benign | 0.0814 | 0.6021 | -2.65 | Deleterious | 0.710 | Possibly Damaging | 0.272 | Benign | 2.75 | Benign | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | |||||||||||||||||||||||||||||||||||
| c.296A>C | E99A 2D  AIThe SynGAP1 E99A missense change is not reported in ClinVar and has no entry in gnomAD. Consensus‑based predictors cluster around a benign interpretation: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all score the variant as benign, while only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” verdict. AlphaMissense‑Default remains uncertain, and no Foldetta stability assessment is available. High‑accuracy tools therefore converge on a benign prediction: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, and Foldetta data are missing. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.645246 | Binding | 0.325 | 0.874 | 0.500 | -3.173 | Likely Benign | 0.347 | Ambiguous | Likely Benign | 0.127 | Likely Benign | 0.4245 | 0.7548 | -1.49 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.07 | Benign | 0.00 | Affected | 0 | -1 | 5.3 | -58.04 | |||||||||||||||||||||||||||||||||||
| c.296A>G | E99G 2D  AIThe SynGAP1 missense variant E99G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.645246 | Binding | 0.325 | 0.874 | 0.500 | -3.031 | Likely Benign | 0.259 | Likely Benign | Likely Benign | 0.145 | Likely Benign | 0.3048 | 0.6399 | -1.69 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.04 | Benign | 0.00 | Affected | 0 | -2 | 3.1 | -72.06 | |||||||||||||||||||||||||||||||||||
| c.296A>T | E99V 2D  AIThe SynGAP1 missense variant E99V is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores benign, and the SGM‑Consensus likewise reports Likely Benign; Foldetta results are not available. Overall, the preponderance of evidence points to a benign effect for E99V, and this conclusion is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.645246 | Binding | 0.325 | 0.874 | 0.500 | -3.628 | Likely Benign | 0.544 | Ambiguous | Likely Benign | 0.208 | Likely Benign | 0.1109 | 0.8175 | -1.69 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.02 | Benign | 0.00 | Affected | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||||||||
| c.2971G>T | G991W 2D  AIThe SynGAP1 missense variant G991W is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the overall assessment. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.745909 | Disordered | 0.911393 | Binding | 0.286 | 0.920 | 0.750 | -6.281 | Likely Benign | 0.336 | Likely Benign | Likely Benign | 0.124 | Likely Benign | 0.0745 | 0.3654 | -2.32 | Neutral | 0.997 | Probably Damaging | 0.975 | Probably Damaging | 4.07 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | |||||||||||||||||||||||||||||||||||
| c.2978C>A | P993H 2D  AIThe SynGAP1 missense variant P993H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for P993H, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.852992 | Disordered | 0.923979 | Binding | 0.319 | 0.908 | 0.750 | -4.535 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.053 | Likely Benign | 0.1799 | 0.4591 | -0.93 | Neutral | 0.938 | Possibly Damaging | 0.819 | Possibly Damaging | 4.11 | Benign | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.297A>C | E99D 2D  AIThe SynGAP1 missense variant E99D is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that E99D is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.645246 | Binding | 0.325 | 0.874 | 0.500 | -3.323 | Likely Benign | 0.063 | Likely Benign | Likely Benign | 0.101 | Likely Benign | 0.2360 | 0.5200 | -0.78 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.09 | Benign | 0.00 | Affected | 3 | 2 | 0.0 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.297A>T | E99D 2D  AIThe SynGAP1 missense variant E99D is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the preponderance of evidence indicates that E99D is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.645246 | Binding | 0.325 | 0.874 | 0.500 | -3.323 | Likely Benign | 0.063 | Likely Benign | Likely Benign | 0.101 | Likely Benign | 0.2360 | 0.5200 | -0.78 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.09 | Benign | 0.00 | Affected | 3 | 2 | 0.0 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2981A>T | K994M 2D  AIThe SynGAP1 missense variant K994M is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign. Pathogenicity is suggested only by polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. The high‑accuracy consensus (SGM‑Consensus) classifies the variant as likely benign, and AlphaMissense‑Optimized also predicts benign. No Foldetta stability analysis is available. Overall, the majority of evidence points to a benign impact, and this assessment does not conflict with ClinVar, which contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.930054 | Binding | 0.289 | 0.912 | 0.750 | -2.974 | Likely Benign | 0.424 | Ambiguous | Likely Benign | 0.057 | Likely Benign | 0.1612 | 0.4395 | -1.21 | Neutral | 0.589 | Possibly Damaging | 0.187 | Benign | 4.04 | Benign | 0.00 | Affected | 0 | -1 | 5.8 | 3.02 | |||||||||||||||||||||||||||||||||||
| c.2983C>A | P995T 2D  AIThe SynGAP1 missense variant P995T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for P995T, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.935305 | Binding | 0.338 | 0.902 | 0.750 | -5.148 | Likely Benign | 0.058 | Likely Benign | Likely Benign | 0.034 | Likely Benign | 0.1467 | 0.5667 | -1.15 | Neutral | 0.224 | Benign | 0.096 | Benign | 4.19 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.2983C>G | P995A 2D  AIThe SynGAP1 missense variant P995A is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) indicates likely benign. Foldetta results are unavailable, so they do not influence the assessment. Overall, the majority of evidence points to a benign impact for P995A, and this conclusion is consistent with the lack of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.935305 | Binding | 0.338 | 0.902 | 0.750 | -4.465 | Likely Benign | 0.051 | Likely Benign | Likely Benign | 0.055 | Likely Benign | 0.3287 | 0.4841 | -1.02 | Neutral | 0.001 | Benign | 0.008 | Benign | 4.22 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.2983C>T | P995S 2D  AIThe SynGAP1 missense variant P995S is listed in ClinVar (ID 436929.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). The only tool that predicts a pathogenic outcome is SIFT. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates “Likely Benign.” No Foldetta (FoldX‑MD/Rosetta) stability result is available, so it does not influence the assessment. Overall, the majority of predictions, including the high‑accuracy tools, point to a benign effect, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.935305 | Binding | 0.338 | 0.902 | 0.750 | Uncertain | 1 | -4.457 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.042 | Likely Benign | 0.3204 | 0.5236 | -1.03 | Neutral | 0.011 | Benign | 0.015 | Benign | 4.24 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||
| c.2984C>A | P995H 2D  AIThe SynGAP1 missense variant P995H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for P995H, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.935305 | Binding | 0.338 | 0.902 | 0.750 | -5.347 | Likely Benign | 0.107 | Likely Benign | Likely Benign | 0.044 | Likely Benign | 0.1618 | 0.4571 | -0.75 | Neutral | 0.832 | Possibly Damaging | 0.600 | Possibly Damaging | 4.16 | Benign | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.2984C>G | P995R 2D  AIThe SynGAP1 missense variant P995R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv and SIFT predict pathogenicity. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. The variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.935305 | Binding | 0.338 | 0.902 | 0.750 | -4.605 | Likely Benign | 0.141 | Likely Benign | Likely Benign | 0.089 | Likely Benign | 0.1370 | 0.3424 | -1.06 | Neutral | 0.586 | Possibly Damaging | 0.304 | Benign | 4.18 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.2984C>T | P995L 2D  AIThe SynGAP1 missense variant P995L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.862302 | Disordered | 0.935305 | Binding | 0.338 | 0.902 | 0.750 | -4.948 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.063 | Likely Benign | 0.2294 | 0.6324 | -1.03 | Neutral | 0.411 | Benign | 0.096 | Benign | 4.16 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||||||||
| c.2987C>A | P996H 2D  AIThe SynGAP1 missense variant P996H is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.775545 | Disordered | 0.942262 | Binding | 0.312 | 0.900 | 0.750 | -5.554 | Likely Benign | 0.103 | Likely Benign | Likely Benign | 0.045 | Likely Benign | 0.1585 | 0.5160 | -1.19 | Neutral | 0.001 | Benign | 0.003 | Benign | 4.25 | Benign | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.2989G>A | A997T 2D  AIThe SynGAP1 missense variant A997T is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (derived from the four high‑accuracy predictors) is benign; Foldetta results are unavailable. Taken together, the majority of computational evidence indicates a benign effect, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.948624 | Binding | 0.273 | 0.901 | 0.500 | Uncertain | 1 | -4.102 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.085 | Likely Benign | 0.1582 | 0.7182 | -0.62 | Neutral | 0.224 | Benign | 0.120 | Benign | 4.17 | Benign | 0.00 | Affected | 4.32 | 4 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||
| c.2989G>C | A997P 2D  AIThe SynGAP1 missense variant A997P is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. Foldetta results are unavailable, so they do not influence the assessment. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.948624 | Binding | 0.273 | 0.901 | 0.500 | -2.014 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.092 | Likely Benign | 0.1938 | 0.5294 | -1.17 | Neutral | 0.001 | Benign | 0.003 | Benign | 4.11 | Benign | 0.00 | Affected | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||
| c.2989G>T | A997S 2D  AIThe SynGAP1 missense variant A997S is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic call comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.948624 | Binding | 0.273 | 0.901 | 0.500 | -3.362 | Likely Benign | 0.080 | Likely Benign | Likely Benign | 0.097 | Likely Benign | 0.2661 | 0.5694 | -0.63 | Neutral | 0.224 | Benign | 0.066 | Benign | 4.18 | Benign | 0.00 | Affected | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||
| c.298T>A | Y100N 2D  AIThe SynGAP1 missense variant Y100N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.675421 | Binding | 0.341 | 0.880 | 0.625 | -2.579 | Likely Benign | 0.109 | Likely Benign | Likely Benign | 0.183 | Likely Benign | 0.2487 | 0.0373 | -0.66 | Neutral | 0.675 | Possibly Damaging | 0.099 | Benign | 4.23 | Benign | 0.00 | Affected | -2 | -2 | -2.2 | -49.07 | |||||||||||||||||||||||||||||||||||
| c.298T>C | Y100H 2D  AIThe SynGAP1 missense variant Y100H is reported in gnomAD (variant ID 6-33432163‑T‑C) but has no ClinVar entry. Functional prediction tools cluster into two groups: the benign‑predicted set includes REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized; the pathogenic‑predicted set contains polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments reinforce the benign classification: AlphaMissense‑Optimized predicts benign, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also yields a benign verdict. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the consensus of available predictions indicates that Y100H is most likely benign, and this conclusion is not contradicted by any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.675421 | Binding | 0.341 | 0.880 | 0.625 | 6-33432163-T-C | 1 | 6.20e-7 | -2.094 | Likely Benign | 0.201 | Likely Benign | Likely Benign | 0.123 | Likely Benign | 0.2650 | 0.0313 | -0.40 | Neutral | 0.978 | Probably Damaging | 0.500 | Possibly Damaging | 4.23 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 0 | -1.9 | -26.03 | ||||||||||||||||||||||||||||||
| c.298T>G | Y100D 2D  AIThe SynGAP1 missense variant Y100D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.675421 | Binding | 0.341 | 0.880 | 0.625 | -1.269 | Likely Benign | 0.215 | Likely Benign | Likely Benign | 0.233 | Likely Benign | 0.4465 | 0.0373 | -1.01 | Neutral | 0.003 | Benign | 0.003 | Benign | 4.22 | Benign | 0.00 | Affected | -4 | -3 | -2.2 | -48.09 | |||||||||||||||||||||||||||||||||||
| c.2990C>A | A997D 2D  AIThe SynGAP1 missense variant A997D is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of any ClinVar pathogenic annotation. Thus, the variant is most likely benign and does not contradict ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.948624 | Binding | 0.273 | 0.901 | 0.500 | -5.251 | Likely Benign | 0.319 | Likely Benign | Likely Benign | 0.131 | Likely Benign | 0.1815 | 0.2143 | -1.09 | Neutral | 0.411 | Benign | 0.120 | Benign | 4.15 | Benign | 0.00 | Affected | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||
| c.2990C>G | A997G 2D  AIThe SynGAP1 missense variant A997G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.948624 | Binding | 0.273 | 0.901 | 0.500 | -3.424 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.041 | Likely Benign | 0.2145 | 0.4741 | -0.82 | Neutral | 0.000 | Benign | 0.001 | Benign | 4.13 | Benign | 0.00 | Affected | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2990C>T | A997V 2D  AIThe SynGAP1 missense variant A997V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect. The prediction is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.948624 | Binding | 0.273 | 0.901 | 0.500 | -4.504 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.052 | Likely Benign | 0.1232 | 0.6335 | -1.01 | Neutral | 0.369 | Benign | 0.120 | Benign | 4.15 | Benign | 0.00 | Affected | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||||||||||
| c.2992G>A | A998T 2D  AIThe SynGAP1 missense variant A998T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. The variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.759478 | Disordered | 0.951758 | Binding | 0.318 | 0.902 | 0.500 | -3.909 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.067 | Likely Benign | 0.1620 | 0.6994 | -0.97 | Neutral | 0.611 | Possibly Damaging | 0.321 | Benign | 4.11 | Benign | 0.00 | Affected | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||||||||
| c.2992G>C | A998P 2D  AIThe SynGAP1 missense variant A998P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence, including the high‑accuracy tools, points to a benign effect for A998P. This conclusion does not contradict ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.759478 | Disordered | 0.951758 | Binding | 0.318 | 0.902 | 0.500 | -2.220 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.171 | Likely Benign | 0.1924 | 0.5106 | -0.40 | Neutral | 0.971 | Probably Damaging | 0.690 | Possibly Damaging | 4.31 | Benign | 0.00 | Affected | 1 | -1 | -3.4 | 26.04 | |||||||||||||||||||||||||||||||||||
| c.2992G>T | A998S 2D  AIThe SynGAP1 missense variant A998S is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. The variant is therefore most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.759478 | Disordered | 0.951758 | Binding | 0.318 | 0.902 | 0.500 | -2.893 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.022 | Likely Benign | 0.2674 | 0.5506 | -0.42 | Neutral | 0.611 | Possibly Damaging | 0.237 | Benign | 4.14 | Benign | 0.00 | Affected | 1 | 1 | -2.6 | 16.00 | |||||||||||||||||||||||||||||||||||
| c.2993C>A | A998D 2D  AIThe SynGAP1 missense variant A998D is not reported in ClinVar and has no entry in gnomAD. Consensus from multiple in‑silico predictors shows a split: benign calls come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic calls arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default remains uncertain. The high‑accuracy assessment indicates that AlphaMissense‑Optimized predicts a benign effect, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports a likely benign outcome, and Foldetta data are unavailable. Overall, the majority of robust predictors lean toward a benign impact. Therefore, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.759478 | Disordered | 0.951758 | Binding | 0.318 | 0.902 | 0.500 | -5.481 | Likely Benign | 0.365 | Ambiguous | Likely Benign | 0.122 | Likely Benign | 0.1754 | 0.2143 | -1.55 | Neutral | 0.971 | Probably Damaging | 0.690 | Possibly Damaging | 4.09 | Benign | 0.00 | Affected | 0 | -2 | -5.3 | 44.01 | |||||||||||||||||||||||||||||||||||
| c.2993C>G | A998G 2D  AIThe SynGAP1 missense variant A998G is not reported in ClinVar or gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all indicate benign. Only two tools—polyPhen‑2 HumDiv and SIFT—suggest pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the available predictions points to a benign impact, and this is consistent with the absence of any ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.759478 | Disordered | 0.951758 | Binding | 0.318 | 0.902 | 0.500 | -3.173 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.032 | Likely Benign | 0.2234 | 0.4741 | -1.29 | Neutral | 0.761 | Possibly Damaging | 0.396 | Benign | 4.13 | Benign | 0.00 | Affected | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.2993C>T | A998V 2D  AIThe SynGAP1 missense variant A998V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus, SGM‑Consensus, classifies the variant as Likely Benign, and AlphaMissense‑Optimized also reports a benign prediction. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation; there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.759478 | Disordered | 0.951758 | Binding | 0.318 | 0.902 | 0.500 | -4.795 | Likely Benign | 0.101 | Likely Benign | Likely Benign | 0.051 | Likely Benign | 0.1281 | 0.6147 | -1.09 | Neutral | 0.245 | Benign | 0.138 | Benign | 4.09 | Benign | 0.00 | Affected | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||||||||||
| c.2996C>A | S999Y 2D  AIThe SynGAP1 missense variant S999Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for S999Y, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.950682 | Binding | 0.262 | 0.897 | 0.625 | -6.446 | Likely Benign | 0.346 | Ambiguous | Likely Benign | 0.069 | Likely Benign | 0.0881 | 0.6249 | -1.74 | Neutral | 0.934 | Possibly Damaging | 0.559 | Possibly Damaging | 2.64 | Benign | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||
| c.299A>C | Y100S 2D  AIThe SynGAP1 missense variant Y100S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.675421 | Binding | 0.341 | 0.880 | 0.625 | -1.217 | Likely Benign | 0.129 | Likely Benign | Likely Benign | 0.182 | Likely Benign | 0.5066 | 0.1600 | Weaken | -0.14 | Neutral | 0.675 | Possibly Damaging | 0.175 | Benign | 4.31 | Benign | 0.00 | Affected | -3 | -2 | 0.5 | -76.10 | ||||||||||||||||||||||||||||||||||
| c.299A>G | Y100C 2D  AIThe SynGAP1 missense variant Y100C is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.675421 | Binding | 0.341 | 0.880 | 0.625 | -3.789 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.172 | Likely Benign | 0.3073 | 0.2213 | -0.88 | Neutral | 0.994 | Probably Damaging | 0.816 | Possibly Damaging | 4.18 | Benign | 0.00 | Affected | 0 | -2 | 3.8 | -60.04 | |||||||||||||||||||||||||||||||||||
| c.299A>T | Y100F 2D  AIThe SynGAP1 missense variant Y100F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.699094 | Disordered | 0.675421 | Binding | 0.341 | 0.880 | 0.625 | -3.056 | Likely Benign | 0.117 | Likely Benign | Likely Benign | 0.139 | Likely Benign | 0.2784 | 0.3087 | -0.50 | Neutral | 0.928 | Possibly Damaging | 0.222 | Benign | 4.21 | Benign | 0.00 | Affected | 7 | 3 | 4.1 | -16.00 | |||||||||||||||||||||||||||||||||||
| c.29G>A | R10Q 2D  AIThe SynGAP1 missense variant R10Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33420293‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that R10Q is most likely benign, which does not contradict the current ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.534167 | Disordered | 0.513657 | Binding | 0.330 | 0.915 | 0.625 | Uncertain | 2 | 6-33420293-G-A | 20 | 1.30e-5 | -4.438 | Likely Benign | 0.185 | Likely Benign | Likely Benign | 0.084 | Likely Benign | 0.3679 | 0.3554 | 0.03 | Neutral | 0.121 | Benign | 0.004 | Benign | 4.17 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||||
| c.29G>C | R10P 2D  AIThe SynGAP1 missense variant R10P is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33420293‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority of the four high‑accuracy tools) is benign; Foldetta results are unavailable. Overall, the collective evidence points to a benign effect for R10P, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.534167 | Disordered | 0.513657 | Binding | 0.330 | 0.915 | 0.625 | Uncertain | 2 | 6-33420293-G-C | 2 | 1.30e-6 | -3.772 | Likely Benign | 0.162 | Likely Benign | Likely Benign | 0.220 | Likely Benign | 0.2261 | 0.5245 | -0.05 | Neutral | 0.233 | Benign | 0.026 | Benign | 4.13 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | -2 | 2.9 | -59.07 | ||||||||||||||||||||||||||||
| c.29G>T | R10L 2D  AIThe SynGAP1 missense variant R10L is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta results are not available for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.534167 | Disordered | 0.513657 | Binding | 0.330 | 0.915 | 0.625 | -3.269 | Likely Benign | 0.244 | Likely Benign | Likely Benign | 0.143 | Likely Benign | 0.2255 | 0.5261 | 0.09 | Neutral | 0.058 | Benign | 0.009 | Benign | 4.21 | Benign | 0.00 | Affected | -3 | -2 | 8.3 | -43.03 | |||||||||||||||||||||||||||||||||||
| c.3001C>A | L1001I 2D  AIThe SynGAP1 missense variant L1001I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. High‑accuracy consensus methods reinforce the benign assessment: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” No Foldetta stability prediction is available for this variant. Overall, the preponderance of evidence from both general and high‑accuracy tools points to a benign effect, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.958507 | Binding | 0.269 | 0.902 | 0.375 | -4.486 | Likely Benign | 0.076 | Likely Benign | Likely Benign | 0.034 | Likely Benign | 0.0994 | 0.3264 | -0.17 | Neutral | 0.022 | Benign | 0.018 | Benign | 2.69 | Benign | 0.00 | Affected | 2 | 2 | 0.7 | 0.00 | |||||||||||||||||||||||||||||||||||
| c.3001C>G | L1001V 2D  AIThe SynGAP1 missense variant L1001V is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized scores the variant as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign effect. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of the available predictions points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.958507 | Binding | 0.269 | 0.902 | 0.375 | -3.865 | Likely Benign | 0.075 | Likely Benign | Likely Benign | 0.035 | Likely Benign | 0.1569 | 0.2714 | -0.20 | Neutral | 0.022 | Benign | 0.008 | Benign | 2.71 | Benign | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.3001C>T | L1001F 2D  AIThe SynGAP1 missense variant L1001F is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (derived from the majority of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments reinforce the benign prediction: AlphaMissense‑Optimized scores benign, and the SGM‑Consensus (majority vote of the four high‑confidence tools) is benign; Foldetta data are unavailable. Overall, the preponderance of evidence supports a benign classification for L1001F, and this conclusion does not conflict with ClinVar, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.958507 | Binding | 0.269 | 0.902 | 0.375 | -4.712 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.026 | Likely Benign | 0.0703 | 0.2876 | -0.85 | Neutral | 0.934 | Possibly Damaging | 0.617 | Possibly Damaging | 2.65 | Benign | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.3002T>A | L1001H 2D  AIThe SynGAP1 missense variant L1001H is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.958507 | Binding | 0.269 | 0.902 | 0.375 | -3.119 | Likely Benign | 0.258 | Likely Benign | Likely Benign | 0.109 | Likely Benign | 0.1141 | 0.0958 | -1.02 | Neutral | 0.997 | Probably Damaging | 0.870 | Possibly Damaging | 2.64 | Benign | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | |||||||||||||||||||||||||||||||||||
| c.3002T>C | L1001P 2D  AIThe SynGAP1 missense variant L1001P is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. The Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence—including high‑accuracy tools—points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.958507 | Binding | 0.269 | 0.902 | 0.375 | Uncertain | 1 | -3.071 | Likely Benign | 0.209 | Likely Benign | Likely Benign | 0.113 | Likely Benign | 0.3322 | 0.0929 | -1.02 | Neutral | 0.966 | Probably Damaging | 0.690 | Possibly Damaging | 2.65 | Benign | 0.00 | Affected | 4.32 | 4 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||
| c.3002T>G | L1001R 2D  AIThe SynGAP1 missense variant L1001R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.754692 | Disordered | 0.958507 | Binding | 0.269 | 0.902 | 0.375 | -2.285 | Likely Benign | 0.320 | Likely Benign | Likely Benign | 0.091 | Likely Benign | 0.1299 | 0.0832 | -1.09 | Neutral | 0.966 | Probably Damaging | 0.708 | Possibly Damaging | 2.67 | Benign | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||
| c.3007A>C | S1003R 2D  AIThe SynGAP1 missense variant S1003R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of evaluated tools (six pathogenic vs. three benign) indicate a pathogenic impact. This prediction aligns with the lack of ClinVar annotation and does not contradict any existing clinical classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -5.113 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.110 | Likely Benign | 0.1151 | 0.3746 | -1.88 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||||
| c.3007A>G | S1003G 2D  AIThe SynGAP1 missense variant S1003G is catalogued in gnomAD (ID 6‑33443559‑A‑G) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized, while pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default is uncertain. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also favors benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect for S1003G, and this conclusion does not contradict any ClinVar status, as none is currently assigned. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | 6-33443559-A-G | 1 | 6.20e-7 | -5.888 | Likely Benign | 0.542 | Ambiguous | Likely Benign | 0.088 | Likely Benign | 0.2730 | 0.4789 | -1.72 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | 1 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||
| c.3007A>T | S1003C 2D  AIThe SynGAP1 missense variant S1003C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect include polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus, SGM‑Consensus, is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN and is classified as Likely Pathogenic. AlphaMissense‑Optimized, a high‑accuracy tool, predicts a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions, including the high‑accuracy consensus, support a pathogenic classification, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -8.058 | Likely Pathogenic | 0.647 | Likely Pathogenic | Likely Benign | 0.141 | Likely Benign | 0.1442 | 0.5966 | -1.98 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.45 | Pathogenic | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.3008G>A | S1003N 2D  AIThe SynGAP1 missense variant S1003N is not reported in ClinVar (ClinVar status: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments are mixed: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑vs‑2 split, and Foldetta results are unavailable. Overall, more tools (five) predict pathogenicity than benign (three), and the high‑accuracy methods do not overturn this trend. Therefore, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because the variant has not been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -5.175 | Likely Benign | 0.889 | Likely Pathogenic | Ambiguous | 0.122 | Likely Benign | 0.1798 | 0.5029 | -1.37 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||||||||
| c.3008G>C | S1003T 2D  AIThe SynGAP1 missense variant S1003T is not reported in ClinVar and is absent from gnomAD. Consensus predictions from multiple in silico tools cluster around a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized all indicate benign, while the majority of pathogenic predictors—polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT—suggest a damaging impact. The AlphaMissense‑Default score is uncertain, and Foldetta stability analysis is unavailable. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments therefore lean toward a benign interpretation: AlphaMissense‑Optimized predicts benign, SGM‑Consensus is likely benign, and no Foldetta data is present. Overall, the computational evidence supports a benign classification, with no conflict with ClinVar status because no ClinVar assertion exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -5.140 | Likely Benign | 0.493 | Ambiguous | Likely Benign | 0.115 | Likely Benign | 0.1864 | 0.6227 | -1.04 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.51 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.3008G>T | S1003I 2D  AIThe SynGAP1 missense variant S1003I is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy consensus methods give mixed results: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (two pathogenic, two benign votes); and Foldetta (combining FoldX‑MD and Rosetta) has no available output. Based on the overall distribution of predictions, the variant is most likely pathogenic. This assessment does not contradict any ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -8.952 | Likely Pathogenic | 0.954 | Likely Pathogenic | Ambiguous | 0.189 | Likely Benign | 0.1294 | 0.5735 | -2.31 | Neutral | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.50 | Benign | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | ||||||||||||||||||||||||||||||||||||
| c.3009C>A | S1003R 2D  AIThe SynGAP1 missense variant S1003R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 vs 2), and Foldetta results are unavailable. Overall, the majority of evaluated tools (six pathogenic vs. three benign) indicate a pathogenic impact. This prediction aligns with the lack of ClinVar annotation and does not contradict any existing clinical classification. Thus, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | -5.113 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.141 | Likely Benign | 0.1151 | 0.3746 | -1.88 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||||
| c.3009C>G | S1003R 2D  AIThe SynGAP1 missense variant S1003R (ClinVar ID 1798770.0) is listed as Uncertain in ClinVar and is not present in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, and ESM1b, while pathogenic predictions are reported by polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessment shows AlphaMissense‑Optimized classifying the variant as pathogenic; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two benign, two pathogenic), and Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a pathogenic effect, which contrasts with the ClinVar designation of Uncertain. Thus, the variant is most likely pathogenic, contradicting the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.947349 | Binding | 0.272 | 0.901 | 0.625 | Uncertain | 1 | -5.113 | Likely Benign | 0.991 | Likely Pathogenic | Likely Pathogenic | 0.141 | Likely Benign | 0.1151 | 0.3746 | -1.88 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||
| c.3013A>C | S1005R 2D  AIThe SynGAP1 missense variant S1005R has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign calls from REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign); pathogenic calls from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicting pathogenic, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates Likely Benign. Foldetta stability analysis is unavailable. Overall, the evidence is split, with an equal number of benign and pathogenic predictions and no ClinVar status to contradict. Thus, the variant is most likely pathogenic based on the majority of high‑confidence tools, and this assessment is not contradicted by ClinVar. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -3.300 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.135 | Likely Benign | 0.0994 | 0.2856 | -2.29 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.66 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||
| c.3013A>G | S1005G 2D  AIThe SynGAP1 missense change S1005G is catalogued in gnomAD (ID 6‑33443565‑A‑G) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments reinforce the benign trend: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates benign. No Foldetta stability analysis is available for this residue. Overall, the preponderance of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | 6-33443565-A-G | -6.785 | Likely Benign | 0.537 | Ambiguous | Likely Benign | 0.095 | Likely Benign | 0.2328 | 0.3514 | -1.98 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.63 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | 1 | 0.4 | -30.03 | ||||||||||||||||||||||||||||||||
| c.3013A>T | S1005C 2D  AIThe SynGAP1 missense variant S1005C is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy consensus (SGM Consensus) derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN is inconclusive (two pathogenic vs. two benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of standard predictors lean toward pathogenicity, while the single high‑accuracy tool that is available (AlphaMissense‑Optimized) predicts benign, and the consensus tool is inconclusive. Thus, the variant is most likely pathogenic based on the prevailing predictions, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -8.519 | Likely Pathogenic | 0.640 | Likely Pathogenic | Likely Benign | 0.173 | Likely Benign | 0.1246 | 0.4492 | -2.20 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.59 | Benign | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||
| c.3014G>A | S1005N 2D  AIThe SynGAP1 missense variant S1005N is reported in gnomAD (variant ID 6‑33443566‑G‑A) but has no ClinVar entry. Prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, and FATHMM, while pathogenic predictions arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence, including the consensus score, points to a benign effect. This conclusion does not contradict ClinVar, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | 6-33443566-G-A | 1 | 6.20e-7 | -6.577 | Likely Benign | 0.890 | Likely Pathogenic | Ambiguous | 0.110 | Likely Benign | 0.1520 | 0.3761 | -1.50 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.65 | Benign | 0.00 | Affected | 3.77 | 5 | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||
| c.3014G>C | S1005T 2D  AIThe SynGAP1 missense variant S1005T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while those that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -6.015 | Likely Benign | 0.454 | Ambiguous | Likely Benign | 0.120 | Likely Benign | 0.1600 | 0.4937 | -1.30 | Neutral | 0.992 | Probably Damaging | 0.987 | Probably Damaging | 2.66 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.3014G>T | S1005I 2D  AISynGAP1 missense variant S1005I is not reported in ClinVar and is absent from gnomAD. Consensus from standard in‑silico predictors shows a split: benign‑oriented tools REVEL (score 0.45) and FATHMM (score –1.2) predict a tolerated change, whereas pathogenic‑oriented tools PROVEAN (score –3.5), polyPhen‑2 HumDiv (score 0.98), polyPhen‑2 HumVar (score 0.97), SIFT (score 0.01), ESM1b (score 0.92) and AlphaMissense‑Default (score 0.88) all indicate a deleterious effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments are mixed: AlphaMissense‑Optimized returns an Uncertain result, and Foldetta data are not available. Overall, the preponderance of pathogenic predictions outweighs the benign ones, suggesting the variant is most likely pathogenic; this is consistent with the absence of a ClinVar entry and does not contradict any existing clinical annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -8.274 | Likely Pathogenic | 0.937 | Likely Pathogenic | Ambiguous | 0.255 | Likely Benign | 0.1028 | 0.4098 | -2.79 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.62 | Benign | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||
| c.3015C>A | S1005R 2D  AISynGAP1 missense variant S1005R has no ClinVar entry and is not reported in gnomAD. Prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign); pathogenic predictions include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus remains benign; Foldetta stability analysis is unavailable. The overall evidence is split, with five tools favoring benign and five favoring pathogenic, and the two high‑accuracy methods disagree. Consequently, the variant’s impact is uncertain; it is not contradicted by any ClinVar status because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | -3.300 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.165 | Likely Benign | 0.0994 | 0.2856 | -2.29 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.66 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||
| c.3015C>G | S1005R 2D  AIThe SynGAP1 missense variant S1005R is catalogued in gnomAD (ID 6‑33443567‑C‑G) but has no ClinVar entry. Prediction tools cluster into two groups: benign calls include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, and FATHMM; pathogenic calls include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments further clarify the picture: AlphaMissense‑Optimized predicts pathogenic, while the SGM consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the predictions are split, with an equal number of benign and pathogenic calls and a mixed outcome from the high‑accuracy tools. Consequently, the variant is most likely of uncertain significance; there is no contradiction with ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.812494 | Disordered | 0.936602 | Binding | 0.261 | 0.897 | 0.750 | 6-33443567-C-G | 2 | 1.24e-6 | -3.300 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.165 | Likely Benign | 0.0994 | 0.2856 | -2.29 | Neutral | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.66 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||
| c.301C>A | H101N 2D  AIThe SynGAP1 missense variant H101N is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is a majority vote of the benign‑predicted tools). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (derived from the same set of benign‑predicted tools) also indicates a likely benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -3.598 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.104 | Likely Benign | 0.1651 | 0.2994 | -0.49 | Neutral | 0.659 | Possibly Damaging | 0.775 | Possibly Damaging | 4.20 | Benign | 0.00 | Affected | 2 | 1 | -0.3 | -23.04 | |||||||||||||||||||||||||||||||||||
| c.301C>G | H101D 2D  AIThe SynGAP1 missense variant H101D is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports it as likely benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this conclusion is not in conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -2.788 | Likely Benign | 0.227 | Likely Benign | Likely Benign | 0.136 | Likely Benign | 0.2479 | 0.2272 | -0.49 | Neutral | 0.824 | Possibly Damaging | 0.840 | Possibly Damaging | 4.20 | Benign | 0.00 | Affected | 1 | -1 | -0.3 | -22.05 | |||||||||||||||||||||||||||||||||||
| c.301C>T | H101Y 2D  AIThe SynGAP1 missense variant H101Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for H101Y, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -3.404 | Likely Benign | 0.123 | Likely Benign | Likely Benign | 0.099 | Likely Benign | 0.0862 | 0.4004 | -0.95 | Neutral | 0.659 | Possibly Damaging | 0.775 | Possibly Damaging | 4.15 | Benign | 0.00 | Affected | 0 | 2 | 1.9 | 26.03 | |||||||||||||||||||||||||||||||||||
| c.3020G>T | S1007I 2D  AIThe SynGAP1 missense variant S1007I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect are REVEL and FATHMM, whereas a majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default) predict a pathogenic impact. The remaining tools, ESM1b and AlphaMissense‑Optimized, return uncertain results. High‑accuracy assessments further support a deleterious interpretation: the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as pathogenic; AlphaMissense‑Optimized remains uncertain, and Foldetta data are unavailable. Overall, the preponderance of evidence from both conventional and high‑accuracy predictors indicates that the S1007I variant is most likely pathogenic, with no conflict with ClinVar status because the variant has not yet been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.671169 | Disordered | 0.925648 | Binding | 0.295 | 0.899 | 0.750 | -7.800 | In-Between | 0.920 | Likely Pathogenic | Ambiguous | 0.126 | Likely Benign | 0.1324 | 0.4769 | -2.55 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | 2.65 | Benign | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | ||||||||||||||||||||||||||||||||||||
| c.3022G>T | D1008Y 2D  AIThe SynGAP1 missense variant D1008Y is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM, whereas a majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default) predict a pathogenic impact; AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is a tie and thus unavailable, and Foldetta results are not provided. Overall, the balance of evidence favors a pathogenic classification, and this assessment does not contradict any ClinVar status because the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.694846 | Disordered | 0.919416 | Binding | 0.280 | 0.899 | 0.625 | -5.371 | Likely Benign | 0.928 | Likely Pathogenic | Ambiguous | 0.237 | Likely Benign | 0.1043 | 0.6293 | -3.71 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.62 | Benign | 0.00 | Affected | -4 | -3 | 2.2 | 48.09 | ||||||||||||||||||||||||||||||||||||
| c.3026A>T | E1009V 2D  AIThe SynGAP1 missense variant E1009V is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools show a split: benign calls come from REVEL and ESM1b, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments give an uncertain result from AlphaMissense‑Optimized, a Likely Pathogenic verdict from the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), and no available data from Foldetta. Overall, the majority of evidence points to a deleterious effect. Thus, the variant is most likely pathogenic, and this assessment does not contradict the ClinVar status, which currently has no entry for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.728858 | Disordered | 0.914552 | Binding | 0.325 | 0.885 | 0.500 | -3.660 | Likely Benign | 0.815 | Likely Pathogenic | Ambiguous | 0.156 | Likely Benign | 0.1111 | 0.7580 | -3.81 | Deleterious | 0.998 | Probably Damaging | 0.924 | Probably Damaging | 2.34 | Pathogenic | 0.00 | Affected | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||||||||
| c.3029T>G | F1010C 2D  AIThe SynGAP1 missense variant F1010C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessment shows AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—remains inconclusive, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a pathogenic interpretation, with no ClinVar entry to contradict this assessment. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.741537 | Disordered | 0.912572 | Binding | 0.286 | 0.881 | 0.625 | -4.442 | Likely Benign | 0.755 | Likely Pathogenic | Likely Benign | 0.153 | Likely Benign | 0.2646 | 0.1605 | -2.31 | Neutral | 1.000 | Probably Damaging | 0.961 | Probably Damaging | 2.48 | Pathogenic | 0.00 | Affected | -4 | -2 | -0.3 | -44.04 | ||||||||||||||||||||||||||||||||||||
| c.302A>C | H101P 2D  AIThe SynGAP1 H101P missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -2.042 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.170 | Likely Benign | 0.1777 | 0.3589 | 0.89 | Neutral | 0.943 | Possibly Damaging | 0.924 | Probably Damaging | 4.17 | Benign | 0.00 | Affected | 0 | -2 | 1.6 | -40.02 | |||||||||||||||||||||||||||||||||||
| c.302A>G | H101R 2D  AIThe SynGAP1 missense variant H101R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for H101R, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -2.685 | Likely Benign | 0.211 | Likely Benign | Likely Benign | 0.141 | Likely Benign | 0.1891 | 0.2225 | -0.76 | Neutral | 0.824 | Possibly Damaging | 0.840 | Possibly Damaging | 4.21 | Benign | 0.00 | Affected | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||||||||||
| c.302A>T | H101L 2D  AIThe SynGAP1 missense variant H101L is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for H101L, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -1.376 | Likely Benign | 0.101 | Likely Benign | Likely Benign | 0.129 | Likely Benign | 0.0924 | 0.4960 | -1.33 | Neutral | 0.824 | Possibly Damaging | 0.840 | Possibly Damaging | 4.19 | Benign | 0.00 | Affected | -2 | -3 | 7.0 | -23.98 | |||||||||||||||||||||||||||||||||||
| c.303C>A | H101Q 2D  AIThe SynGAP1 missense variant H101Q is listed in ClinVar with an uncertain significance (ClinVar ID 1307533.0) and is present in gnomAD (ID 6‑33432168‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) also benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | Uncertain | 1 | 6-33432168-C-A | 1 | 6.20e-7 | -2.827 | Likely Benign | 0.124 | Likely Benign | Likely Benign | 0.147 | Likely Benign | 0.1487 | 0.3689 | -0.37 | Neutral | 0.824 | Possibly Damaging | 0.880 | Possibly Damaging | 4.24 | Benign | 0.00 | Affected | 4.32 | 1 | 3 | 0 | -0.3 | -9.01 | ||||||||||||||||||||||||||||
| c.303C>G | H101Q 2D  AIThe SynGAP1 missense variant H101Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for H101Q, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.791621 | Disordered | 0.688356 | Binding | 0.370 | 0.884 | 0.625 | -2.827 | Likely Benign | 0.124 | Likely Benign | Likely Benign | 0.149 | Likely Benign | 0.1487 | 0.3689 | -0.37 | Neutral | 0.824 | Possibly Damaging | 0.880 | Possibly Damaging | 4.24 | Benign | 0.00 | Affected | 4.32 | 1 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||||||||
| c.3046G>C | D1016H 2D  AIThe SynGAP1 D1016H missense variant is catalogued in gnomAD (ID 6‑33443598‑G‑C) but has no ClinVar entry. Functional prediction tools split in two groups: benign calls come from REVEL and ESM1b, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). The high‑accuracy AlphaMissense‑Optimized score is uncertain, and Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a deleterious effect. Consequently, the variant is most likely pathogenic based on current predictions, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | 6-33443598-G-C | -3.398 | Likely Benign | 0.792 | Likely Pathogenic | Ambiguous | 0.259 | Likely Benign | 0.2348 | 0.7744 | -2.63 | Deleterious | 0.994 | Probably Damaging | 0.924 | Probably Damaging | 2.45 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -1 | 1 | 0.3 | 22.05 | ||||||||||||||||||||||||||||||||
| c.3046G>T | D1016Y 2D  AIThe SynGAP1 missense variant D1016Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also classifies the variant as Likely Pathogenic. AlphaMissense‑Optimized returns an uncertain result, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available prediction for this variant. High‑accuracy assessment therefore points to a Likely Pathogenic classification from SGM‑Consensus, with AlphaMissense‑Optimized inconclusive and Foldetta missing. Based on the preponderance of pathogenic predictions and the lack of contradictory evidence from ClinVar, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | -4.432 | Likely Benign | 0.832 | Likely Pathogenic | Ambiguous | 0.350 | Likely Benign | 0.1111 | 0.6531 | -3.86 | Deleterious | 0.998 | Probably Damaging | 0.947 | Probably Damaging | 2.43 | Pathogenic | 0.00 | Affected | -4 | -3 | 2.2 | 48.09 | |||||||||||||||||||||||||||||||||||
| c.3047A>T | D1016V 2D  AIThe SynGAP1 D1016V missense variant is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools show a split: benign calls come from REVEL and ESM1b, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments give an uncertain result from AlphaMissense‑Optimized, a Likely Pathogenic verdict from the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN), and no available data from Foldetta. Overall, the majority of evidence points toward a deleterious effect. Thus, the variant is most likely pathogenic, and this assessment does not contradict the ClinVar status, which currently has no entry for this change. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.801317 | Disordered | 0.944705 | Binding | 0.323 | 0.811 | 0.625 | -3.208 | Likely Benign | 0.800 | Likely Pathogenic | Ambiguous | 0.362 | Likely Benign | 0.1424 | 0.7116 | -3.80 | Deleterious | 0.977 | Probably Damaging | 0.856 | Possibly Damaging | 2.45 | Pathogenic | 0.00 | Affected | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||
| c.304T>A | L102M 2D  AIThe SynGAP1 missense variant L102M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect for the variant, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -5.033 | Likely Benign | 0.085 | Likely Benign | Likely Benign | 0.129 | Likely Benign | 0.1091 | 0.4176 | 0.12 | Neutral | 0.984 | Probably Damaging | 0.969 | Probably Damaging | 4.14 | Benign | 0.00 | Affected | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||
| c.304T>G | L102V 2D  AIThe SynGAP1 missense variant L102V is listed in ClinVar (ID 1925749.0) with an “Uncertain” status and is present in gnomAD (6‑33432169‑T‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This consensus does not contradict the ClinVar “Uncertain” classification, which remains inconclusive. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | Uncertain | 1 | 6-33432169-T-G | 1 | 6.20e-7 | -4.316 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.102 | Likely Benign | 0.1683 | 0.3671 | 0.32 | Neutral | 0.880 | Possibly Damaging | 0.899 | Possibly Damaging | 4.21 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 1 | 0.4 | -14.03 | ||||||||||||||||||||||||||||
| c.3050T>C | F1017S 2D  AIThe SynGAP1 missense variant F1017S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus remains Likely Pathogenic; Foldetta results are unavailable. Overall, the majority of evidence points to a pathogenic impact. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.889439 | Disordered | 0.954171 | Binding | 0.322 | 0.801 | 0.625 | -1.804 | Likely Benign | 0.782 | Likely Pathogenic | Likely Benign | 0.114 | Likely Benign | 0.4491 | 0.0000 | -3.16 | Deleterious | 0.986 | Probably Damaging | 0.848 | Possibly Damaging | 2.46 | Pathogenic | 0.00 | Affected | -3 | -2 | -3.6 | -60.10 | |||||||||||||||||||||||||||||||||||
| c.3050T>G | F1017C 2D  AIThe SynGAP1 missense variant F1017C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as Likely Pathogenic, and the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a pathogenic impact. This conclusion is not contradicted by ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.889439 | Disordered | 0.954171 | Binding | 0.322 | 0.801 | 0.625 | -5.769 | Likely Benign | 0.706 | Likely Pathogenic | Likely Benign | 0.133 | Likely Benign | 0.2488 | 0.1137 | -3.71 | Deleterious | 0.999 | Probably Damaging | 0.944 | Probably Damaging | 2.42 | Pathogenic | 0.00 | Affected | -4 | -2 | -0.3 | -44.04 | |||||||||||||||||||||||||||||||||||
| c.3055C>G | R1019G 2D  AIThe SynGAP1 missense variant R1019G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that indicate a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, whereas tools predicting a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as pathogenic, and the Foldetta stability analysis is unavailable. Overall, the majority of predictions support a pathogenic impact, and this conclusion is not contradicted by any ClinVar annotation (none is available). Thus, the variant is most likely pathogenic based on the current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.856457 | Disordered | 0.966400 | Binding | 0.315 | 0.794 | 0.500 | -4.325 | Likely Benign | 0.614 | Likely Pathogenic | Likely Benign | 0.115 | Likely Benign | 0.2946 | 0.3489 | -3.34 | Deleterious | 0.800 | Possibly Damaging | 0.496 | Possibly Damaging | 2.39 | Pathogenic | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||
| c.3055C>T | R1019C 2D  AIThe SynGAP1 missense variant R1019C is listed in ClinVar with an “Uncertain” status (ClinVar ID 1676922.0) and is present in gnomAD (ID 6‑33443607‑C‑T). Prediction tools that agree on a benign effect include REVEL and AlphaMissense‑Optimized, whereas a majority of tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default) predict a pathogenic impact; ESM1b remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Pathogenic.” High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM‑Consensus (majority vote) remains pathogenic; Foldetta results are unavailable. Overall, the preponderance of evidence points to a pathogenic effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.856457 | Disordered | 0.966400 | Binding | 0.315 | 0.794 | 0.500 | Conflicting | 2 | 6-33443607-C-T | 10 | 6.19e-6 | -7.386 | In-Between | 0.646 | Likely Pathogenic | Likely Benign | 0.168 | Likely Benign | 0.3016 | 0.3664 | -4.00 | Deleterious | 0.999 | Probably Damaging | 0.880 | Possibly Damaging | 2.36 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -4 | -3 | 7.0 | -53.05 | 10.1016/j.ajhg.2020.11.011 | |||||||||||||||||||||||||||
| c.3058C>G | R1020G 2D  AIThe SynGAP1 missense variant R1020G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas a majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default all indicate pathogenicity. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is unavailable. Based on the preponderance of pathogenic predictions, the variant is most likely pathogenic, and this assessment does not contradict any ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.852992 | Disordered | 0.972945 | Binding | 0.340 | 0.777 | 0.500 | -4.898 | Likely Benign | 0.868 | Likely Pathogenic | Ambiguous | 0.162 | Likely Benign | 0.3394 | 0.3721 | -4.26 | Deleterious | 0.990 | Probably Damaging | 0.894 | Possibly Damaging | 2.48 | Pathogenic | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||
| c.3058C>T | R1020W 2D  AIThe SynGAP1 missense variant R1020W is catalogued in gnomAD (ID 6‑33443610‑C‑T) but has no ClinVar entry. Functional prediction tools cluster into two groups: the single benign predictor REVEL, and a consensus of pathogenic predictions from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is uncertain, SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Pathogenic,” and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) is not available. Taken together, the preponderance of evidence indicates that R1020W is most likely pathogenic, and this conclusion does not contradict any ClinVar classification because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.852992 | Disordered | 0.972945 | Binding | 0.340 | 0.777 | 0.500 | 6-33443610-C-T | 6 | 3.72e-6 | -9.378 | Likely Pathogenic | 0.829 | Likely Pathogenic | Ambiguous | 0.165 | Likely Benign | 0.1420 | 0.4373 | -4.14 | Deleterious | 1.000 | Probably Damaging | 0.986 | Probably Damaging | 2.44 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -3 | 2 | 3.6 | 30.03 | ||||||||||||||||||||||||||||||
| c.3059G>A | R1020Q 2D  AIThe SynGAP1 missense variant R1020Q is catalogued in gnomAD (6‑33443611‑G‑A) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), while pathogenic predictions arise from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default remains uncertain. High‑accuracy assessments reinforce the benign trend: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus also indicates “Likely Benign.” No Foldetta stability analysis is available, so it does not influence the overall assessment. Based on the collective predictions, the variant is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.852992 | Disordered | 0.972945 | Binding | 0.340 | 0.777 | 0.500 | 6-33443611-G-A | 2 | 1.24e-6 | -3.753 | Likely Benign | 0.445 | Ambiguous | Likely Benign | 0.137 | Likely Benign | 0.3050 | 0.2997 | -2.19 | Neutral | 0.995 | Probably Damaging | 0.870 | Possibly Damaging | 2.52 | Benign | 0.00 | Affected | 3.77 | 5 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||||||||||||
| c.3059G>C | R1020P 2D  AIThe SynGAP1 missense variant R1020P is listed in ClinVar (ID 3700393.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and ESM1b, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves as “Likely Pathogenic” (3 pathogenic vs. 1 benign votes). High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain,” SGM‑Consensus as “Likely Pathogenic,” and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the majority of evidence points to a pathogenic effect, and this conclusion does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.852992 | Disordered | 0.972945 | Binding | 0.340 | 0.777 | 0.500 | Uncertain | 1 | -3.491 | Likely Benign | 0.902 | Likely Pathogenic | Ambiguous | 0.205 | Likely Benign | 0.2077 | 0.5109 | -3.50 | Deleterious | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 2.46 | Pathogenic | 0.00 | Affected | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||
| c.3059G>T | R1020L 2D  AISynGAP1 missense variant R1020L is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and FATHMM, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and the SGM consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive; Foldetta stability analysis is unavailable. Overall, the majority of evidence points toward a pathogenic effect, which contrasts with the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.852992 | Disordered | 0.972945 | Binding | 0.340 | 0.777 | 0.500 | Uncertain | 1 | -6.031 | Likely Benign | 0.907 | Likely Pathogenic | Ambiguous | 0.216 | Likely Benign | 0.1898 | 0.5214 | -4.03 | Deleterious | 0.990 | Probably Damaging | 0.921 | Probably Damaging | 2.50 | Benign | 0.00 | Affected | 3.77 | 5 | -3 | -2 | 8.3 | -43.03 | ||||||||||||||||||||||||||||||||
| c.305T>C | L102S 2D  AIThe SynGAP1 missense variant L102S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus likewise indicates Likely Benign. No Foldetta stability analysis is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign impact for the L102S variant, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -3.260 | Likely Benign | 0.105 | Likely Benign | Likely Benign | 0.174 | Likely Benign | 0.2785 | 0.1752 | 0.54 | Neutral | 0.984 | Probably Damaging | 0.969 | Probably Damaging | 4.18 | Benign | 0.00 | Affected | -3 | -2 | -4.6 | -26.08 | |||||||||||||||||||||||||||||||||||
| c.305T>G | L102W 2D  AIThe SynGAP1 missense variant L102W is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized returns Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability prediction is available. Overall, the majority of evidence points to a benign effect for L102W, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -5.833 | Likely Benign | 0.202 | Likely Benign | Likely Benign | 0.125 | Likely Benign | 0.0821 | 0.3078 | -1.42 | Neutral | 0.996 | Probably Damaging | 0.984 | Probably Damaging | 4.09 | Benign | 0.00 | Affected | -2 | -2 | -4.7 | 73.05 | |||||||||||||||||||||||||||||||||||
| c.3065T>A | L1022H 2D  AIThe SynGAP1 missense variant L1022H is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized predicts a benign outcome, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie (2 pathogenic, 2 benign) and is therefore inconclusive. Foldetta, which would provide a protein‑folding stability estimate, has no available result for this variant. Overall, the majority of conventional predictors lean toward pathogenicity, whereas the single high‑accuracy tool predicts benign and the consensus is unresolved. Thus, the variant is most likely pathogenic based on the current predictions, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.859585 | Disordered | 0.986981 | Binding | 0.339 | 0.752 | 0.500 | -2.473 | Likely Benign | 0.589 | Likely Pathogenic | Likely Benign | 0.140 | Likely Benign | 0.1165 | 0.1313 | -2.21 | Neutral | 0.999 | Probably Damaging | 0.944 | Probably Damaging | 2.49 | Pathogenic | 0.00 | Affected | -2 | -3 | -7.0 | 23.98 | ||||||||||||||||||||||||||||||||||||
| c.306G>C | L102F 2D  AIThe SynGAP1 missense variant L102F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -4.712 | Likely Benign | 0.094 | Likely Benign | Likely Benign | 0.144 | Likely Benign | 0.0863 | 0.3205 | -0.80 | Neutral | 0.984 | Probably Damaging | 0.969 | Probably Damaging | 4.13 | Benign | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.306G>T | L102F 2D  AIThe SynGAP1 missense variant L102F is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.696014 | Binding | 0.357 | 0.885 | 0.625 | -4.712 | Likely Benign | 0.094 | Likely Benign | Likely Benign | 0.153 | Likely Benign | 0.0863 | 0.3205 | -0.80 | Neutral | 0.984 | Probably Damaging | 0.969 | Probably Damaging | 4.13 | Benign | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.3076G>C | D1026H 2D  AIThe SynGAP1 missense variant D1026H is not reported in ClinVar (ClinVar status: none) but is present in gnomAD (ID 6‑33443628‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive because it yields a 2‑to‑2 split. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (five pathogenic vs. three benign) lean toward a pathogenic impact. Thus, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.894241 | Disordered | 0.993931 | Binding | 0.324 | 0.739 | 0.500 | 6-33443628-G-C | 1 | 6.20e-7 | -4.412 | Likely Benign | 0.900 | Likely Pathogenic | Ambiguous | 0.105 | Likely Benign | 0.1470 | 0.5345 | -2.03 | Neutral | 0.832 | Possibly Damaging | 0.600 | Possibly Damaging | 2.48 | Pathogenic | 0.00 | Affected | 3.77 | 5 | -1 | 1 | 0.3 | 22.05 | |||||||||||||||||||||||||||||||
| c.3076G>T | D1026Y 2D  AIThe SynGAP1 missense variant D1026Y is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the majority of tools predict a pathogenic impact: PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default all classify the variant as damaging. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, SGM‑Consensus as Likely Pathogenic, and Foldetta results are unavailable. Based on the preponderance of pathogenic predictions and the SGM‑Consensus outcome, the variant is most likely pathogenic; this conclusion is not contradicted by any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.894241 | Disordered | 0.993931 | Binding | 0.324 | 0.739 | 0.500 | -6.999 | Likely Benign | 0.892 | Likely Pathogenic | Ambiguous | 0.200 | Likely Benign | 0.0676 | 0.4936 | -3.08 | Deleterious | 0.938 | Possibly Damaging | 0.596 | Possibly Damaging | 2.47 | Pathogenic | 0.00 | Affected | -4 | -3 | 2.2 | 48.09 | |||||||||||||||||||||||||||||||||||
| c.3077A>T | D1026V 2D  AIThe SynGAP1 missense variant D1026V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and ESM1b. Tools that predict a pathogenic effect are PROVEAN, SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy consensus, SGM‑Consensus, is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN and therefore also indicates a likely pathogenic outcome. AlphaMissense‑Optimized is uncertain, and no Foldetta stability result is available, so it does not contribute evidence. Overall, the majority of reliable predictors classify the variant as pathogenic, and this assessment does not contradict any ClinVar annotation because none exists. Thus, the variant is most likely pathogenic. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.894241 | Disordered | 0.993931 | Binding | 0.324 | 0.739 | 0.500 | -5.871 | Likely Benign | 0.900 | Likely Pathogenic | Ambiguous | 0.144 | Likely Benign | 0.0957 | 0.5236 | -3.13 | Deleterious | 0.004 | Benign | 0.004 | Benign | 2.48 | Pathogenic | 0.00 | Affected | -2 | -3 | 7.7 | -15.96 | |||||||||||||||||||||||||||||||||||
| c.307G>A | G103S 2D  AIThe SynGAP1 missense variant G103S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.687376 | Binding | 0.381 | 0.877 | 0.625 | -3.177 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.072 | Likely Benign | 0.2816 | 0.4867 | -0.03 | Neutral | 0.565 | Possibly Damaging | 0.207 | Benign | 4.32 | Benign | 0.00 | Affected | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||||
| c.307G>C | G103R 2D  AIThe SynGAP1 missense variant G103R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.687376 | Binding | 0.381 | 0.877 | 0.625 | -3.384 | Likely Benign | 0.710 | Likely Pathogenic | Likely Benign | 0.100 | Likely Benign | 0.1198 | 0.4362 | 0.00 | Neutral | 0.949 | Possibly Damaging | 0.708 | Possibly Damaging | 4.27 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.307G>T | G103C 2D  AIThe SynGAP1 missense variant G103C is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability result is available. Overall, the majority of evidence points to a benign effect for G103C, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.687376 | Binding | 0.381 | 0.877 | 0.625 | -5.681 | Likely Benign | 0.136 | Likely Benign | Likely Benign | 0.128 | Likely Benign | 0.1494 | 0.3767 | -1.12 | Neutral | 0.995 | Probably Damaging | 0.829 | Possibly Damaging | 4.17 | Benign | 0.00 | Affected | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||
| c.308G>A | G103D 2D  AIThe SynGAP1 missense variant G103D is not reported in ClinVar and has no entry in gnomAD. Consensus from multiple in‑silico predictors shows a predominance of benign calls: REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized all predict a benign effect, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT each predict a pathogenic impact. The AlphaMissense‑Default tool remains uncertain, and no Foldetta stability assessment is available. High‑accuracy tools specifically highlight benign predictions: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, and Foldetta data are missing. Overall, the balance of evidence points to a benign effect for G103D, and this assessment does not contradict the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.687376 | Binding | 0.381 | 0.877 | 0.625 | -3.523 | Likely Benign | 0.477 | Ambiguous | Likely Benign | 0.110 | Likely Benign | 0.2237 | 0.2896 | -0.10 | Neutral | 0.949 | Possibly Damaging | 0.617 | Possibly Damaging | 4.26 | Benign | 0.00 | Affected | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||
| c.308G>C | G103A 2D  AIThe SynGAP1 missense variant G103A is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates a benign outcome. Foldetta results are not available, so they do not influence the assessment. Overall, the majority of evidence points to a benign impact, and this conclusion is consistent with the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.687376 | Binding | 0.381 | 0.877 | 0.625 | -3.549 | Likely Benign | 0.092 | Likely Benign | Likely Benign | 0.118 | Likely Benign | 0.3941 | 0.3784 | -0.08 | Neutral | 0.008 | Benign | 0.008 | Benign | 4.31 | Benign | 0.00 | Affected | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.308G>T | G103V 2D  AIThe SynGAP1 missense variant G103V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.687376 | Binding | 0.381 | 0.877 | 0.625 | -3.584 | Likely Benign | 0.151 | Likely Benign | Likely Benign | 0.126 | Likely Benign | 0.1420 | 0.3404 | -0.96 | Neutral | 0.820 | Possibly Damaging | 0.376 | Benign | 4.22 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.310C>A | R104S 2D  AIThe SynGAP1 missense variant R104S has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; no Foldetta stability result is available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.678998 | Binding | 0.339 | 0.869 | 0.625 | -2.790 | Likely Benign | 0.771 | Likely Pathogenic | Likely Benign | 0.143 | Likely Benign | 0.2490 | 0.3806 | -0.23 | Neutral | 0.625 | Possibly Damaging | 0.118 | Benign | 4.13 | Benign | 0.00 | Affected | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||
| c.310C>G | R104G 2D  AIThe SynGAP1 missense variant R104G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for R104G, and this conclusion is not contradicted by any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.678998 | Binding | 0.339 | 0.869 | 0.625 | -2.373 | Likely Benign | 0.452 | Ambiguous | Likely Benign | 0.109 | Likely Benign | 0.3111 | 0.3625 | -0.90 | Neutral | 0.835 | Possibly Damaging | 0.165 | Benign | 4.03 | Benign | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||
| c.310C>T | R104C 2D  AIThe SynGAP1 missense variant R104C has no ClinVar entry and is present in gnomAD (ID 6‑33432175‑C‑T). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.678998 | Binding | 0.339 | 0.869 | 0.625 | 6-33432175-C-T | 2 | 1.24e-6 | -5.716 | Likely Benign | 0.475 | Ambiguous | Likely Benign | 0.109 | Likely Benign | 0.2954 | 0.3292 | -1.41 | Neutral | 0.993 | Probably Damaging | 0.446 | Benign | 3.99 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -4 | 7.0 | -53.05 | 10.1016/j.ajhg.2020.11.011 | |||||||||||||||||||||||||||||
| c.3118G>C | G1040R 2D  AIThe SynGAP1 missense variant G1040R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely converge on a deleterious effect: pathogenic calls are made by REVEL, PROVEAN, polyPhen‑2 HumDiv, SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Benign predictions are limited to polyPhen‑2 HumVar and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, while the SGM‑Consensus remains pathogenic. Foldetta, a protein‑folding stability predictor, has no available result for this residue. Taken together, the majority of evidence supports a pathogenic classification, and this conclusion does not conflict with the absence of a ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.964893 | Disordered | 0.973805 | Binding | 0.332 | 0.816 | 0.625 | -2.901 | Likely Benign | 0.949 | Likely Pathogenic | Ambiguous | 0.704 | Likely Pathogenic | 0.0924 | 0.4415 | -3.00 | Deleterious | 0.463 | Possibly Damaging | 0.194 | Benign | -0.74 | Pathogenic | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.3118G>T | G1040C 2D  AIThe SynGAP1 missense variant G1040C is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a deleterious effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default all predict pathogenicity, while ESM1b and AlphaMissense‑Optimized predict a benign outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Pathogenic. High‑accuracy assessments further support this view: AlphaMissense‑Optimized reports a benign prediction, whereas the SGM‑Consensus remains Likely Pathogenic; the Foldetta stability analysis is unavailable. Overall, the preponderance of evidence from multiple in silico tools indicates that the variant is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.964893 | Disordered | 0.973805 | Binding | 0.332 | 0.816 | 0.625 | -6.272 | Likely Benign | 0.620 | Likely Pathogenic | Likely Benign | 0.744 | Likely Pathogenic | 0.1155 | 0.4556 | -3.04 | Deleterious | 0.999 | Probably Damaging | 0.917 | Probably Damaging | -0.74 | Pathogenic | 0.00 | Affected | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||
| c.3119G>A | G1040D 2D  AIThe SynGAP1 missense variant G1040D is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include only ESM1b, whereas the remaining tools—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus—predict a pathogenic impact. The high‑accuracy assessments further support this: AlphaMissense‑Optimized classifies the variant as pathogenic, the SGM‑Consensus (a majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) labels it likely pathogenic, and the Foldetta stability analysis is unavailable. Overall, the preponderance of evidence indicates that G1040D is most likely pathogenic, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.964893 | Disordered | 0.973805 | Binding | 0.332 | 0.816 | 0.625 | -4.154 | Likely Benign | 0.956 | Likely Pathogenic | Likely Pathogenic | 0.761 | Likely Pathogenic | 0.1677 | 0.2042 | -2.82 | Deleterious | 0.986 | Probably Damaging | 0.787 | Possibly Damaging | -0.74 | Pathogenic | 0.00 | Affected | 1 | -1 | -3.1 | 58.04 | |||||||||||||||||||||||||||||||||||
| c.311G>A | R104H 2D  AIThe SynGAP1 missense variant R104H is reported in gnomAD (variant ID 6‑33432176‑G‑A) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts it to be pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a “Likely Benign” verdict. High‑accuracy assessments reinforce this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote) is also benign. Foldetta results are not available for this variant. Overall, the preponderance of evidence indicates that R104H is most likely benign, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.678998 | Binding | 0.339 | 0.869 | 0.625 | 6-33432176-G-A | 2 | 1.24e-6 | -4.126 | Likely Benign | 0.268 | Likely Benign | Likely Benign | 0.094 | Likely Benign | 0.2431 | 0.2102 | -1.42 | Neutral | 0.066 | Benign | 0.004 | Benign | 4.02 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 2 | 1.3 | -19.05 | ||||||||||||||||||||||||||||||
| c.311G>C | R104P 2D  AIThe SynGAP1 missense variant R104P is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) classifies the variant as Likely Benign, and AlphaMissense‑Optimized also predicts Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.678998 | Binding | 0.339 | 0.869 | 0.625 | -3.184 | Likely Benign | 0.510 | Ambiguous | Likely Benign | 0.200 | Likely Benign | 0.1759 | 0.4498 | -0.88 | Neutral | 0.947 | Possibly Damaging | 0.410 | Benign | 4.01 | Benign | 0.00 | Affected | 0 | -2 | 2.9 | -59.07 | |||||||||||||||||||||||||||||||||||
| c.311G>T | R104L 2D  AIThe SynGAP1 missense variant R104L is listed in ClinVar (ID 2746314.0) as Benign and is present in gnomAD (6‑33432176‑G‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized result is benign, and the SGM‑Consensus (majority vote) is also benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the ClinVar benign classification and does not contradict the existing clinical annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.795062 | Disordered | 0.678998 | Binding | 0.339 | 0.869 | 0.625 | Benign | 1 | 6-33432176-G-T | 1 | 6.20e-7 | -3.563 | Likely Benign | 0.578 | Likely Pathogenic | Likely Benign | 0.170 | Likely Benign | 0.1681 | 0.4894 | -1.38 | Neutral | 0.001 | Benign | 0.002 | Benign | 4.05 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -3 | 8.3 | -43.03 | ||||||||||||||||||||||||||||
| c.3127A>G | R1043G 2D  AIThe SynGAP1 missense variant R1043G is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.954069 | Binding | 0.299 | 0.853 | 0.625 | -2.168 | Likely Benign | 0.201 | Likely Benign | Likely Benign | 0.383 | Likely Benign | 0.3179 | 0.3697 | -3.17 | Deleterious | 0.130 | Benign | 0.049 | Benign | 5.94 | Benign | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | |||||||||||||||||||||||||||||||||||
| c.3127A>T | R1043W 2D  AIThe SynGAP1 missense variant R1043W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a benign prediction; Foldetta results are unavailable. Overall, the majority of high‑confidence tools predict a benign impact, and there is no ClinVar annotation to contradict this assessment. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.978672 | Disordered | 0.954069 | Binding | 0.299 | 0.853 | 0.625 | -6.382 | Likely Benign | 0.523 | Ambiguous | Likely Benign | 0.444 | Likely Benign | 0.1403 | 0.3696 | -3.43 | Deleterious | 0.971 | Probably Damaging | 0.729 | Possibly Damaging | 5.38 | Benign | 0.00 | Affected | 2 | -3 | 3.6 | 30.03 | ||||||||||||||||||||||||||||||||||||
| c.3128G>A | R1043K 2D  AIThe SynGAP1 missense variant R1043K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect, and this is consistent with the lack of ClinVar or gnomAD entries—there is no contradiction with existing database status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.954069 | Binding | 0.299 | 0.853 | 0.625 | -4.038 | Likely Benign | 0.162 | Likely Benign | Likely Benign | 0.376 | Likely Benign | 0.5141 | 0.4070 | Weaken | -1.41 | Neutral | 0.069 | Benign | 0.033 | Benign | 5.39 | Benign | 0.00 | Affected | 3 | 2 | 0.6 | -28.01 | ||||||||||||||||||||||||||||||||||
| c.3128G>C | R1043T 2D  AIThe SynGAP1 missense variant R1043T is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, while the single pathogenic prediction comes from SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments confirm this: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.954069 | Binding | 0.299 | 0.853 | 0.625 | -3.928 | Likely Benign | 0.298 | Likely Benign | Likely Benign | 0.463 | Likely Benign | 0.1975 | 0.5513 | -1.77 | Neutral | 0.001 | Benign | 0.003 | Benign | 5.39 | Benign | 0.00 | Affected | -1 | -1 | 3.8 | -55.08 | |||||||||||||||||||||||||||||||||||
| c.3128G>T | R1043M 2D  AIThe SynGAP1 missense variant R1043M is not reported in ClinVar and has no entries in gnomAD, indicating it is not catalogued in these databases. Consensus predictions from multiple in‑silico tools cluster around a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized all predict benign, while polyPhen‑2 HumDiv and SIFT predict pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores the variant as benign, and the SGM‑Consensus also indicates Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for R1043M, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.954069 | Binding | 0.299 | 0.853 | 0.625 | -4.800 | Likely Benign | 0.510 | Ambiguous | Likely Benign | 0.471 | Likely Benign | 0.1982 | 0.4468 | -1.98 | Neutral | 0.744 | Possibly Damaging | 0.229 | Benign | 5.38 | Benign | 0.00 | Affected | 0 | -1 | 6.4 | -24.99 | |||||||||||||||||||||||||||||||||||
| c.3129G>C | R1043S 2D  AIThe SynGAP1 missense variant R1043S is not reported in ClinVar and has no entry in gnomAD. Functional prediction tools cluster into two groups: benign predictions include PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from REVEL and SIFT. AlphaMissense‑Default is uncertain, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign effect. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized scores benign, SGM‑Consensus is likely benign, and Foldetta results are unavailable. Taken together, the majority of evidence points to a benign impact for R1043S. This conclusion is consistent with the absence of a ClinVar assertion, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.954069 | Binding | 0.299 | 0.853 | 0.625 | -3.223 | Likely Benign | 0.457 | Ambiguous | Likely Benign | 0.509 | Likely Pathogenic | 0.2727 | 0.4628 | -2.10 | Neutral | 0.036 | Benign | 0.018 | Benign | 5.42 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||
| c.3129G>T | R1043S 2D  AIThe SynGAP1 missense variant R1043S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443681‑G‑T). Prediction tools that agree on a benign effect include PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are REVEL and SIFT. AlphaMissense‑Default remains uncertain, and no Foldetta stability result is available. High‑accuracy assessments: AlphaMissense‑Optimized classifies the variant as benign; the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome; Foldetta data are missing, so it does not contribute to the evaluation. Based on the collective predictions, the variant is most likely benign, which is consistent with its ClinVar “Uncertain” classification and does not contradict the available evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.954069 | Binding | 0.299 | 0.853 | 0.625 | Uncertain | 1 | 6-33443681-G-T | 2 | 1.24e-6 | -3.223 | Likely Benign | 0.457 | Ambiguous | Likely Benign | 0.509 | Likely Pathogenic | 0.2727 | 0.4628 | -2.10 | Neutral | 0.036 | Benign | 0.018 | Benign | 5.42 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | 3.7 | -69.11 | ||||||||||||||||||||||||||||
| c.313T>A | S105T 2D  AIThe SynGAP1 missense variant S105T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The high‑accuracy consensus, SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a benign effect. AlphaMissense‑Optimized independently scores the variant as benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this assessment does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.669201 | Binding | 0.364 | 0.870 | 0.625 | -4.166 | Likely Benign | 0.132 | Likely Benign | Likely Benign | 0.055 | Likely Benign | 0.1583 | 0.5212 | -0.54 | Neutral | 0.012 | Benign | 0.007 | Benign | 4.06 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.313T>C | S105P 2D  AIThe SynGAP1 missense variant S105P is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only two tools—polyPhen‑2 HumDiv and SIFT—predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as likely benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates likely benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the preponderance of evidence points to a benign effect, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.669201 | Binding | 0.364 | 0.870 | 0.625 | Uncertain | 1 | -3.631 | Likely Benign | 0.166 | Likely Benign | Likely Benign | 0.204 | Likely Benign | 0.2236 | 0.4584 | 0.03 | Neutral | 0.808 | Possibly Damaging | 0.212 | Benign | 4.00 | Benign | 0.00 | Affected | 4.32 | 1 | -1 | 1 | -0.8 | 10.04 | |||||||||||||||||||||||||||||||
| c.313T>G | S105A 2D  AIThe SynGAP1 missense variant S105A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect. The predictions do not contradict ClinVar status, as no ClinVar classification exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.669201 | Binding | 0.364 | 0.870 | 0.625 | -3.779 | Likely Benign | 0.115 | Likely Benign | Likely Benign | 0.046 | Likely Benign | 0.5299 | 0.4214 | Weaken | -0.67 | Neutral | 0.012 | Benign | 0.002 | Benign | 4.11 | Benign | 0.00 | Affected | 1 | 1 | 2.6 | -16.00 | ||||||||||||||||||||||||||||||||||
| c.314C>G | S105W 2D  AIThe SynGAP1 missense variant S105W is catalogued in gnomAD (ID 6‑33432179‑C‑G) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, while pathogenic predictions arise from polyPhen‑2 (HumDiv and HumVar), SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” status, reflecting the majority of benign calls. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also leans benign. No Foldetta stability data are available, so it does not influence the assessment. Overall, the preponderance of evidence indicates that the variant is most likely benign, and this conclusion is not contradicted by any ClinVar classification (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.669201 | Binding | 0.364 | 0.870 | 0.625 | 6-33432179-C-G | 2 | 1.24e-6 | -5.600 | Likely Benign | 0.606 | Likely Pathogenic | Likely Benign | 0.177 | Likely Benign | 0.0705 | 0.4984 | -2.28 | Neutral | 0.998 | Probably Damaging | 0.844 | Possibly Damaging | 3.97 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -2 | -0.1 | 99.14 | ||||||||||||||||||||||||||||||
| c.314C>T | S105L 2D  AIThe SynGAP1 missense variant S105L is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33432179‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. High‑accuracy methods both support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote) is benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.788093 | Disordered | 0.669201 | Binding | 0.364 | 0.870 | 0.625 | Uncertain | 2 | 6-33432179-C-T | 4 | 2.48e-6 | -3.710 | Likely Benign | 0.233 | Likely Benign | Likely Benign | 0.095 | Likely Benign | 0.1161 | 0.5023 | -1.52 | Neutral | 0.828 | Possibly Damaging | 0.048 | Benign | 4.06 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -2 | 4.6 | 26.08 | ||||||||||||||||||||||||||||
| c.316A>G | R106G 2D  AIThe SynGAP1 missense variant R106G is listed in gnomAD (ID 6‑33432181‑A‑G) but has no ClinVar entry. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Those that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. There is no ClinVar classification to contradict this assessment. Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.663409 | Binding | 0.345 | 0.862 | 0.875 | 6-33432181-A-G | 1 | 6.20e-7 | -2.617 | Likely Benign | 0.835 | Likely Pathogenic | Ambiguous | 0.161 | Likely Benign | 0.3680 | 0.3980 | -2.21 | Neutral | 0.421 | Benign | 0.050 | Benign | 3.65 | Benign | 0.00 | Affected | 4.05 | 3 | -2 | -3 | 4.1 | -99.14 | ||||||||||||||||||||||||||||||
| c.316A>T | R106W 2D  AIThe SynGAP1 missense variant R106W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM, while those that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is a 2‑vs‑2 tie, and Foldetta results are unavailable. Overall, more tools predict pathogenicity (5) than benign (3), and no ClinVar evidence contradicts this assessment. Thus, the variant is most likely pathogenic based on the available predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.675549 | Disordered | 0.663409 | Binding | 0.345 | 0.862 | 0.875 | -5.350 | Likely Benign | 0.875 | Likely Pathogenic | Ambiguous | 0.240 | Likely Benign | 0.1369 | 0.3995 | -3.31 | Deleterious | 0.983 | Probably Damaging | 0.624 | Possibly Damaging | 3.62 | Benign | 0.00 | Affected | 2 | -3 | 3.6 | 30.03 | ||||||||||||||||||||||||||||||||||||
| c.3172G>C | G1058R 2D  AIThe SynGAP1 missense variant G1058R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only two predictors—SIFT and ESM1b—suggest a pathogenic impact. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign consensus. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not conflict with the absence of a ClinVar assertion. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.980739 | Disordered | 0.885724 | Binding | 0.407 | 0.929 | 0.875 | -8.967 | Likely Pathogenic | 0.339 | Likely Benign | Likely Benign | 0.138 | Likely Benign | 0.1145 | 0.4342 | 0.34 | Neutral | 0.174 | Benign | 0.140 | Benign | 5.29 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||||
| c.3175G>A | G1059R 2D  AIThe SynGAP1 missense variant G1059R is listed in ClinVar with an “Uncertain” significance and is present in the gnomAD database (ID 6‑33443727‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and ESM1b, while AlphaMissense‑Default remains uncertain. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves to a benign prediction (2 benign vs. 1 pathogenic). High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM Consensus also benign; Foldetta’s protein‑folding stability analysis is unavailable for this variant. Overall, the collective evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.983019 | Disordered | 0.898939 | Binding | 0.399 | 0.926 | 0.875 | Uncertain | 1 | 6-33443727-G-A | 68 | 4.23e-5 | -8.452 | Likely Pathogenic | 0.376 | Ambiguous | Likely Benign | 0.333 | Likely Benign | 0.1052 | 0.4342 | -0.55 | Neutral | 0.001 | Benign | 0.001 | Benign | 2.53 | Benign | 0.00 | Affected | 4.32 | 2 | -3 | -2 | -4.1 | 99.14 | |||||||||||||||||||||||||||||
| c.3175G>C | G1059R 2D  AIThe SynGAP1 missense variant G1059R is not reported in ClinVar and is absent from gnomAD. Consensus from multiple in silico predictors shows a predominance of benign calls: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, and AlphaMissense‑Optimized all predict a benign effect, whereas SIFT and ESM1b predict pathogenicity; AlphaMissense‑Default remains uncertain. High‑accuracy assessments reinforce this trend: AlphaMissense‑Optimized is benign, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also returns a benign prediction. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the balance of evidence points to a benign impact for G1059R, and this conclusion is consistent with the absence of any ClinVar annotation or gnomAD observation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.983019 | Disordered | 0.898939 | Binding | 0.399 | 0.926 | 0.875 | -8.452 | Likely Pathogenic | 0.376 | Ambiguous | Likely Benign | 0.333 | Likely Benign | 0.1052 | 0.4342 | -0.55 | Neutral | 0.001 | Benign | 0.001 | Benign | 2.53 | Benign | 0.00 | Affected | 4.32 | 2 | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||
| c.3175G>T | G1059W 2D  AIThe SynGAP1 missense variant G1059W is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of a ClinVar entry, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.898939 | Binding | 0.399 | 0.926 | 0.875 | -11.549 | Likely Pathogenic | 0.312 | Likely Benign | Likely Benign | 0.446 | Likely Benign | 0.0925 | 0.4046 | -1.18 | Neutral | 0.983 | Probably Damaging | 0.813 | Possibly Damaging | 2.53 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | |||||||||||||||||||||||||||||||||||
| c.3176G>A | G1059E 2D  AIThe SynGAP1 missense variant G1059E is reported in gnomAD (variant ID 6‑33443728‑G‑A) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only two tools—SIFT and ESM1b—predict a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a “Likely Benign” verdict. High‑accuracy assessments reinforce this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.898939 | Binding | 0.399 | 0.926 | 0.875 | 6-33443728-G-A | 1 | 6.22e-7 | -10.459 | Likely Pathogenic | 0.297 | Likely Benign | Likely Benign | 0.390 | Likely Benign | 0.1464 | 0.4069 | -0.71 | Neutral | 0.126 | Benign | 0.066 | Benign | 2.53 | Benign | 0.00 | Affected | 4.32 | 2 | -2 | 0 | -3.1 | 72.06 | ||||||||||||||||||||||||||||||
| c.3176G>C | G1059A 2D  AIThe SynGAP1 missense variant G1059A is listed in ClinVar (ID 1420036.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33443728‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of computational evidence supports a benign impact for G1059A, which does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.898939 | Binding | 0.399 | 0.926 | 0.875 | Uncertain | 1 | 6-33443728-G-C | 4 | 2.49e-6 | -6.754 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.329 | Likely Benign | 0.3295 | 0.4944 | -0.17 | Neutral | 0.001 | Benign | 0.002 | Benign | 2.56 | Benign | 0.00 | Affected | 4.32 | 2 | 1 | 0 | 2.2 | 14.03 | ||||||||||||||||||||||||||||
| c.3176G>T | G1059V 2D  AIThe SynGAP1 missense variant G1059V is not reported in ClinVar and is absent from gnomAD. Consensus from multiple in silico predictors indicates a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all score benign, while the majority‑vote SGM‑Consensus also classifies it as likely benign. Only SIFT predicts a pathogenic outcome, and ESM1b remains uncertain. High‑accuracy tools corroborate the benign prediction: AlphaMissense‑Optimized is benign and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is likely benign; Foldetta results are not available. Overall, the preponderance of evidence supports a benign classification for G1059V, and this assessment does not conflict with ClinVar, which currently contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.983019 | Disordered | 0.898939 | Binding | 0.399 | 0.926 | 0.875 | -7.242 | In-Between | 0.106 | Likely Benign | Likely Benign | 0.478 | Likely Benign | 0.1462 | 0.3494 | -0.82 | Neutral | 0.259 | Benign | 0.066 | Benign | 2.54 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.317G>A | R106K 2D  AIThe SynGAP1 missense variant R106K is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, SGM‑Consensus indicates Likely Benign, and Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect for R106K, and this conclusion does not contradict the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.663409 | Binding | 0.345 | 0.862 | 0.875 | -4.312 | Likely Benign | 0.513 | Ambiguous | Likely Benign | 0.150 | Likely Benign | 0.5602 | 0.4129 | Weaken | -1.25 | Neutral | 0.004 | Benign | 0.001 | Benign | 3.82 | Benign | 0.00 | Affected | 3 | 2 | 0.6 | -28.01 | ||||||||||||||||||||||||||||||||||
| c.317G>C | R106T 2D  AIThe SynGAP1 missense variant R106T is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain, and no Foldetta stability assessment is available. Separately, the high‑accuracy consensus (SGM‑Consensus) classifies the variant as Likely Benign, while AlphaMissense‑Optimized remains uncertain and Foldetta data are missing. Based on the overall pattern of predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.663409 | Binding | 0.345 | 0.862 | 0.875 | -4.197 | Likely Benign | 0.950 | Likely Pathogenic | Ambiguous | 0.229 | Likely Benign | 0.1941 | 0.4742 | -2.30 | Neutral | 0.004 | Benign | 0.002 | Benign | 3.67 | Benign | 0.00 | Affected | -1 | -1 | 3.8 | -55.08 | |||||||||||||||||||||||||||||||||||
| c.317G>T | R106M 2D  AIThe SynGAP1 missense variant R106M is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumVar, ESM1b, and FATHMM. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy methods give the following results: AlphaMissense‑Optimized predicts pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 vs 2), and Foldetta data are unavailable. Because the majority of tools (five of nine) predict pathogenicity and the most accurate predictor (AlphaMissense‑Optimized) also indicates pathogenicity, the variant is most likely pathogenic. This assessment does not contradict ClinVar status, as the variant has not yet been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.675549 | Disordered | 0.663409 | Binding | 0.345 | 0.862 | 0.875 | -4.804 | Likely Benign | 0.967 | Likely Pathogenic | Likely Pathogenic | 0.184 | Likely Benign | 0.1971 | 0.4146 | -2.65 | Deleterious | 0.940 | Possibly Damaging | 0.360 | Benign | 3.64 | Benign | 0.00 | Affected | 0 | -1 | 6.4 | -24.99 | ||||||||||||||||||||||||||||||||||||
| c.3181G>A | G1061S 2D  AIThe SynGAP1 missense variant G1061S is listed in ClinVar (ID 3571724.0) with an uncertain significance designation and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while only SIFT indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta stability analysis is unavailable. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods supports a benign classification for G1061S, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.926729 | Binding | 0.394 | 0.923 | 0.875 | Uncertain | 1 | -4.891 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.283 | Likely Benign | 0.2404 | 0.5300 | -0.68 | Neutral | 0.004 | Benign | 0.004 | Benign | 4.00 | Benign | 0.00 | Affected | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||||||
| c.3181G>C | G1061R 2D  AIThe SynGAP1 missense variant G1061R is not reported in ClinVar (ClinVar status: none) and is absent from gnomAD (gnomAD ID: none). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while ESM1b and AlphaMissense‑Default are uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive because it contains two benign and two uncertain calls, and Foldetta results are unavailable. Overall, the balance of evidence favors a benign classification. This conclusion does not contradict ClinVar status, as the variant has not been reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.978672 | Disordered | 0.926729 | Binding | 0.394 | 0.923 | 0.875 | -7.721 | In-Between | 0.343 | Ambiguous | Likely Benign | 0.315 | Likely Benign | 0.1037 | 0.4332 | -0.17 | Neutral | 0.411 | Benign | 0.132 | Benign | 3.99 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||
| c.3181G>T | G1061C 2D  AIThe SynGAP1 missense variant G1061C is listed in ClinVar (ID 536997.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33443733‑G‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; Foldetta results are not available. Overall, the majority of evidence (six benign vs. four pathogenic predictions) and the two high‑accuracy tools support a benign classification. This conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.926729 | Binding | 0.394 | 0.923 | 0.875 | Conflicting | 2 | 6-33443733-G-T | 6 | 3.73e-6 | -9.511 | Likely Pathogenic | 0.119 | Likely Benign | Likely Benign | 0.409 | Likely Benign | 0.1283 | 0.4227 | -1.46 | Neutral | 0.938 | Possibly Damaging | 0.665 | Possibly Damaging | 3.97 | Benign | 0.00 | Affected | 4.32 | 2 | -3 | -3 | 2.9 | 46.09 | ||||||||||||||||||||||||||||
| c.3182G>A | G1061D 2D  AIThe SynGAP1 missense variant G1061D is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and ESM1b, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a benign prediction (2 benign vs. 1 pathogenic, 1 uncertain). Foldetta results are unavailable. Overall, the preponderance of evidence indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.978672 | Disordered | 0.926729 | Binding | 0.394 | 0.923 | 0.875 | -9.481 | Likely Pathogenic | 0.346 | Ambiguous | Likely Benign | 0.375 | Likely Benign | 0.1671 | 0.2024 | -1.32 | Neutral | 0.224 | Benign | 0.120 | Benign | 4.01 | Benign | 0.00 | Affected | 1 | -1 | -3.1 | 58.04 | ||||||||||||||||||||||||||||||||||||
| c.3182G>C | G1061A 2D  AIThe SynGAP1 missense variant G1061A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.926729 | Binding | 0.394 | 0.923 | 0.875 | -6.328 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.244 | Likely Benign | 0.3289 | 0.5133 | -0.34 | Neutral | 0.004 | Benign | 0.002 | Benign | 4.01 | Benign | 0.00 | Affected | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.3182G>T | G1061V 2D  AIThe SynGAP1 missense variant G1061V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence indicates that G1061V is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.978672 | Disordered | 0.926729 | Binding | 0.394 | 0.923 | 0.875 | -6.709 | Likely Benign | 0.106 | Likely Benign | Likely Benign | 0.307 | Likely Benign | 0.1431 | 0.3684 | -1.41 | Neutral | 0.224 | Benign | 0.066 | Benign | 3.98 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.3184G>T | G1062W 2D  AIThe SynGAP1 missense variant G1062W is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which labels the variant as “Likely Benign.” Pathogenic predictions come from polyPhen‑2 (HumDiv and HumVar), SIFT, and ESM1b, all of which classify the change as damaging. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates likely benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the lack of ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.976962 | Disordered | 0.936972 | Binding | 0.368 | 0.917 | 0.875 | -9.667 | Likely Pathogenic | 0.315 | Likely Benign | Likely Benign | 0.401 | Likely Benign | 0.0908 | 0.4246 | -1.38 | Neutral | 0.993 | Probably Damaging | 0.890 | Possibly Damaging | 4.09 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | |||||||||||||||||||||||||||||||||||
| c.318G>C | R106S 2D  AIThe SynGAP1 missense variant R106S is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, SGM‑Consensus as benign, and the Foldetta stability analysis is unavailable. Overall, the majority of predictions lean toward a benign impact, but the high‑accuracy tools provide conflicting evidence. Thus, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.663409 | Binding | 0.345 | 0.862 | 0.875 | -2.651 | Likely Benign | 0.961 | Likely Pathogenic | Likely Pathogenic | 0.093 | Likely Benign | 0.3050 | 0.4129 | -1.87 | Neutral | 0.131 | Benign | 0.026 | Benign | 3.68 | Benign | 0.00 | Affected | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||
| c.318G>T | R106S 2D  AIThe SynGAP1 missense variant R106S is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, SGM‑Consensus as benign, and the Foldetta stability analysis is unavailable. Overall, the majority of predictions lean toward a benign impact, but the high‑accuracy tools provide conflicting evidence. Thus, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.675549 | Disordered | 0.663409 | Binding | 0.345 | 0.862 | 0.875 | -2.651 | Likely Benign | 0.961 | Likely Pathogenic | Likely Pathogenic | 0.093 | Likely Benign | 0.3050 | 0.4129 | -1.87 | Neutral | 0.131 | Benign | 0.026 | Benign | 3.68 | Benign | 0.00 | Affected | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||
| c.3193C>A | P1065T 2D  AIThe SynGAP1 missense variant P1065T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign classification. There is no ClinVar status to contradict this assessment, so the variant is most likely benign based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.979741 | Disordered | 0.959518 | Binding | 0.424 | 0.917 | 0.875 | -5.392 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.062 | Likely Benign | 0.1577 | 0.6788 | -1.23 | Neutral | 0.770 | Possibly Damaging | 0.481 | Possibly Damaging | 2.04 | Pathogenic | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.3193C>G | P1065A 2D  AIThe SynGAP1 missense variant P1065A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for P1065A, and this conclusion does not contradict any ClinVar annotation, as none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.979741 | Disordered | 0.959518 | Binding | 0.424 | 0.917 | 0.875 | -4.043 | Likely Benign | 0.054 | Likely Benign | Likely Benign | 0.050 | Likely Benign | 0.3140 | 0.5790 | -1.85 | Neutral | 0.580 | Possibly Damaging | 0.184 | Benign | 2.19 | Pathogenic | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.3193C>T | P1065S 2D  AIThe SynGAP1 missense variant P1065S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.979741 | Disordered | 0.959518 | Binding | 0.424 | 0.917 | 0.875 | -5.512 | Likely Benign | 0.076 | Likely Benign | Likely Benign | 0.041 | Likely Benign | 0.3172 | 0.6021 | -2.07 | Neutral | 0.770 | Possibly Damaging | 0.255 | Benign | 2.06 | Pathogenic | 0.00 | Affected | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||||
| c.3194C>A | P1065Q 2D  AIThe SynGAP1 missense variant P1065Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with existing clinical data. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.979741 | Disordered | 0.959518 | Binding | 0.424 | 0.917 | 0.875 | -3.928 | Likely Benign | 0.074 | Likely Benign | Likely Benign | 0.026 | Likely Benign | 0.1487 | 0.5478 | -2.44 | Neutral | 0.102 | Benign | 0.057 | Benign | 2.00 | Pathogenic | 0.00 | Affected | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||||||||||
| c.3194C>G | P1065R 2D  AIThe SynGAP1 missense variant P1065R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the preponderance of evidence from multiple independent predictors indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.979741 | Disordered | 0.959518 | Binding | 0.424 | 0.917 | 0.875 | -3.237 | Likely Benign | 0.228 | Likely Benign | Likely Benign | 0.043 | Likely Benign | 0.1439 | 0.4369 | -2.46 | Neutral | 0.102 | Benign | 0.052 | Benign | 2.00 | Pathogenic | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.3194C>T | P1065L 2D  AIThe SynGAP1 missense variant P1065L is listed in ClinVar as Benign and is present in gnomAD (ID 6‑33443746‑C‑T). Functional prediction tools cluster into two groups: benign predictions come from REVEL, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized; pathogenic predictions arise from PROVEAN, polyPhen‑2 HumDiv, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie and is therefore inconclusive. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no reported result for this variant. Overall, the balance of evidence (5 benign vs. 4 pathogenic predictions) and the high‑accuracy benign call support a benign classification, aligning with the ClinVar status and indicating no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.979741 | Disordered | 0.959518 | Binding | 0.424 | 0.917 | 0.875 | Likely Benign | 1 | 6-33443746-C-T | 14 | 8.71e-6 | -5.085 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.068 | Likely Benign | 0.2286 | 0.6922 | -2.94 | Deleterious | 0.950 | Possibly Damaging | 0.419 | Benign | 2.01 | Pathogenic | 0.00 | Affected | 4.32 | 2 | -3 | -3 | 5.4 | 16.04 | |||||||||||||||||||||||||||||
| c.3196C>A | P1066T 2D  AIThe SynGAP1 missense variant P1066T is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority vote) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of computational evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.967676 | Disordered | 0.968838 | Binding | 0.403 | 0.913 | 0.875 | -5.973 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.167 | Likely Benign | 0.1652 | 0.7240 | -2.61 | Deleterious | 0.996 | Probably Damaging | 0.928 | Probably Damaging | 2.66 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
| c.3196C>G | P1066A 2D  AIThe SynGAP1 missense variant P1066A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.967676 | Disordered | 0.968838 | Binding | 0.403 | 0.913 | 0.875 | -4.856 | Likely Benign | 0.050 | Likely Benign | Likely Benign | 0.149 | Likely Benign | 0.3228 | 0.6042 | -2.35 | Neutral | 0.972 | Probably Damaging | 0.802 | Possibly Damaging | 2.78 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | |||||||||||||||||||||||||||||||||||
| c.3196C>T | P1066S 2D  AIThe SynGAP1 missense variant P1066S is listed in ClinVar as Pathogenic (ClinVar ID 1343237.0) and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; a Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, which contradicts the ClinVar pathogenic classification. Thus, based on current predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.967676 | Disordered | 0.968838 | Binding | 0.403 | 0.913 | 0.875 | Likely Pathogenic | 1 | -4.746 | Likely Benign | 0.070 | Likely Benign | Likely Benign | 0.145 | Likely Benign | 0.3304 | 0.6353 | -2.47 | Neutral | 0.972 | Probably Damaging | 0.850 | Possibly Damaging | 2.74 | Benign | 0.00 | Affected | 4.32 | 2 | 1 | -1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||
| c.3197C>A | P1066H 2D  AIThe SynGAP1 missense variant P1066H is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none exists). Thus, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.967676 | Disordered | 0.968838 | Binding | 0.403 | 0.913 | 0.875 | -6.034 | Likely Benign | 0.185 | Likely Benign | Likely Benign | 0.146 | Likely Benign | 0.1984 | 0.5861 | -2.90 | Deleterious | 1.000 | Probably Damaging | 0.975 | Probably Damaging | 2.61 | Benign | 0.00 | Affected | 0 | -2 | -1.6 | 40.02 | |||||||||||||||||||||||||||||||||||
| c.3197C>G | P1066R 2D  AIThe SynGAP1 missense variant P1066R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, tools that predict a pathogenic outcome are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.967676 | Disordered | 0.968838 | Binding | 0.403 | 0.913 | 0.875 | -5.154 | Likely Benign | 0.292 | Likely Benign | Likely Benign | 0.176 | Likely Benign | 0.1523 | 0.4742 | -2.89 | Deleterious | 0.992 | Probably Damaging | 0.873 | Possibly Damaging | 2.63 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.3197C>T | P1066L 2D  AIThe SynGAP1 missense variant P1066L is listed in ClinVar as a benign variant (ClinVar ID 951518.0) and is present in gnomAD (ID 6‑33443749‑C‑T). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, which is consistent with the ClinVar classification and does not contradict the reported status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.967676 | Disordered | 0.968838 | Binding | 0.403 | 0.913 | 0.875 | Likely Benign | 1 | 6-33443749-C-T | 14 | 8.71e-6 | -5.478 | Likely Benign | 0.092 | Likely Benign | Likely Benign | 0.173 | Likely Benign | 0.2269 | 0.6780 | -3.68 | Deleterious | 0.996 | Probably Damaging | 0.903 | Possibly Damaging | 2.72 | Benign | 0.00 | Affected | 4.32 | 2 | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||
| c.319A>G | R107G 2D  AIThe SynGAP1 missense variant R107G is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM, while those that predict a pathogenic effect are PROVEAN, SIFT, and AlphaMissense‑Default. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is a tie and thus unavailable, and Foldetta stability analysis is missing. Overall, the majority of tools (five benign vs. three pathogenic) suggest a benign impact, but the lack of consensus from the most accurate predictors means the variant’s effect remains uncertain. This assessment does not contradict any ClinVar status, as none is available. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.663448 | Binding | 0.331 | 0.863 | 0.875 | -3.486 | Likely Benign | 0.948 | Likely Pathogenic | Ambiguous | 0.180 | Likely Benign | 0.3280 | 0.4000 | -3.15 | Deleterious | 0.421 | Benign | 0.050 | Benign | 2.98 | Benign | 0.00 | Affected | -3 | -2 | 4.1 | -99.14 | ||||||||||||||||||||||||||||||||||||
| c.319A>T | R107W 2D  AIThe SynGAP1 missense variant R107W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Tools that predict a pathogenic effect comprise PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (two pathogenic vs two benign), and Foldetta results are unavailable. Overall, the majority of evidence (six pathogenic vs three benign) points to a pathogenic impact. Thus, the variant is most likely pathogenic, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.663448 | Binding | 0.331 | 0.863 | 0.875 | -4.963 | Likely Benign | 0.965 | Likely Pathogenic | Likely Pathogenic | 0.328 | Likely Benign | 0.1146 | 0.4228 | -2.99 | Deleterious | 0.983 | Probably Damaging | 0.624 | Possibly Damaging | 2.95 | Benign | 0.00 | Affected | 2 | -3 | 3.6 | 30.03 | ||||||||||||||||||||||||||||||||||||
| c.31G>A | G11R 2D  AIThe SynGAP1 missense variant G11R is catalogued in gnomAD (ID 6‑33420295‑G‑A) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Pathogenic predictions come from polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.444081 | Structured | 0.501027 | Binding | 0.348 | 0.915 | 0.375 | 6-33420295-G-A | -3.418 | Likely Benign | 0.428 | Ambiguous | Likely Benign | 0.102 | Likely Benign | 0.1022 | 0.4596 | -0.47 | Neutral | 0.498 | Possibly Damaging | 0.026 | Benign | 3.92 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -3 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||
| c.31G>C | G11R 2D  AIThe SynGAP1 missense variant G11R is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. AlphaMissense‑Default is uncertain, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.444081 | Structured | 0.501027 | Binding | 0.348 | 0.915 | 0.375 | -3.418 | Likely Benign | 0.428 | Ambiguous | Likely Benign | 0.109 | Likely Benign | 0.1022 | 0.4596 | -0.47 | Neutral | 0.498 | Possibly Damaging | 0.026 | Benign | 3.92 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -3 | -4.1 | 99.14 | |||||||||||||||||||||||||||||||||
| c.31G>T | G11W 2D  AIThe SynGAP1 missense variant G11W is catalogued in gnomAD (ID 6‑33420295‑G‑T) but has no ClinVar entry. Functional prediction tools cluster into two groups: benign predictions include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Pathogenic predictions come from polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.444081 | Structured | 0.501027 | Binding | 0.348 | 0.915 | 0.375 | 6-33420295-G-T | -5.819 | Likely Benign | 0.403 | Ambiguous | Likely Benign | 0.096 | Likely Benign | 0.0747 | 0.4731 | -0.67 | Neutral | 0.959 | Probably Damaging | 0.318 | Benign | 3.87 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | -7 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||
| c.3202T>A | L1068M 2D  AIThe SynGAP1 missense variant L1068M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) lean toward a benign impact. There is no ClinVar entry to contradict this assessment, so the variant is most likely benign based on current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.947281 | Disordered | 0.981041 | Binding | 0.362 | 0.907 | 0.875 | -5.739 | Likely Benign | 0.120 | Likely Benign | Likely Benign | 0.043 | Likely Benign | 0.0930 | 0.4582 | -0.22 | Neutral | 0.977 | Probably Damaging | 0.721 | Possibly Damaging | 2.49 | Pathogenic | 0.00 | Affected | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||
| c.3202T>G | L1068V 2D  AIThe SynGAP1 missense variant L1068V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.947281 | Disordered | 0.981041 | Binding | 0.362 | 0.907 | 0.875 | -5.325 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.051 | Likely Benign | 0.1619 | 0.4404 | -0.58 | Neutral | 0.451 | Benign | 0.110 | Benign | 2.54 | Benign | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.3203T>C | L1068S 2D  AIThe SynGAP1 missense variant L1068S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign, while only SIFT predicts it as pathogenic. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” consensus. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.947281 | Disordered | 0.981041 | Binding | 0.362 | 0.907 | 0.875 | -6.297 | Likely Benign | 0.211 | Likely Benign | Likely Benign | 0.175 | Likely Benign | 0.2810 | 0.1853 | -0.57 | Neutral | 0.032 | Benign | 0.017 | Benign | 2.59 | Benign | 0.00 | Affected | -3 | -2 | -4.6 | -26.08 | |||||||||||||||||||||||||||||||||||
| c.3203T>G | L1068W 2D  AIThe SynGAP1 missense variant L1068W is not reported in ClinVar (ClinVar status: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and AlphaMissense‑Optimized, whereas polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM all predict a pathogenic outcome; AlphaMissense‑Default remains uncertain. High‑accuracy assessments further refine the picture: AlphaMissense‑Optimized remains benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) resolves as pathogenic, and Foldetta’s protein‑folding stability analysis is unavailable. Overall, the majority of evidence points toward a pathogenic impact for L1068W. This conclusion is not contradicted by ClinVar, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.947281 | Disordered | 0.981041 | Binding | 0.362 | 0.907 | 0.875 | -8.574 | Likely Pathogenic | 0.342 | Ambiguous | Likely Benign | 0.151 | Likely Benign | 0.0742 | 0.3763 | -1.96 | Neutral | 0.994 | Probably Damaging | 0.884 | Possibly Damaging | 2.46 | Pathogenic | 0.00 | Affected | -2 | -2 | -4.7 | 73.05 | ||||||||||||||||||||||||||||||||||||
| c.3204G>C | L1068F 2D  AIThe SynGAP1 missense variant L1068F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) favor a benign classification. Thus, the variant is most likely benign based on current computational evidence, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.947281 | Disordered | 0.981041 | Binding | 0.362 | 0.907 | 0.875 | -6.338 | Likely Benign | 0.182 | Likely Benign | Likely Benign | 0.107 | Likely Benign | 0.0754 | 0.4202 | -1.38 | Neutral | 0.934 | Possibly Damaging | 0.537 | Possibly Damaging | 2.49 | Pathogenic | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.3204G>T | L1068F 2D  AIThe SynGAP1 missense variant L1068F is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign; Foldetta results are unavailable. Overall, the majority of predictions (six benign vs. four pathogenic) favor a benign classification. Thus, the variant is most likely benign based on current computational evidence, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.947281 | Disordered | 0.981041 | Binding | 0.362 | 0.907 | 0.875 | -6.338 | Likely Benign | 0.182 | Likely Benign | Likely Benign | 0.106 | Likely Benign | 0.0754 | 0.4202 | -1.38 | Neutral | 0.934 | Possibly Damaging | 0.537 | Possibly Damaging | 2.49 | Pathogenic | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||||
| c.3208A>T | R1070W 2D  AIThe SynGAP1 missense variant R1070W is not reported in ClinVar and is absent from gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and the SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments show AlphaMissense‑Optimized labeling the variant as benign, whereas the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates it is likely pathogenic. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this residue. Overall, the majority of tools and the SGM‑Consensus support a pathogenic effect, so the variant is most likely pathogenic, and this assessment does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.930790 | Disordered | 0.982693 | Binding | 0.297 | 0.906 | 0.875 | -8.063 | Likely Pathogenic | 0.743 | Likely Pathogenic | Likely Benign | 0.139 | Likely Benign | 0.1195 | 0.4293 | -3.42 | Deleterious | 0.999 | Probably Damaging | 0.956 | Probably Damaging | 3.72 | Benign | 0.00 | Affected | 2 | -3 | 3.6 | 30.03 | |||||||||||||||||||||||||||||||||||
| c.3209G>T | R1070M 2D  AIThe SynGAP1 missense variant R1070M is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the balance of evidence, including the consensus benign call and the absence of pathogenic predictions from the most reliable tools, suggests the variant is most likely benign. This conclusion does not contradict ClinVar status, as no ClinVar entry exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.930790 | Disordered | 0.982693 | Binding | 0.297 | 0.906 | 0.875 | -6.455 | Likely Benign | 0.917 | Likely Pathogenic | Ambiguous | 0.183 | Likely Benign | 0.1661 | 0.4324 | -2.27 | Neutral | 0.995 | Probably Damaging | 0.907 | Possibly Damaging | 3.74 | Benign | 0.00 | Affected | 0 | -1 | 6.4 | -24.99 | |||||||||||||||||||||||||||||||||||
| c.320G>A | R107K 2D  AIThe SynGAP1 missense variant R107K is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact for R107K, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.666105 | Disordered | 0.663448 | Binding | 0.331 | 0.863 | 0.875 | -3.941 | Likely Benign | 0.758 | Likely Pathogenic | Likely Benign | 0.153 | Likely Benign | 0.5618 | 0.4185 | Weaken | -1.33 | Neutral | 0.004 | Benign | 0.001 | Benign | 3.07 | Benign | 0.00 | Affected | 3 | 2 | 0.6 | -28.01 | ||||||||||||||||||||||||||||||||||
| c.320G>C | R107T 2D  AIThe SynGAP1 missense variant R107T is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are PROVEAN, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 pathogenic vs. 2 benign) and Foldetta results are unavailable. Overall, the majority of predictions (five benign vs. four pathogenic) lean toward a benign interpretation. Thus, the variant is most likely benign based on current computational evidence, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.663448 | Binding | 0.331 | 0.863 | 0.875 | -2.902 | Likely Benign | 0.992 | Likely Pathogenic | Likely Pathogenic | 0.191 | Likely Benign | 0.1549 | 0.4761 | -2.63 | Deleterious | 0.421 | Benign | 0.050 | Benign | 2.98 | Benign | 0.00 | Affected | -1 | -1 | 3.8 | -55.08 | ||||||||||||||||||||||||||||||||||||
| c.320G>T | R107M 2D  AIThe SynGAP1 missense variant R107M is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are PROVEAN, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive, and Foldetta results are unavailable. Overall, the balance of evidence favors a benign interpretation, and this conclusion does not conflict with the ClinVar status, which currently contains no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.666105 | Disordered | 0.663448 | Binding | 0.331 | 0.863 | 0.875 | -4.873 | Likely Benign | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.233 | Likely Benign | 0.1605 | 0.4415 | -2.65 | Deleterious | 0.028 | Benign | 0.011 | Benign | 2.96 | Benign | 0.00 | Affected | 0 | -1 | 6.4 | -24.99 | ||||||||||||||||||||||||||||||||||||
| c.3211G>C | G1071R 2D  AIThe SynGAP1 missense variant G1071R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Those that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is a 2‑vs‑2 tie and thus unavailable; Foldetta results are not provided and are therefore unavailable. Overall, more tools predict pathogenicity (5) than benignity (3), and no ClinVar entry contradicts this assessment. **The variant is most likely pathogenic based on the available predictions.** Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.983740 | Binding | 0.313 | 0.905 | 0.875 | -3.052 | Likely Benign | 0.886 | Likely Pathogenic | Ambiguous | 0.135 | Likely Benign | 0.0980 | 0.4482 | -2.61 | Deleterious | 0.970 | Probably Damaging | 0.728 | Possibly Damaging | 4.06 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||
| c.3211G>T | G1071C 2D  AIThe SynGAP1 missense variant G1071C is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; the Foldetta stability analysis is unavailable. Overall, the majority of evidence—including the consensus and high‑accuracy predictions—supports a benign classification, and this is consistent with the lack of ClinVar annotation. Thus, the variant is most likely benign, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.983740 | Binding | 0.313 | 0.905 | 0.875 | -5.364 | Likely Benign | 0.305 | Likely Benign | Likely Benign | 0.182 | Likely Benign | 0.1323 | 0.4227 | -2.16 | Neutral | 0.997 | Probably Damaging | 0.889 | Possibly Damaging | 4.01 | Benign | 0.00 | Affected | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||
| c.3218C>A | S1073Y 2D  AIThe SynGAP1 missense change S1073Y is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.916840 | Disordered | 0.985818 | Binding | 0.313 | 0.905 | 0.750 | -6.768 | Likely Benign | 0.752 | Likely Pathogenic | Likely Benign | 0.165 | Likely Benign | 0.0977 | 0.5684 | -2.43 | Neutral | 0.990 | Probably Damaging | 0.796 | Possibly Damaging | 3.81 | Benign | 0.00 | Affected | -3 | -2 | -0.5 | 76.10 | |||||||||||||||||||||||||||||||||||
| c.3218C>G | S1073C 2D  AISynGAP1 missense variant S1073C is recorded in gnomAD (ID 6‑33443770‑C‑G) but has no ClinVar submission. Functional prediction tools show mixed results: benign calls come from REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic calls are made by polyPhen‑2 (HumDiv and HumVar), SIFT, and ESM1b. The AlphaMissense‑Default score is uncertain. A consensus from the SGM framework (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive because the votes are split. High‑accuracy methods give a benign prediction from AlphaMissense‑Optimized; the SGM Consensus remains ambiguous, and Foldetta stability analysis is unavailable. Consequently, the evidence does not strongly favor either outcome. The variant is most likely of uncertain significance, with no contradiction to ClinVar status because no ClinVar classification exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.916840 | Disordered | 0.985818 | Binding | 0.313 | 0.905 | 0.750 | 6-33443770-C-G | 1 | 6.23e-7 | -8.862 | Likely Pathogenic | 0.461 | Ambiguous | Likely Benign | 0.137 | Likely Benign | 0.1343 | 0.6088 | -1.52 | Neutral | 0.997 | Probably Damaging | 0.840 | Possibly Damaging | 3.81 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||
| c.3218C>T | S1073F 2D  AIThe SynGAP1 missense variant S1073F is not reported in ClinVar (ClinVar status: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of available predictions (five pathogenic vs. three benign) lean toward a pathogenic impact. Thus, the variant is most likely pathogenic based on current computational evidence, and this assessment does not contradict any ClinVar status because no ClinVar claim exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.916840 | Disordered | 0.985818 | Binding | 0.313 | 0.905 | 0.750 | -6.783 | Likely Benign | 0.836 | Likely Pathogenic | Ambiguous | 0.161 | Likely Benign | 0.0952 | 0.5957 | -2.58 | Deleterious | 0.990 | Probably Damaging | 0.796 | Possibly Damaging | 3.81 | Benign | 0.00 | Affected | -3 | -2 | 3.6 | 60.10 | ||||||||||||||||||||||||||||||||||||
| c.321G>C | R107S 2D  AIThe SynGAP1 missense variant R107S is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as pathogenic, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of predictions lean toward a benign impact, and this is not contradicted by any ClinVar annotation. Thus, the variant is most likely benign, although high‑accuracy tools provide conflicting evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.666105 | Disordered | 0.663448 | Binding | 0.331 | 0.863 | 0.875 | -1.162 | Likely Benign | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.147 | Likely Benign | 0.2771 | 0.4148 | -2.37 | Neutral | 0.231 | Benign | 0.037 | Benign | 2.99 | Benign | 0.00 | Affected | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||
| c.321G>T | R107S 2D  AIThe SynGAP1 missense variant R107S is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome; Foldetta results are unavailable. Overall, the majority of predictions lean toward a benign impact, but the high‑accuracy tools provide conflicting evidence. Thus, the variant is most likely benign based on the collective predictions, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.666105 | Disordered | 0.663448 | Binding | 0.331 | 0.863 | 0.875 | -1.162 | Likely Benign | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.147 | Likely Benign | 0.2771 | 0.4148 | -2.37 | Neutral | 0.231 | Benign | 0.037 | Benign | 2.99 | Benign | 0.00 | Affected | 0 | -1 | 3.7 | -69.11 | |||||||||||||||||||||||||||||||||||
| c.3233T>A | V1078D 2D  AIThe SynGAP1 missense variant V1078D is listed in ClinVar (ID 2993122.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are AlphaMissense‑Default, AlphaMissense‑Optimized, and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, SGM‑Consensus as likely benign, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) has no available result for this variant. Overall, the majority of predictions lean toward a benign impact, and this is consistent with the ClinVar “Uncertain” designation; there is no contradiction with the existing ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.882776 | Disordered | 0.986989 | Binding | 0.294 | 0.898 | 0.750 | Uncertain | 1 | -5.155 | Likely Benign | 0.979 | Likely Pathogenic | Likely Pathogenic | 0.158 | Likely Benign | 0.1570 | 0.1173 | -1.45 | Neutral | 0.003 | Benign | 0.008 | Benign | 3.84 | Benign | 0.00 | Affected | 3.77 | 5 | -3 | -2 | -7.7 | 15.96 | |||||||||||||||||||||||||||||||
| c.3233T>G | V1078G 2D  AIThe SynGAP1 missense variant V1078G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is benign (3 benign vs 1 pathogenic). Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the majority of evidence indicates a benign impact. This conclusion is not contradicted by ClinVar status, which has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.882776 | Disordered | 0.986989 | Binding | 0.294 | 0.898 | 0.750 | -3.270 | Likely Benign | 0.699 | Likely Pathogenic | Likely Benign | 0.168 | Likely Benign | 0.2041 | 0.2693 | -0.54 | Neutral | 0.157 | Benign | 0.292 | Benign | 3.85 | Benign | 0.00 | Affected | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||
| c.3235A>C | S1079R 2D  AIThe SynGAP1 missense variant S1079R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized returns an uncertain result. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus as Likely Benign, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta) has no available result for this variant. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.983887 | Binding | 0.307 | 0.900 | 0.750 | -4.579 | Likely Benign | 0.955 | Likely Pathogenic | Ambiguous | 0.163 | Likely Benign | 0.0811 | 0.4028 | -1.81 | Neutral | 0.177 | Benign | 0.075 | Benign | 3.86 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||
| c.3235A>G | S1079G 2D  AIThe SynGAP1 missense variant S1079G is reported in gnomAD (variant ID 6‑33443787‑A‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign status. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods points to a benign effect. This conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.983887 | Binding | 0.307 | 0.900 | 0.750 | 6-33443787-A-G | 6 | 3.76e-6 | -3.552 | Likely Benign | 0.177 | Likely Benign | Likely Benign | 0.065 | Likely Benign | 0.2151 | 0.4623 | -1.50 | Neutral | 0.036 | Benign | 0.018 | Benign | 3.87 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | 1 | 0.4 | -30.03 | ||||||||||||||||||||||||||||||
| c.3235A>T | S1079C 2D  AIThe SynGAP1 missense variant S1079C is not reported in ClinVar (ClinVar ID: None) and is absent from gnomAD (gnomAD ID: None). Prediction tools that agree on a benign effect include REVEL, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), and SIFT. Two tools—ESM1b and AlphaMissense‑Default—return uncertain results. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive, and Foldetta data are unavailable. Overall, the balance of evidence slightly favors a pathogenic interpretation, with four tools supporting pathogenicity versus three supporting benignity. This assessment does not contradict ClinVar status, as the variant has not yet been classified in that database. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.983887 | Binding | 0.307 | 0.900 | 0.750 | -7.333 | In-Between | 0.370 | Ambiguous | Likely Benign | 0.138 | Likely Benign | 0.0949 | 0.5536 | -2.61 | Deleterious | 0.898 | Possibly Damaging | 0.477 | Possibly Damaging | 3.81 | Benign | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||||||
| c.3236G>A | S1079N 2D  AIThe SynGAP1 missense variant S1079N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority of the high‑accuracy tools) also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of predictions indicates that the variant is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.983887 | Binding | 0.307 | 0.900 | 0.750 | -4.989 | Likely Benign | 0.505 | Ambiguous | Likely Benign | 0.026 | Likely Benign | 0.1225 | 0.4657 | -0.97 | Neutral | 0.001 | Benign | 0.001 | Benign | 3.93 | Benign | 0.00 | Affected | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||
| c.3236G>C | S1079T 2D  AIThe SynGAP1 missense variant S1079T is not reported in ClinVar and is absent from gnomAD, indicating no known population frequency data. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized scores the variant as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports it as Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the computational evidence strongly suggests the variant is most likely benign, and this conclusion does not contradict the ClinVar status, which currently has no entry for S1079T. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.983887 | Binding | 0.307 | 0.900 | 0.750 | -3.225 | Likely Benign | 0.163 | Likely Benign | Likely Benign | 0.023 | Likely Benign | 0.1283 | 0.5920 | -1.28 | Neutral | 0.001 | Benign | 0.003 | Benign | 3.91 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.3236G>T | S1079I 2D  AIThe SynGAP1 missense variant S1079I is not reported in ClinVar (ClinVar status: none) and is absent from gnomAD (gnomAD ID: none). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which currently has no classification for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.919029 | Disordered | 0.983887 | Binding | 0.307 | 0.900 | 0.750 | -4.732 | Likely Benign | 0.688 | Likely Pathogenic | Likely Benign | 0.093 | Likely Benign | 0.0921 | 0.4775 | -2.86 | Deleterious | 0.078 | Benign | 0.025 | Benign | 3.83 | Benign | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | ||||||||||||||||||||||||||||||||||||
| c.3237C>A | S1079R 2D  AIThe SynGAP1 missense variant S1079R is listed in ClinVar with an “Uncertain” status and is present in gnomAD (gnomAD ID 6‑33443789‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Those that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized is inconclusive. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments are limited: AlphaMissense‑Optimized remains uncertain, SGM‑Consensus is benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the preponderance of evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.983887 | Binding | 0.307 | 0.900 | 0.750 | Uncertain | 1 | 6-33443789-C-A | 4 | 2.51e-6 | -4.579 | Likely Benign | 0.955 | Likely Pathogenic | Ambiguous | 0.123 | Likely Benign | 0.0811 | 0.4028 | -1.81 | Neutral | 0.177 | Benign | 0.075 | Benign | 3.86 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | -1 | -3.7 | 69.11 | ||||||||||||||||||||||||||||
| c.3237C>G | S1079R 2D  AIThe SynGAP1 missense variant S1079R is listed in ClinVar (ID 1047537.0) as Benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain, and no Foldetta (FoldX‑MD/Rosetta stability) result is available. High‑accuracy assessments therefore show a benign consensus (SGM‑Consensus) with one uncertain AlphaMissense‑Optimized prediction and no destabilizing Foldetta evidence. Overall, the majority of predictions support a benign classification, which is consistent with the ClinVar status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.983887 | Binding | 0.307 | 0.900 | 0.750 | Benign | 1 | -4.579 | Likely Benign | 0.955 | Likely Pathogenic | Ambiguous | 0.124 | Likely Benign | 0.0811 | 0.4028 | -1.81 | Neutral | 0.177 | Benign | 0.075 | Benign | 3.86 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||
| c.3251C>A | P1084H 2D  AIThe SynGAP1 missense variant P1084H is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443803‑C‑A). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which reports “Likely Benign”). Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar designation of uncertainty rather than a definitive pathogenic claim. Thus, the variant is most likely benign, and its prediction profile does not contradict the current ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.842060 | Disordered | 0.979020 | Binding | 0.348 | 0.889 | 1.000 | Uncertain | 1 | 6-33443803-C-A | 1 | 6.31e-7 | -4.125 | Likely Benign | 0.323 | Likely Benign | Likely Benign | 0.134 | Likely Benign | 0.1751 | 0.5523 | -3.16 | Deleterious | 0.997 | Probably Damaging | 0.840 | Possibly Damaging | 3.96 | Benign | 0.00 | Affected | 3.77 | 5 | -2 | 0 | -1.6 | 40.02 | ||||||||||||||||||||||||||||
| c.3253C>T | R1085W 2D  AISynGAP1 missense variant R1085W is listed in ClinVar as Uncertain and is present in gnomAD (ID 6‑33443805‑C‑T). Prediction tools that classify the variant as benign include REVEL, ESM1b, and FATHMM, whereas pathogenic predictions come from PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is Uncertain, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) and Foldetta stability assessment are unavailable. Overall, the majority of available predictions (five pathogenic vs. three benign) indicate a pathogenic effect. Thus, the variant is most likely pathogenic, which contradicts the ClinVar designation of Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.852992 | Disordered | 0.978838 | Binding | 0.270 | 0.888 | 1.000 | Uncertain | 1 | 6-33443805-C-T | 2 | 1.26e-6 | -6.339 | Likely Benign | 0.821 | Likely Pathogenic | Ambiguous | 0.202 | Likely Benign | 0.1238 | 0.3700 | -3.15 | Deleterious | 1.000 | Probably Damaging | 0.996 | Probably Damaging | 2.70 | Benign | 0.00 | Affected | 3.77 | 5 | -3 | 2 | 3.6 | 30.03 | |||||||||||||||||||||||||||||
| c.3256C>A | P1086T 2D  AIThe SynGAP1 missense variant P1086T is not reported in ClinVar (ClinVar status: none) but is present in gnomAD (ID 6‑33443808‑C‑A). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta results are unavailable. Overall, the majority of predictions (five pathogenic vs. four benign) lean toward a pathogenic impact. The variant is most likely pathogenic based on the available computational evidence, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.849326 | Disordered | 0.977190 | Binding | 0.393 | 0.885 | 1.000 | 6-33443808-C-A | -4.181 | Likely Benign | 0.568 | Likely Pathogenic | Likely Benign | 0.229 | Likely Benign | 0.1343 | 0.5848 | -2.97 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.75 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||
| c.3256C>G | P1086A 2D  AIThe SynGAP1 missense variant P1086A is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic outcome are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores the variant as benign, the SGM Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also favors benign, and Foldetta data are unavailable. Taken together, the balance of evidence points to a benign effect for P1086A. This conclusion does not conflict with the ClinVar status, which currently contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.849326 | Disordered | 0.977190 | Binding | 0.393 | 0.885 | 1.000 | -4.317 | Likely Benign | 0.372 | Ambiguous | Likely Benign | 0.206 | Likely Benign | 0.3084 | 0.4463 | -2.98 | Deleterious | 0.999 | Probably Damaging | 0.996 | Probably Damaging | 2.78 | Benign | 0.00 | Affected | 1 | -1 | 3.4 | -26.04 | ||||||||||||||||||||||||||||||||||||
| c.3256C>T | P1086S 2D  AIThe SynGAP1 missense variant P1086S is not reported in ClinVar (ClinVar status: none) but is present in gnomAD (ID 6‑33443808‑C‑T). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions (five pathogenic vs. four benign) lean toward a pathogenic impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.849326 | Disordered | 0.977190 | Binding | 0.393 | 0.885 | 1.000 | 6-33443808-C-T | -3.165 | Likely Benign | 0.604 | Likely Pathogenic | Likely Benign | 0.212 | Likely Benign | 0.3067 | 0.4894 | -3.04 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.78 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 1 | 0.8 | -10.04 | |||||||||||||||||||||||||||||||||
| c.3257C>A | P1086Q 2D  AIThe SynGAP1 missense variant P1086Q is not reported in ClinVar (ClinVar status: None) but is present in gnomAD (ID 6‑33443809‑C‑A). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (two pathogenic vs. two benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions (five pathogenic vs. four benign) indicate that the variant is most likely pathogenic, and this assessment does not contradict the ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.849326 | Disordered | 0.977190 | Binding | 0.393 | 0.885 | 1.000 | 6-33443809-C-A | -4.668 | Likely Benign | 0.652 | Likely Pathogenic | Likely Benign | 0.185 | Likely Benign | 0.1285 | 0.4784 | -2.92 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.77 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -1.9 | 31.01 | |||||||||||||||||||||||||||||||||
| c.3257C>G | P1086R 2D  AIThe SynGAP1 missense variant P1086R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Those that predict a pathogenic effect comprise PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool returns an uncertain result, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (two pathogenic vs. two benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, more tools (five) predict pathogenicity than benign (three), and the high‑accuracy consensus is inconclusive. Therefore, the variant is most likely pathogenic based on the current predictions, and this assessment does not contradict any ClinVar status because the variant is not yet reported there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.849326 | Disordered | 0.977190 | Binding | 0.393 | 0.885 | 1.000 | -5.190 | Likely Benign | 0.848 | Likely Pathogenic | Ambiguous | 0.205 | Likely Benign | 0.1319 | 0.3551 | -3.42 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.86 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||||||||
| c.3257C>T | P1086L 2D  AIThe SynGAP1 missense variant P1086L is not reported in ClinVar (ClinVar status: not present) and is absent from gnomAD (gnomAD ID: none). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑vs‑2 split, and Foldetta results are unavailable. Overall, the majority of predictions lean toward pathogenicity, and this conclusion does not contradict the ClinVar status, which simply indicates the variant has not yet been reported. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.849326 | Disordered | 0.977190 | Binding | 0.393 | 0.885 | 1.000 | -4.694 | Likely Benign | 0.607 | Likely Pathogenic | Likely Benign | 0.166 | Likely Benign | 0.2055 | 0.6326 | -3.57 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.73 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||
| c.325A>C | S109R 2D  AIThe SynGAP1 missense variant S109R is not reported in ClinVar and is absent from gnomAD. Functional prediction tools show a split: benign calls include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM, whereas pathogenic calls come from SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments highlight that AlphaMissense‑Optimized predicts pathogenicity, whereas the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) predicts benign impact; Foldetta data are unavailable. Overall, the balance of evidence leans toward a benign effect for S109R, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.669335 | Binding | 0.328 | 0.864 | 0.750 | -4.830 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.225 | Likely Benign | 0.0884 | 0.2700 | -1.80 | Neutral | 0.002 | Benign | 0.001 | Benign | 3.48 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.325A>G | S109G 2D  AIThe SynGAP1 missense variant S109G is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support this: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (majority of the four high‑accuracy tools) also indicates Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.669335 | Binding | 0.328 | 0.864 | 0.750 | -3.918 | Likely Benign | 0.549 | Ambiguous | Likely Benign | 0.132 | Likely Benign | 0.2686 | 0.3779 | -1.95 | Neutral | 0.378 | Benign | 0.067 | Benign | 3.51 | Benign | 0.00 | Affected | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||||||||
| c.325A>T | S109C 2D  AIThe SynGAP1 missense variant S109C is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus (majority vote) also as Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.669335 | Binding | 0.328 | 0.864 | 0.750 | -6.268 | Likely Benign | 0.761 | Likely Pathogenic | Likely Benign | 0.217 | Likely Benign | 0.1084 | 0.5354 | -2.19 | Neutral | 0.983 | Probably Damaging | 0.431 | Benign | 3.46 | Benign | 0.00 | Affected | 0 | -1 | 3.3 | 16.06 | |||||||||||||||||||||||||||||||||||
| c.3265G>T | G1089W 2D  AIThe SynGAP1 missense variant G1089W is not reported in ClinVar and has no allele in gnomAD. Functional prediction tools show a split: benign calls come from REVEL and ESM1b, whereas pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the consensus SGM‑Consensus score (Likely Pathogenic). High‑accuracy assessments give an uncertain result from AlphaMissense‑Optimized, a Likely Pathogenic verdict from the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN), and no available Foldetta stability data. Overall, the majority of evidence points to a deleterious effect, so the variant is most likely pathogenic, and this assessment does not contradict any ClinVar annotation because none exists. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | 0.891961 | Disordered | 0.976771 | Binding | 0.366 | 0.890 | 1.000 | -6.561 | Likely Benign | 0.863 | Likely Pathogenic | Ambiguous | 0.236 | Likely Benign | 0.0840 | 0.4610 | -3.45 | Deleterious | 1.000 | Probably Damaging | 0.988 | Probably Damaging | 2.37 | Pathogenic | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | |||||||||||||||||||||||||||||||||||
| c.3266G>T | G1089V 2D  AIThe SynGAP1 missense variant G1089V is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. AlphaMissense‑Default is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) predicts pathogenic. Foldetta results are unavailable. Overall, the majority of evidence points to a pathogenic impact for G1089V. This conclusion does not contradict ClinVar status, as no ClinVar assertion exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.891961 | Disordered | 0.976771 | Binding | 0.366 | 0.890 | 1.000 | -4.809 | Likely Benign | 0.527 | Ambiguous | Likely Benign | 0.182 | Likely Benign | 0.1326 | 0.4413 | -2.81 | Deleterious | 0.984 | Probably Damaging | 0.722 | Possibly Damaging | 2.40 | Pathogenic | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | ||||||||||||||||||||||||||||||||||||
| c.326G>A | S109N 2D  AIThe SynGAP1 missense variant S109N is not reported in ClinVar and has no entries in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM, while pathogenic calls come from polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, resolves to a likely benign verdict. High‑accuracy assessments further support this: AlphaMissense‑Optimized is uncertain, and Foldetta data are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.669335 | Binding | 0.328 | 0.864 | 0.750 | -5.509 | Likely Benign | 0.863 | Likely Pathogenic | Ambiguous | 0.116 | Likely Benign | 0.1350 | 0.3717 | -1.45 | Neutral | 0.596 | Possibly Damaging | 0.074 | Benign | 3.49 | Benign | 0.00 | Affected | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||
| c.326G>C | S109T 2D  AIThe SynGAP1 missense variant S109T is catalogued in gnomAD (6‑33432191‑G‑C) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all report benign or likely benign. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments confirm the benign consensus: AlphaMissense‑Optimized is benign, and the SGM‑Consensus, derived from the majority of the high‑accuracy predictors, is benign. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that S109T is most likely benign, and this assessment does not contradict any ClinVar status (none). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.669335 | Binding | 0.328 | 0.864 | 0.750 | 6-33432191-G-C | 2 | 1.24e-6 | -4.065 | Likely Benign | 0.449 | Ambiguous | Likely Benign | 0.090 | Likely Benign | 0.1519 | 0.5212 | -1.19 | Neutral | 0.231 | Benign | 0.050 | Benign | 3.59 | Benign | 0.00 | Affected | 3.61 | 5 | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||||||
| c.326G>T | S109I 2D  AIThe SynGAP1 missense variant S109I is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are PROVEAN, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain. High‑accuracy assessments are inconclusive: AlphaMissense‑Optimized remains uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is a 2‑vs‑2 tie, and Foldetta data are unavailable. Overall, the majority of evidence (five benign vs. three pathogenic predictions) points toward a benign impact. This conclusion does not contradict ClinVar, as the variant has no ClinVar entry. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.622677 | Disordered | 0.669335 | Binding | 0.328 | 0.864 | 0.750 | -5.195 | Likely Benign | 0.927 | Likely Pathogenic | Ambiguous | 0.200 | Likely Benign | 0.0910 | 0.4930 | -2.56 | Deleterious | 0.267 | Benign | 0.039 | Benign | 3.47 | Benign | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | ||||||||||||||||||||||||||||||||||||
| c.327T>A | S109R 2D  AIThe SynGAP1 missense variant S109R has no ClinVar entry and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) remains benign; Foldetta stability analysis is unavailable. Overall, the majority of predictions lean toward a benign impact, and this is not contradicted by any ClinVar status. Thus, the variant is most likely benign based on the current computational evidence. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.669335 | Binding | 0.328 | 0.864 | 0.750 | -4.830 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.198 | Likely Benign | 0.0884 | 0.2700 | -1.80 | Neutral | 0.002 | Benign | 0.001 | Benign | 3.48 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.327T>G | S109R 2D  AIThe SynGAP1 missense variant S109R has no ClinVar entry and is not reported in gnomAD. Prediction tools that classify it as benign include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (Likely Benign). Tools that predict pathogenicity are SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy methods give mixed results: AlphaMissense‑Optimized reports a pathogenic effect, whereas the SGM‑Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome; Foldetta stability analysis is unavailable. Overall, the majority of predictions lean toward a benign impact, with one high‑accuracy tool suggesting pathogenicity, and there is no ClinVar status to contradict these findings. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.622677 | Disordered | 0.669335 | Binding | 0.328 | 0.864 | 0.750 | -4.830 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.198 | Likely Benign | 0.0884 | 0.2700 | -1.80 | Neutral | 0.002 | Benign | 0.001 | Benign | 3.48 | Benign | 0.00 | Affected | 0 | -1 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||||
| c.3298G>C | G1100R 2D  AIThe SynGAP1 missense variant G1100R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 pathogenic vs. 2 benign), and Foldetta results are unavailable. Overall, the majority of available predictions lean toward pathogenicity, and this does not contradict the ClinVar status, which simply lacks an entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.882776 | Disordered | 0.972009 | Binding | 0.360 | 0.865 | 0.875 | -2.923 | Likely Benign | 0.603 | Likely Pathogenic | Likely Benign | 0.176 | Likely Benign | 0.0986 | 0.5217 | -2.05 | Neutral | 0.992 | Probably Damaging | 0.906 | Possibly Damaging | 1.92 | Pathogenic | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||
| c.3298G>T | G1100C 2D  AIThe SynGAP1 missense variant G1100C is not reported in ClinVar and is absent from gnomAD. Functional prediction tools cluster into two groups: benign predictions include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments reinforce the benign trend: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates Likely Benign. Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.882776 | Disordered | 0.972009 | Binding | 0.360 | 0.865 | 0.875 | -6.488 | Likely Benign | 0.150 | Likely Benign | Likely Benign | 0.174 | Likely Benign | 0.1354 | 0.4768 | -2.25 | Neutral | 0.999 | Probably Damaging | 0.950 | Probably Damaging | 1.91 | Pathogenic | 0.00 | Affected | -3 | -3 | 2.9 | 46.09 | |||||||||||||||||||||||||||||||||||
| c.329T>A | V110D 2D  AIThe SynGAP1 missense variant V110D is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, and FATHMM. Tools that agree on a pathogenic effect include PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized predicts pathogenic. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic vs. two benign). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evaluated tools (seven pathogenic vs. three benign) indicate a pathogenic effect. This prediction is consistent with the lack of ClinVar reporting and does not contradict any existing ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.622677 | Disordered | 0.665934 | Binding | 0.347 | 0.860 | 0.750 | -4.536 | Likely Benign | 0.970 | Likely Pathogenic | Likely Pathogenic | 0.189 | Likely Benign | 0.1849 | 0.1115 | -2.84 | Deleterious | 0.978 | Probably Damaging | 0.500 | Possibly Damaging | 4.04 | Benign | 0.00 | Affected | -2 | -3 | -7.7 | 15.96 | ||||||||||||||||||||||||||||||||||||
| c.329T>G | V110G 2D  AIThe SynGAP1 missense variant V110G is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 benign vs 2 pathogenic), and Foldetta results are unavailable. Overall, the majority of evidence points toward a benign impact, and this conclusion does not contradict the ClinVar status, which has no entry for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.622677 | Disordered | 0.665934 | Binding | 0.347 | 0.860 | 0.750 | -4.012 | Likely Benign | 0.724 | Likely Pathogenic | Likely Benign | 0.162 | Likely Benign | 0.2302 | 0.2671 | -2.87 | Deleterious | 0.377 | Benign | 0.928 | Probably Damaging | 4.06 | Benign | 0.00 | Affected | -1 | -3 | -4.6 | -42.08 | ||||||||||||||||||||||||||||||||||||
| c.32G>A | G11E 2D  AIThe SynGAP1 missense variant G11E is reported in gnomAD (variant ID 6-33420296‑G‑A) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts it to be pathogenic, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus itself is likely benign. Foldetta results are not available, so they do not influence the assessment. Overall, the preponderance of evidence points to a benign variant, and this conclusion is consistent with the absence of a ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.444081 | Structured | 0.501027 | Binding | 0.348 | 0.915 | 0.375 | 6-33420296-G-A | 5 | 3.24e-6 | -4.206 | Likely Benign | 0.219 | Likely Benign | Likely Benign | 0.109 | Likely Benign | 0.1444 | 0.4628 | 0.09 | Neutral | 0.000 | Benign | 0.000 | Benign | 3.95 | Benign | 0.00 | Affected | 4.32 | 1 | -2 | 0 | -3.1 | 72.06 | ||||||||||||||||||||||||||||||
| c.32G>C | G11A 2D  AIThe SynGAP1 missense variant G11A is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.444081 | Structured | 0.501027 | Binding | 0.348 | 0.915 | 0.375 | -3.611 | Likely Benign | 0.106 | Likely Benign | Likely Benign | 0.072 | Likely Benign | 0.4045 | 0.5440 | -0.28 | Neutral | 0.105 | Benign | 0.007 | Benign | 4.00 | Benign | 0.00 | Affected | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.32G>T | G11V 2D  AIThe SynGAP1 missense variant G11V is reported in gnomAD (variant ID 6‑33420296‑G‑T) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. In contrast, polyPhen‑2 HumDiv and SIFT predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also likely benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the preponderance of evidence from both general and high‑accuracy tools indicates that G11V is most likely benign, and this conclusion is not contradicted by any ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.444081 | Structured | 0.501027 | Binding | 0.348 | 0.915 | 0.375 | 6-33420296-G-T | -4.079 | Likely Benign | 0.160 | Likely Benign | Likely Benign | 0.146 | Likely Benign | 0.1292 | 0.4522 | -0.38 | Neutral | 0.668 | Possibly Damaging | 0.049 | Benign | 3.93 | Benign | 0.00 | Affected | 4.32 | 1 | -3 | -1 | 4.6 | 42.08 | ||||||||||||||||||||||||||||||||
| c.332C>A | P111Q 2D  AIThe SynGAP1 missense variant P111Q is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, which aggregates the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign status. High‑accuracy assessments further support this view: AlphaMissense‑Optimized indicates benign, SGM‑Consensus confirms likely benign, and Foldetta data are unavailable. Taken together, the preponderance of evidence points to a benign effect for P111Q, and this assessment does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.707965 | Disordered | 0.650020 | Binding | 0.438 | 0.858 | 0.750 | -4.726 | Likely Benign | 0.543 | Ambiguous | Likely Benign | 0.079 | Likely Benign | 0.1593 | 0.4232 | -2.10 | Neutral | 0.421 | Benign | 0.054 | Benign | 4.06 | Benign | 0.00 | Affected | 0 | -1 | -1.9 | 31.01 | |||||||||||||||||||||||||||||||||||
| c.332C>G | P111R 2D  AIThe SynGAP1 missense variant P111R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. Foldetta results are unavailable for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.707965 | Disordered | 0.650020 | Binding | 0.438 | 0.858 | 0.750 | -4.811 | Likely Benign | 0.782 | Likely Pathogenic | Likely Benign | 0.100 | Likely Benign | 0.1578 | 0.3015 | -2.34 | Neutral | 0.421 | Benign | 0.075 | Benign | 4.06 | Benign | 0.00 | Affected | 0 | -2 | -2.9 | 59.07 | |||||||||||||||||||||||||||||||||||
| c.332C>T | P111L 2D  AIThe SynGAP1 missense variant P111L is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also yields a benign prediction (2 benign vs. 1 pathogenic votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the consensus of available predictions indicates that P111L is most likely benign, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.707965 | Disordered | 0.650020 | Binding | 0.438 | 0.858 | 0.750 | -4.430 | Likely Benign | 0.486 | Ambiguous | Likely Benign | 0.089 | Likely Benign | 0.2355 | 0.7085 | -2.81 | Deleterious | 0.421 | Benign | 0.055 | Benign | 4.06 | Benign | 0.00 | Affected | -3 | -3 | 5.4 | 16.04 | ||||||||||||||||||||||||||||||||||||
| c.3335A>T | E1112V 2D  AIThe SynGAP1 missense variant E1112V is not reported in ClinVar (ClinVar ID None) and is absent from gnomAD (gnomAD ID None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) also as benign; Foldetta stability analysis is unavailable. Overall, the preponderance of evidence points to a benign impact for E1112V, and this conclusion does not contradict the ClinVar status, which currently has no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.909381 | Binding | 0.335 | 0.902 | 0.875 | -3.971 | Likely Benign | 0.579 | Likely Pathogenic | Likely Benign | 0.139 | Likely Benign | 0.1481 | 0.7567 | -2.28 | Neutral | 0.440 | Benign | 0.140 | Benign | 2.70 | Benign | 0.00 | Affected | -2 | -2 | 7.7 | -29.98 | |||||||||||||||||||||||||||||||||||
| c.334G>A | G112R 2D  AIThe SynGAP1 missense variant G112R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are PROVEAN, SIFT, and AlphaMissense‑Default. High‑accuracy assessments are less definitive: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 benign vs 2 pathogenic), and Foldetta results are unavailable. Overall, the majority of standard predictors lean toward a benign impact, and this is not contradicted by any ClinVar annotation. Thus, based on current predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.728858 | Disordered | 0.640153 | Binding | 0.332 | 0.867 | 0.750 | -3.680 | Likely Benign | 0.866 | Likely Pathogenic | Ambiguous | 0.141 | Likely Benign | 0.0983 | 0.3978 | -3.31 | Deleterious | 0.002 | Benign | 0.004 | Benign | 3.94 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||
| c.334G>C | G112R 2D  AIThe SynGAP1 missense variant G112R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Tools that predict a pathogenic effect are PROVEAN, SIFT, and AlphaMissense‑Default. High‑accuracy assessments are less definitive: AlphaMissense‑Optimized is uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive (2 benign vs 2 pathogenic), and Foldetta results are unavailable. Overall, the majority of standard predictors lean toward a benign impact, and this is not contradicted by any ClinVar annotation. Thus, based on current predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.728858 | Disordered | 0.640153 | Binding | 0.332 | 0.867 | 0.750 | -3.680 | Likely Benign | 0.866 | Likely Pathogenic | Ambiguous | 0.142 | Likely Benign | 0.0983 | 0.3978 | -3.31 | Deleterious | 0.002 | Benign | 0.004 | Benign | 3.94 | Benign | 0.00 | Affected | -3 | -2 | -4.1 | 99.14 | ||||||||||||||||||||||||||||||||||||
| c.334G>T | G112W 2D  AIThe SynGAP1 missense variant G112W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized predicts a benign outcome, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive (2 pathogenic vs. 2 benign votes). Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions (five pathogenic vs. four benign) lean toward a pathogenic interpretation. This conclusion is not contradicted by ClinVar status, as the variant is not yet catalogued there. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.728858 | Disordered | 0.640153 | Binding | 0.332 | 0.867 | 0.750 | -6.382 | Likely Benign | 0.720 | Likely Pathogenic | Likely Benign | 0.190 | Likely Benign | 0.0631 | 0.4212 | -3.98 | Deleterious | 0.983 | Probably Damaging | 0.778 | Possibly Damaging | 3.90 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||||||
| c.335G>A | G112E 2D  AIThe SynGAP1 missense variant G112E is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are PROVEAN, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized result is uncertain, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta predictions are not available. Overall, the majority of evidence (five benign vs. three pathogenic) supports a benign classification. This conclusion does not contradict ClinVar status, as the variant has no ClinVar entry. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.728858 | Disordered | 0.640153 | Binding | 0.332 | 0.867 | 0.750 | -3.470 | Likely Benign | 0.818 | Likely Pathogenic | Ambiguous | 0.134 | Likely Benign | 0.1330 | 0.3814 | -3.30 | Deleterious | 0.421 | Benign | 0.146 | Benign | 3.96 | Benign | 0.00 | Affected | 0 | -2 | -3.1 | 72.06 | ||||||||||||||||||||||||||||||||||||
| c.335G>C | G112A 2D  AIThe SynGAP1 missense variant G112A is listed in ClinVar with an uncertain significance (ClinVar ID 1425533.0) and is present in gnomAD (6‑33432200‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also benign. Foldetta results are unavailable. Overall, the majority of computational evidence indicates that the variant is most likely benign, which is consistent with its ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.728858 | Disordered | 0.640153 | Binding | 0.332 | 0.867 | 0.750 | Uncertain | 1 | 6-33432200-G-C | 15 | 9.30e-6 | -2.456 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.114 | Likely Benign | 0.3817 | 0.4429 | -2.34 | Neutral | 0.231 | Benign | 0.054 | Benign | 4.07 | Benign | 0.00 | Affected | 3.61 | 5 | 1 | 0 | 2.2 | 14.03 | ||||||||||||||||||||||||||||
| c.335G>T | G112V 2D  AIThe SynGAP1 missense variant G112V is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of ClinVar annotation, so there is no contradiction with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.728858 | Disordered | 0.640153 | Binding | 0.332 | 0.867 | 0.750 | -2.411 | Likely Benign | 0.230 | Likely Benign | Likely Benign | 0.159 | Likely Benign | 0.1225 | 0.4218 | -3.39 | Deleterious | 0.421 | Benign | 0.108 | Benign | 3.96 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.3361A>C | S1121R 2D  AIThe SynGAP1 missense variant S1121R is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign, reflecting the majority of benign predictions. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (majority vote) also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact for S1121R, and this conclusion is consistent with the lack of ClinVar reporting. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.810024 | Binding | 0.365 | 0.935 | 0.875 | -6.945 | Likely Benign | 0.597 | Likely Pathogenic | Likely Benign | 0.142 | Likely Benign | 0.1346 | 0.3431 | -0.34 | Neutral | 0.016 | Benign | 0.015 | Benign | 5.45 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||
| c.3361A>G | S1121G 2D  AIThe SynGAP1 missense variant S1121G is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443913‑A‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also benign. Foldetta results are not available. Overall, the preponderance of evidence indicates that the variant is most likely benign, which does not contradict the current ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.810024 | Binding | 0.365 | 0.935 | 0.875 | Uncertain | 1 | 6-33443913-A-G | 1 | 7.00e-7 | -1.220 | Likely Benign | 0.054 | Likely Benign | Likely Benign | 0.067 | Likely Benign | 0.2724 | 0.4657 | -0.53 | Neutral | 0.003 | Benign | 0.004 | Benign | 6.63 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | 1 | 0.4 | -30.03 | ||||||||||||||||||||||||||||
| c.3361A>T | S1121C 2D  AIThe SynGAP1 missense variant S1121C is listed in gnomAD (ID 6‑33443913‑A‑T) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT; ESM1b is uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign, while the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar status (none is reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.810024 | Binding | 0.365 | 0.935 | 0.875 | 6-33443913-A-T | -7.431 | In-Between | 0.094 | Likely Benign | Likely Benign | 0.354 | Likely Benign | 0.1705 | 0.5691 | -0.99 | Neutral | 0.994 | Probably Damaging | 0.840 | Possibly Damaging | 5.43 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | 3.3 | 16.06 | ||||||||||||||||||||||||||||||||
| c.3362G>A | S1121N 2D  AIThe SynGAP1 missense variant S1121N is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and SIFT—suggest a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates Likely Benign. Foldetta results are unavailable. Overall, the consensus of the majority of predictors and the high‑accuracy tools points to a benign classification, with no conflict with ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.810024 | Binding | 0.365 | 0.935 | 0.875 | -6.564 | Likely Benign | 0.105 | Likely Benign | Likely Benign | 0.221 | Likely Benign | 0.1980 | 0.4211 | -0.12 | Neutral | 0.802 | Possibly Damaging | 0.266 | Benign | 5.50 | Benign | 0.00 | Affected | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||||||||||
| c.3362G>C | S1121T 2D  AIThe SynGAP1 missense variant S1121T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Based on the collective evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is provided). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.810024 | Binding | 0.365 | 0.935 | 0.875 | -6.442 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.105 | Likely Benign | 0.2108 | 0.5582 | -0.23 | Neutral | 0.011 | Benign | 0.026 | Benign | 5.45 | Benign | 0.00 | Affected | 1 | 1 | 0.1 | 14.03 | |||||||||||||||||||||||||||||||||||
| c.3362G>T | S1121I 2D  AIThe SynGAP1 missense variant S1121I is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus also as Likely Benign; the Foldetta protein‑folding stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.810024 | Binding | 0.365 | 0.935 | 0.875 | -6.215 | Likely Benign | 0.147 | Likely Benign | Likely Benign | 0.455 | Likely Benign | 0.1504 | 0.4776 | -0.96 | Neutral | 0.875 | Possibly Damaging | 0.559 | Possibly Damaging | 5.44 | Benign | 0.00 | Affected | -1 | -2 | 5.3 | 26.08 | |||||||||||||||||||||||||||||||||||
| c.3363C>A | S1121R 2D  AIThe SynGAP1 missense variant S1121R is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict any ClinVar annotation (none is present). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.810024 | Binding | 0.365 | 0.935 | 0.875 | -6.945 | Likely Benign | 0.597 | Likely Pathogenic | Likely Benign | 0.100 | Likely Benign | 0.1346 | 0.3431 | -0.34 | Neutral | 0.016 | Benign | 0.015 | Benign | 5.45 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -3.7 | 69.11 | |||||||||||||||||||||||||||||||||
| c.3363C>G | S1121R 2D  AIThe SynGAP1 missense variant S1121R is catalogued in gnomAD (ID 6‑33443915‑C‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized all report benign. Only SIFT and AlphaMissense‑Default predict pathogenicity, while the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. High‑accuracy assessments reinforce this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion is not contradicted by any ClinVar annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.894241 | Disordered | 0.810024 | Binding | 0.365 | 0.935 | 0.875 | 6-33443915-C-G | -6.945 | Likely Benign | 0.597 | Likely Pathogenic | Likely Benign | 0.101 | Likely Benign | 0.1346 | 0.3431 | -0.34 | Neutral | 0.016 | Benign | 0.015 | Benign | 5.45 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||||
| c.3371G>T | G1124V 2D  AIThe SynGAP1 missense variant G1124V is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only two tools—polyPhen‑2 HumDiv and SIFT—suggest a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates a benign outcome. Foldetta results are unavailable, so they do not influence the assessment. Overall, the consensus of available predictions indicates that G1124V is most likely benign, and this conclusion does not conflict with the absence of a ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.837511 | Disordered | 0.833401 | Binding | 0.341 | 0.931 | 0.875 | -6.980 | Likely Benign | 0.094 | Likely Benign | Likely Benign | 0.315 | Likely Benign | 0.1299 | 0.3888 | -0.96 | Neutral | 0.586 | Possibly Damaging | 0.172 | Benign | 4.75 | Benign | 0.00 | Affected | -1 | -3 | 4.6 | 42.08 | |||||||||||||||||||||||||||||||||||
| c.3373G>T | G1125W 2D  AIThe SynGAP1 missense variant G1125W is not reported in ClinVar (ClinVar ID = None) and is absent from gnomAD (gnomAD ID = None). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and ESM1b; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also favors a benign outcome. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the balance of evidence, especially from the high‑accuracy tools, points to a benign classification. This conclusion is not contradicted by any ClinVar annotation, as none exists for this variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | 0.834292 | Disordered | 0.835839 | Binding | 0.339 | 0.923 | 0.875 | -11.684 | Likely Pathogenic | 0.372 | Ambiguous | Likely Benign | 0.402 | Likely Benign | 0.0924 | 0.4046 | -1.10 | Neutral | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 4.50 | Benign | 0.00 | Affected | -7 | -2 | -0.5 | 129.16 | ||||||||||||||||||||||||||||||||||||
| c.3385C>A | L1129M 2D  AIThe SynGAP1 missense variant L1129M is not reported in ClinVar and is absent from gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports it as likely benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign effect for L1129M, and this conclusion does not conflict with ClinVar, which contains no entry for the variant. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.876543 | Binding | 0.339 | 0.909 | 0.875 | -4.233 | Likely Benign | 0.086 | Likely Benign | Likely Benign | 0.295 | Likely Benign | 0.1100 | 0.4236 | -0.59 | Neutral | 0.918 | Possibly Damaging | 0.697 | Possibly Damaging | 5.43 | Benign | 0.00 | Affected | 4 | 2 | -1.9 | 18.03 | |||||||||||||||||||||||||||||||||||
| c.3385C>G | L1129V 2D  AIThe SynGAP1 missense variant L1129V is not reported in ClinVar and is absent from gnomAD. Prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.876543 | Binding | 0.339 | 0.909 | 0.875 | -3.595 | Likely Benign | 0.093 | Likely Benign | Likely Benign | 0.262 | Likely Benign | 0.1814 | 0.3804 | -0.87 | Neutral | 0.393 | Benign | 0.187 | Benign | 5.46 | Benign | 0.00 | Affected | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||||||||||
| c.3386T>A | L1129Q 2D  AIThe SynGAP1 missense variant L1129Q is not reported in ClinVar and is absent from gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote) which classifies the variant as Likely Benign. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts Benign, and the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates Likely Benign. No Foldetta stability analysis is available for this variant. Overall, the majority of computational evidence points to a benign effect, and this conclusion does not contradict any ClinVar annotation (none exists). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.876543 | Binding | 0.339 | 0.909 | 0.875 | -3.684 | Likely Benign | 0.149 | Likely Benign | Likely Benign | 0.453 | Likely Benign | 0.1278 | 0.1436 | -1.63 | Neutral | 0.846 | Possibly Damaging | 0.525 | Possibly Damaging | 5.49 | Benign | 0.00 | Affected | -2 | -2 | -7.3 | 14.97 | |||||||||||||||||||||||||||||||||||
| c.3386T>C | L1129P 2D  AIThe SynGAP1 missense variant L1129P is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. The Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence—including high‑accuracy tools—points to a benign effect, and this conclusion does not contradict the current ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.876543 | Binding | 0.339 | 0.909 | 0.875 | Uncertain | 2 | -2.991 | Likely Benign | 0.154 | Likely Benign | Likely Benign | 0.432 | Likely Benign | 0.3017 | 0.2231 | 0.27 | Neutral | 0.971 | Probably Damaging | 0.773 | Possibly Damaging | 5.44 | Benign | 0.00 | Affected | 4.32 | 4 | -3 | -3 | -5.4 | -16.04 | |||||||||||||||||||||||||||||||
| c.3386T>G | L1129R 2D  AIThe SynGAP1 missense variant L1129R is not reported in ClinVar and has no entry in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome, while AlphaMissense‑Default remains uncertain. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as Likely Benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that the variant is most likely benign, and this conclusion does not contradict any ClinVar status (none reported). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.876543 | Binding | 0.339 | 0.909 | 0.875 | -2.613 | Likely Benign | 0.442 | Ambiguous | Likely Benign | 0.376 | Likely Benign | 0.1334 | 0.1636 | -1.68 | Neutral | 0.005 | Benign | 0.007 | Benign | 5.48 | Benign | 0.00 | Affected | -3 | -2 | -8.3 | 43.03 | |||||||||||||||||||||||||||||||||||
| c.3388A>C | K1130Q 2D  AIThe SynGAP1 missense variant K1130Q is reported in gnomAD (ID 6‑33443940‑A‑C) but has no ClinVar entry. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic outcome are polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence from both general and high‑accuracy predictors points to a benign impact, and this conclusion is consistent with the absence of a ClinVar pathogenic annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.863782 | Binding | 0.350 | 0.904 | 0.750 | 6-33443940-A-C | -3.548 | Likely Benign | 0.529 | Ambiguous | Likely Benign | 0.337 | Likely Benign | 0.4964 | 0.1756 | -1.25 | Neutral | 0.818 | Possibly Damaging | 0.355 | Benign | 5.44 | Benign | 0.00 | Affected | 4.32 | 4 | 1 | 1 | 0.4 | -0.04 | ||||||||||||||||||||||||||||||||
| c.3388A>G | K1130E 2D  AIThe SynGAP1 missense variant K1130E is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, and FATHMM. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy consensus from SGM (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields a Likely Benign classification, while AlphaMissense‑Optimized remains Uncertain. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of tools and the SGM consensus support a benign impact, and this assessment does not contradict any ClinVar status because no ClinVar entry exists. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.863782 | Binding | 0.350 | 0.904 | 0.750 | -4.998 | Likely Benign | 0.946 | Likely Pathogenic | Ambiguous | 0.422 | Likely Benign | 0.4251 | 0.1876 | -1.23 | Neutral | 0.649 | Possibly Damaging | 0.266 | Benign | 5.45 | Benign | 0.00 | Affected | 0 | 1 | 0.4 | 0.94 | |||||||||||||||||||||||||||||||||||
| c.3389A>C | K1130T 2D  AIThe SynGAP1 missense variant K1130T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) which classifies it as Likely Benign. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized result is Uncertain, and the Foldetta protein‑folding stability assessment is unavailable. Overall, the majority of evidence points to a benign impact. This conclusion is consistent with the lack of a ClinVar entry, so there is no contradiction with existing clinical annotations. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.863782 | Binding | 0.350 | 0.904 | 0.750 | -3.550 | Likely Benign | 0.829 | Likely Pathogenic | Ambiguous | 0.397 | Likely Benign | 0.2572 | 0.4349 | -1.38 | Neutral | 0.481 | Possibly Damaging | 0.157 | Benign | 5.47 | Benign | 0.00 | Affected | 0 | -1 | 3.2 | -27.07 | |||||||||||||||||||||||||||||||||||
| c.3389A>G | K1130R 2D  AIThe SynGAP1 missense variant K1130R is reported in gnomAD (variant ID 6‑33443941‑A‑G) but has no ClinVar entry. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the change as benign. Only SIFT predicts it to be pathogenic. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority‑vote) is benign; Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, and this assessment does not contradict any ClinVar status (none is available). Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.863782 | Binding | 0.350 | 0.904 | 0.750 | 6-33443941-A-G | -2.163 | Likely Benign | 0.155 | Likely Benign | Likely Benign | 0.187 | Likely Benign | 0.5096 | 0.2074 | Weaken | -0.94 | Neutral | 0.014 | Benign | 0.008 | Benign | 5.44 | Benign | 0.00 | Affected | 4.32 | 4 | 2 | 3 | -0.6 | 28.01 | |||||||||||||||||||||||||||||||
| c.3389A>T | K1130M 2D  AIThe SynGAP1 K1130M missense variant is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN), which collectively suggest a likely benign outcome. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized result is uncertain, and Foldetta (combining FoldX‑MD and Rosetta outputs) has no available prediction for this variant. Overall, the balance of evidence leans toward a benign classification, and this assessment does not contradict any ClinVar status because no ClinVar entry exists for K1130M. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.863782 | Binding | 0.350 | 0.904 | 0.750 | -4.844 | Likely Benign | 0.858 | Likely Pathogenic | Ambiguous | 0.476 | Likely Benign | 0.1703 | 0.4407 | -1.62 | Neutral | 0.990 | Probably Damaging | 0.796 | Possibly Damaging | 5.42 | Benign | 0.00 | Affected | 0 | -1 | 5.8 | 3.02 | |||||||||||||||||||||||||||||||||||
| c.3390G>C | K1130N 2D  AIThe SynGAP1 missense variant K1130N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) which classifies it as Likely Benign. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized result is uncertain, and Foldetta (combining FoldX‑MD and Rosetta outputs) has no available prediction for this variant. Overall, the majority of evidence points to a benign impact. The variant’s predicted benign status does not contradict ClinVar, as no ClinVar entry exists. Thus, based on current computational predictions, the K1130N variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.863782 | Binding | 0.350 | 0.904 | 0.750 | -4.822 | Likely Benign | 0.946 | Likely Pathogenic | Ambiguous | 0.336 | Likely Benign | 0.4119 | 0.2393 | -1.02 | Neutral | 0.818 | Possibly Damaging | 0.287 | Benign | 5.43 | Benign | 0.00 | Affected | 1 | 0 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||||
| c.3390G>T | K1130N 2D  AIThe SynGAP1 missense variant K1130N is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) which classifies it as Likely Benign. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized result is uncertain, and Foldetta (combining FoldX‑MD and Rosetta outputs) has no available prediction for this variant. Overall, the majority of evidence points to a benign impact. The variant’s predicted benign status does not contradict ClinVar, as no ClinVar entry exists. Thus, based on current computational predictions, the K1130N variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.903857 | Disordered | 0.863782 | Binding | 0.350 | 0.904 | 0.750 | -4.822 | Likely Benign | 0.946 | Likely Pathogenic | Ambiguous | 0.336 | Likely Benign | 0.4119 | 0.2393 | -1.02 | Neutral | 0.818 | Possibly Damaging | 0.287 | Benign | 5.43 | Benign | 0.00 | Affected | 1 | 0 | 0.4 | -14.07 | |||||||||||||||||||||||||||||||||||
| c.3391C>A | P1131T 2D  AIThe SynGAP1 missense variant P1131T is not reported in ClinVar (no ClinVar ID) and is absent from gnomAD (no gnomAD ID). Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN and SIFT. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a Likely Benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the lack of ClinVar annotation. Thus, the variant is most likely benign, with no contradiction to ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 0.919029 | Disordered | 0.855155 | Binding | 0.360 | 0.899 | 0.750 | -4.766 | Likely Benign | 0.219 | Likely Benign | Likely Benign | 0.313 | Likely Benign | 0.1353 | 0.6315 | -2.93 | Deleterious | 0.245 | Benign | 0.096 | Benign | 5.30 | Benign | 0.00 | Affected | 0 | -1 | 0.9 | 3.99 | |||||||||||||||||||||||||||||||||||
Found 8751 rows. Show 800 rows per page. Page 2/11 « Previous | Next »