

Table of SynGAP1 Isoform α2 (UniProt Q96PV0-1) Missense Variants.
| c.dna | Variant | SGM Consensus | Domain | ClinVar | gnomAD | ESM1b | AlphaMissense | REVEL | FoldX | Rosetta | Foldetta | PremPS | PROVEAN | PolyPhen-2 HumDiv | PolyPhen-2 HumVar | FATHMM | SIFT | PAM | Physical | SASA | Normalized B-factor backbone | Normalized B-factor sidechain | SynGAP Structural Annotation | DOI | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Status | Review | Subm. | ID | Allele count | Allele freq. | LLR score | Prediction | Pathogenicity | Class | Optimized | Score | Prediction | Average ΔΔG | Prediction | StdDev | ΔΔG | Prediction | ΔΔG | Prediction | ΔΔG | Prediction | Score | Prediction | pph2_prob | Prediction | pph2_prob | Prediction | Nervous System Score | Prediction | Prediction | Status | Conservation | Sequences | PAM250 | PAM120 | Hydropathy Δ | MW Δ | Average | Δ | Δ | StdDev | Δ | StdDev | Secondary | Tertiary bonds | Inside out | GAP-Ras interface | At membrane | No effect | MD Alert | Verdict | Description | |||||
| c.2029A>T | S677C 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S677C is reported in ClinVar as Benign (ClinVar ID 2825814.0) and is not present in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, Rosetta, Foldetta, premPS, PROVEAN, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, polyPhen‑2 HumVar, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 HumDiv, SIFT, and ESM1b predict a pathogenic impact. High‑accuracy predictors all support a benign outcome: AlphaMissense‑Optimized is benign, the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is Likely Benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) is benign. No prediction or folding‑stability result is missing or inconclusive. Based on the preponderance of evidence, the variant is most likely benign, and this assessment aligns with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign | 1 | -8.496 | Likely Pathogenic | 0.076 | Likely Benign | Likely Benign | 0.153 | Likely Benign | -0.51 | Ambiguous | 0.3 | -0.30 | Likely Benign | -0.41 | Likely Benign | 0.15 | Likely Benign | -2.41 | Neutral | 0.932 | Possibly Damaging | 0.222 | Benign | 3.25 | Benign | 0.04 | Affected | 3.41 | 23 | -1 | 0 | 3.3 | 16.06 | ||||||||||||||||||||
| c.2115G>C | K705N 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant K705N is listed in ClinVar (ID 872011.0) as Pathogenic and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions (REVEL, Rosetta, premPS, FATHMM) and pathogenic predictions (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default). Uncertain results come from FoldX, Foldetta, and AlphaMissense‑Optimized. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is inconclusive, but the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—classifies the variant as Likely Pathogenic, and Foldetta also yields an uncertain stability change. Overall, the preponderance of evidence indicates the variant is most likely pathogenic, which aligns with its ClinVar classification and does not contradict the reported status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Pathogenic | 1 | -9.767 | Likely Pathogenic | 0.925 | Likely Pathogenic | Ambiguous | 0.183 | Likely Benign | 0.74 | Ambiguous | 0.0 | 0.37 | Likely Benign | 0.56 | Ambiguous | 0.44 | Likely Benign | -3.12 | Deleterious | 0.996 | Probably Damaging | 0.876 | Possibly Damaging | 3.37 | Benign | 0.02 | Affected | 3.47 | 10 | 1 | 0 | 0.4 | -14.07 | 221.4 | -20.2 | 0.0 | 0.0 | 0.0 | 0.1 | X | Uncertain | The amino side chain of Lys705, located at the end and outer surface of an α-helix (res. Thr704-Gly712), does not form any interactions in the WT simulations. In the variant simulations, the carboxamide side chain of Asn705 briefly forms a salt bridge with Glu706. However, there is no apparent difference between the systems. Due to the model ending abruptly at the C-terminus, no definite conclusions can be drawn based on the simulations. | |||||||||||
| c.2207G>A | R736H 2D  AIThe SynGAP1 missense variant R736H is listed in ClinVar (ID 1351080.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33441672‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign. Foldetta results are not available. Overall, the majority of computational evidence indicates a benign impact, and this does not contradict the ClinVar “Uncertain” classification. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33441672-G-A | 6 | 3.72e-6 | -5.409 | Likely Benign | 0.067 | Likely Benign | Likely Benign | 0.029 | Likely Benign | -0.12 | Neutral | 0.004 | Benign | 0.001 | Benign | 2.50 | Benign | 0.00 | Affected | 4.07 | 3 | 2 | 0 | 1.3 | -19.05 | |||||||||||||||||||||||||||
| c.2210A>C | Q737P 2D  AIThe SynGAP1 missense variant Q737P is listed in ClinVar (ID 2580571.0) with an uncertain significance designation and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while only SIFT indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta stability analysis is unavailable. Taken together, the preponderance of evidence supports a benign classification for Q737P, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.407 | Likely Benign | 0.054 | Likely Benign | Likely Benign | 0.154 | Likely Benign | -1.22 | Neutral | 0.005 | Benign | 0.013 | Benign | 2.78 | Benign | 0.04 | Affected | 4.07 | 3 | -1 | 0 | 1.9 | -31.01 | ||||||||||||||||||||||||||||||
| c.2270G>C | G757A 2D  AIThe SynGAP1 missense change G757A is catalogued in ClinVar (ID 3635272.0) with an uncertain significance designation and is not reported in gnomAD. Functional prediction algorithms uniformly classify the variant as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores. No tool in the dataset predicts pathogenicity. High‑accuracy consensus methods corroborate this view: the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign effect, and AlphaMissense‑Optimized also predicts benign. The Foldetta stability assessment is unavailable for this variant. Taken together, the evidence overwhelmingly supports a benign interpretation, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.626 | Likely Benign | 0.091 | Likely Benign | Likely Benign | 0.066 | Likely Benign | -0.45 | Neutral | 0.267 | Benign | 0.127 | Benign | 2.73 | Benign | 0.35 | Tolerated | 1 | 0 | 2.2 | 14.03 | ||||||||||||||||||||||||||||||||
| c.2305C>T | L769F 2D  AIThe SynGAP1 missense variant L769F is listed in ClinVar (ID 3617309.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority of the high‑accuracy tools) also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of predictions—including the high‑accuracy tools—suggest the variant is most likely benign, which is consistent with its ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -5.044 | Likely Benign | 0.146 | Likely Benign | Likely Benign | 0.060 | Likely Benign | -0.89 | Neutral | 0.925 | Possibly Damaging | 0.510 | Possibly Damaging | 3.94 | Benign | 0.02 | Affected | 2 | 0 | -1.0 | 34.02 | ||||||||||||||||||||||||||||||||
| c.2324G>C | R775P 2D  AIThe SynGAP1 missense variant R775P (ClinVar ID 2959355.0) is classified as Benign in ClinVar and is not reported in gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, consistent with the ClinVar designation, and there is no contradiction with the reported ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | -5.072 | Likely Benign | 0.452 | Ambiguous | Likely Benign | 0.168 | Likely Benign | -0.79 | Neutral | 0.971 | Probably Damaging | 0.944 | Probably Damaging | 4.13 | Benign | 0.07 | Tolerated | 3.64 | 6 | -2 | 0 | 2.9 | -59.07 | ||||||||||||||||||||||||||||||
| c.2339C>G | S780C 2D  AIThe SynGAP1 missense variant S780C is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33442891‑C‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). No tool in the dataset predicts a pathogenic outcome; ESM1b is inconclusive and therefore treated as unavailable. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign, while Foldetta results are not reported and thus unavailable. Based on the collective predictions, the variant is most likely benign, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 4 | 6-33442891-C-G | 16 | 9.94e-6 | -7.603 | In-Between | 0.278 | Likely Benign | Likely Benign | 0.078 | Likely Benign | -1.41 | Neutral | 0.065 | Benign | 0.043 | Benign | 2.59 | Benign | 0.10 | Tolerated | 3.64 | 6 | -1 | 0 | 3.3 | 16.06 | |||||||||||||||||||||||||||
| c.2350G>A | A784T 2D  AIThe SynGAP1 missense variant A784T is listed in ClinVar (ID 962668.0) as Benign and is not reported in gnomAD. Across the available in‑silico predictors, every tool examined—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—consistently classifies the substitution as benign. No tool predicts pathogenicity. High‑accuracy assessments reinforce this consensus: AlphaMissense‑Optimized reports a benign effect, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign outcome. A Foldetta stability analysis is unavailable, so it does not influence the overall interpretation. Based on the unanimous benign predictions and the ClinVar designation, the variant is most likely benign, with no contradiction to the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | -3.579 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.046 | Likely Benign | 1.23 | Neutral | 0.001 | Benign | 0.006 | Benign | 2.92 | Benign | 1.00 | Tolerated | 3.64 | 6 | 1 | 0 | -2.5 | 30.03 | ||||||||||||||||||||||||||||||
| c.2354G>A | R785H 2D  AIThe SynGAP1 R785H missense variant (ClinVar ID 2321588.0) is listed as “Uncertain” in ClinVar and is present in gnomAD (ID 6‑33442906‑G‑A). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, while those that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, whereas the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a pathogenic outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, does not provide a result for this variant. Overall, the majority of computational predictions (five pathogenic versus three benign) lean toward a pathogenic interpretation. Thus, the variant is most likely pathogenic based on current predictions, and this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | Uncertain | 2 | 6-33442906-G-A | 4 | 2.50e-6 | -4.782 | Likely Benign | 0.388 | Ambiguous | Likely Benign | 0.129 | Likely Benign | -2.61 | Deleterious | 0.999 | Probably Damaging | 0.947 | Probably Damaging | 2.25 | Pathogenic | 0.01 | Affected | 3.64 | 6 | 2 | 0 | 1.3 | -19.05 | |||||||||||||||||||||||||||
| c.2369C>A | T790N 2D 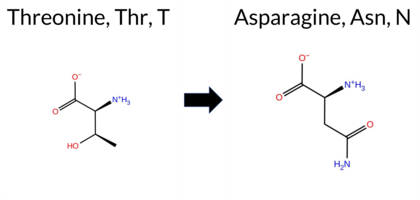 AIThe SynGAP1 missense variant T790N is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (ID 6‑33442921‑C‑A). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive and therefore unavailable, and Foldetta results are not reported. Overall, the majority of conventional tools (5 pathogenic vs. 4 benign) lean toward a pathogenic interpretation, while the single high‑accuracy tool suggests benign. The variant’s ClinVar status remains uncertain, so there is no contradiction with the current clinical classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | Conflicting | 3 | 6-33442921-C-A | 69 | 4.28e-5 | -5.243 | Likely Benign | 0.276 | Likely Benign | Likely Benign | 0.103 | Likely Benign | -2.54 | Deleterious | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.27 | Pathogenic | 0.02 | Affected | 3.64 | 6 | 0 | 0 | -2.8 | 13.00 | |||||||||||||||||||||||||||
| c.2444G>A | R815H 2D  AIThe SynGAP1 missense variant R815H (ClinVar ID 833773.0) is classified as benign in ClinVar and is present in gnomAD (6‑33442996‑G‑A). Functional prediction tools cluster into two groups: benign predictions from REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions from polyPhen‑2 (HumDiv and HumVar) and SIFT. Two tools report uncertainty: ESM1b and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is benign, while the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) and Foldetta stability assessment are unavailable. Overall, the balance of evidence favors a benign effect, consistent with the ClinVar annotation and with no conflict regarding its status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | Likely Benign | 2 | 6-33442996-G-A | 24 | 1.49e-5 | -7.474 | In-Between | 0.553 | Ambiguous | Likely Benign | 0.157 | Likely Benign | -1.81 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 2.61 | Benign | 0.02 | Affected | 4.32 | 4 | 2 | 0 | 1.3 | -19.05 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||
| c.2506A>G | S836G 2D  AIThe SynGAP1 missense variant S836G is listed in ClinVar (ID 537003.0) with an uncertain significance annotation and is observed in the gnomAD database (variant ID 6‑33443058‑A‑G). Consensus from multiple in silico predictors indicates a benign effect: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the substitution as benign. No tool in the dataset predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized reports a benign outcome, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is labeled Likely Benign. Foldetta, a protein‑folding stability predictor, did not return a result for this variant, so its status is unavailable. Overall, the computational evidence strongly supports a benign classification, which does not conflict with the ClinVar uncertain designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443058-A-G | 4 | 2.48e-6 | -4.749 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.066 | Likely Benign | -1.65 | Neutral | 0.006 | Benign | 0.019 | Benign | 2.54 | Benign | 0.39 | Tolerated | 3.77 | 5 | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||
| c.2514C>A | N838K 2D  AIThe SynGAP1 missense variant N838K is listed in ClinVar with an “Uncertain” status (ClinVar ID 1377909.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, SIFT, and FATHMM, whereas those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Pathogenic.” High‑accuracy assessments show AlphaMissense‑Optimized as “Uncertain,” SGM‑Consensus as “Likely Pathogenic,” and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the balance of evidence leans toward a pathogenic interpretation, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 2 | -8.470 | Likely Pathogenic | 0.862 | Likely Pathogenic | Ambiguous | 0.097 | Likely Benign | -2.78 | Deleterious | 0.997 | Probably Damaging | 0.995 | Probably Damaging | 2.69 | Benign | 0.16 | Tolerated | 3.77 | 5 | 1 | 0 | -0.4 | 14.07 | ||||||||||||||||||||||||||||||
| c.2522T>C | V841A 2D 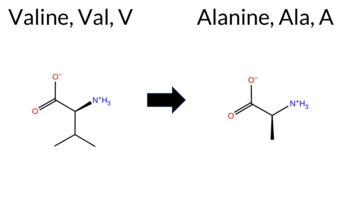 AIThe SynGAP1 missense variant V841A (ClinVar ID 1395978.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33443074‑T‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and FATHMM, whereas those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized tool reports an uncertain outcome, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie (two pathogenic, two benign) and is therefore inconclusive. No Foldetta stability assessment is available for this variant. Overall, the balance of evidence favors a pathogenic interpretation, which does not contradict the current ClinVar designation of Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33443074-T-C | 3 | 1.86e-6 | -8.152 | Likely Pathogenic | 0.901 | Likely Pathogenic | Ambiguous | 0.183 | Likely Benign | -2.13 | Neutral | 0.992 | Probably Damaging | 0.989 | Probably Damaging | 2.57 | Benign | 0.02 | Affected | 3.77 | 5 | 0 | 0 | -2.4 | -28.05 | ||||||||||||||||||||||||||||
| c.2561G>A | R854H 2D  AIThe SynGAP1 missense variant R854H is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33443113‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which is “Likely Benign”). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; the Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443113-G-A | 4 | 2.48e-6 | -3.686 | Likely Benign | 0.094 | Likely Benign | Likely Benign | 0.183 | Likely Benign | -1.38 | Neutral | 0.997 | Probably Damaging | 0.899 | Possibly Damaging | 4.07 | Benign | 0.04 | Affected | 3.88 | 3 | 2 | 0 | 1.3 | -19.05 | |||||||||||||||||||||||||||
| c.2591C>T | A864V 2D  AIThe SynGAP1 missense variant A864V is listed in ClinVar with an uncertain significance (ClinVar ID 655662.0) and is observed in gnomAD (ID 6‑33443143‑C‑T). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv and FATHMM. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is benign, and Foldetta results are unavailable. Based on the preponderance of evidence, the variant is most likely benign, which does not contradict its current ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33443143-C-T | 6 | 3.72e-6 | -4.749 | Likely Benign | 0.126 | Likely Benign | Likely Benign | 0.038 | Likely Benign | -1.35 | Neutral | 0.767 | Possibly Damaging | 0.119 | Benign | 2.45 | Pathogenic | 0.30 | Tolerated | 3.82 | 4 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.2632A>G | T878A 2D  AIThe SynGAP1 missense variant T878A is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. All available in‑silico predictors agree on a benign effect: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” High‑accuracy tools likewise support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is benign; Foldetta results are unavailable. Based on the unanimous benign predictions, the variant is most likely benign, and this assessment does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.154 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.088 | Likely Benign | -0.67 | Neutral | 0.003 | Benign | 0.006 | Benign | 2.73 | Benign | 0.18 | Tolerated | 3.77 | 5 | 1 | 0 | 2.5 | -30.03 | ||||||||||||||||||||||||||||||
| c.263T>C | V88A 2D  AIThe SynGAP1 missense variant V88A is listed in ClinVar (ID 2656486.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. Tools that predict a pathogenic effect are AlphaMissense‑Default, AlphaMissense‑Optimized, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (majority vote) is benign; Foldetta stability analysis is unavailable. Overall, the balance of evidence favors a benign interpretation, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -5.860 | Likely Benign | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.050 | Likely Benign | -1.22 | Neutral | 0.053 | Benign | 0.008 | Benign | 3.75 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 0 | -2.4 | -28.05 | ||||||||||||||||||||||||||||||
| c.2657C>T | A886V 2D  AIThe SynGAP1 missense variant A886V is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (gnomAD ID 6‑33443209‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus call (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also as benign, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443209-C-T | 18 | 1.12e-5 | -4.478 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.061 | Likely Benign | -0.20 | Neutral | 0.888 | Possibly Damaging | 0.314 | Benign | 2.17 | Pathogenic | 0.00 | Affected | 4.32 | 4 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.2669G>A | R890H 2D  AIThe SynGAP1 missense variant R890H is listed in ClinVar as a benign alteration (ClinVar ID 1037885.0) and is observed in gnomAD (6‑33443221‑G‑A). All evaluated in‑silico predictors agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores, and no tool predicts pathogenicity. High‑accuracy assessments reinforce this consensus: AlphaMissense‑Optimized is benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign,” while Foldetta’s protein‑folding stability analysis is unavailable. Overall, the computational evidence strongly supports a benign classification, which is consistent with the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33443221-G-A | 19 | 1.18e-5 | -3.600 | Likely Benign | 0.198 | Likely Benign | Likely Benign | 0.056 | Likely Benign | -1.29 | Neutral | 0.254 | Benign | 0.134 | Benign | 3.97 | Benign | 0.15 | Tolerated | 4.32 | 4 | 2 | 0 | 1.3 | -19.05 | |||||||||||||||||||||||||||
| c.2702C>T | A901V 2D  AIThe SynGAP1 missense variant A901V is listed in ClinVar (ID 934469.0) with an “Uncertain” clinical significance and is present in the gnomAD database (gnomAD ID 6‑33443254‑C‑T). All evaluated in‑silico predictors classify the substitution as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool reports a pathogenic or likely pathogenic outcome. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the computational evidence strongly supports a benign effect, which does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33443254-C-T | 2 | 1.24e-6 | -5.043 | Likely Benign | 0.219 | Likely Benign | Likely Benign | 0.029 | Likely Benign | -1.83 | Neutral | 0.106 | Benign | 0.009 | Benign | 2.64 | Benign | 0.17 | Tolerated | 3.77 | 5 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.2704G>A | A902T 2D  AIThe SynGAP1 missense variant A902T is listed in ClinVar (ID 1027238.0) as benign and is observed in gnomAD (variant ID 6‑33443256‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The high‑accuracy consensus, SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. AlphaMissense‑Optimized also predicts benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, was not available for this variant. Overall, the majority of computational evidence supports a benign impact, consistent with the ClinVar annotation, and there is no contradiction between the predictions and the clinical classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | 6-33443256-G-A | 36 | 2.23e-5 | -4.966 | Likely Benign | 0.116 | Likely Benign | Likely Benign | 0.075 | Likely Benign | -1.11 | Neutral | 0.951 | Possibly Damaging | 0.617 | Possibly Damaging | 2.61 | Benign | 0.01 | Affected | 3.77 | 5 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.2714G>A | R905H 2D  AIThe SynGAP1 missense variant R905H is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443266‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta’s protein‑folding stability analysis is unavailable for this variant. Overall, the majority of computational evidence points to a benign effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443266-G-A | 8 | 4.96e-6 | -4.182 | Likely Benign | 0.457 | Ambiguous | Likely Benign | 0.192 | Likely Benign | -1.11 | Neutral | 1.000 | Probably Damaging | 0.991 | Probably Damaging | 2.59 | Benign | 0.09 | Tolerated | 3.77 | 5 | 2 | 0 | 1.3 | -19.05 | |||||||||||||||||||||||||||
| c.2724G>C | Q908H 2D  AIThe SynGAP1 missense variant Q908H is listed in ClinVar (ID 436926.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33443276‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This consensus does not contradict the ClinVar “Uncertain” classification, which remains inconclusive. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 4 | 6-33443276-G-C | 1 | 6.20e-7 | -4.658 | Likely Benign | 0.311 | Likely Benign | Likely Benign | 0.112 | Likely Benign | -0.74 | Neutral | 0.996 | Probably Damaging | 0.995 | Probably Damaging | 2.58 | Benign | 0.05 | Affected | 3.77 | 5 | 3 | 0 | 0.3 | 9.01 | |||||||||||||||||||||||||||
| c.2729G>C | G910A 2D  AIThe SynGAP1 missense variant G910A is listed in ClinVar with an “Uncertain” status (ClinVar ID 2091237.0) and is present in gnomAD (6‑33443281‑G‑C). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PolyPhen‑2 HumDiv and PolyPhen‑2 HumVar. The remaining predictions are uncertain: AlphaMissense‑Default is inconclusive, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports a likely benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443281-G-C | 1 | 6.20e-7 | -3.587 | Likely Benign | 0.361 | Ambiguous | Likely Benign | 0.209 | Likely Benign | -1.43 | Neutral | 0.999 | Probably Damaging | 0.999 | Probably Damaging | 2.78 | Benign | 0.10 | Tolerated | 3.77 | 5 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||
| c.2735C>A | T912N 2D  AIThe SynGAP1 missense variant T912N is listed in ClinVar with an uncertain significance (ClinVar ID 2337624.0) and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as likely benign. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates likely benign. No Foldetta (FoldX‑MD/Rosetta) stability result is available, so it does not influence the overall assessment. Based on the preponderance of evidence, the variant is most likely benign, and this conclusion does not contradict the current ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -4.260 | Likely Benign | 0.190 | Likely Benign | Likely Benign | 0.116 | Likely Benign | -1.15 | Neutral | 0.999 | Probably Damaging | 0.977 | Probably Damaging | 3.96 | Benign | 0.00 | Affected | 3.77 | 5 | 0 | 0 | -2.8 | 13.00 | ||||||||||||||||||||||||||||||
| c.2743G>A | G915S 2D  AIThe SynGAP1 missense variant G915S is listed in ClinVar as Benign (ClinVar ID 652083.0) and is present in the gnomAD database (gnomAD ID 6‑33443295‑G‑A). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign. Only polyPhen‑2 HumDiv reports a pathogenic prediction, representing the sole discordant signal. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is Likely Benign. No Foldetta stability result is available, so it does not influence the assessment. Overall, the majority of evidence indicates that the variant is most likely benign, and this conclusion is consistent with its ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33443295-G-A | 9 | 5.58e-6 | -3.557 | Likely Benign | 0.083 | Likely Benign | Likely Benign | 0.050 | Likely Benign | -0.88 | Neutral | 0.801 | Possibly Damaging | 0.201 | Benign | 2.73 | Benign | 0.31 | Tolerated | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.2750C>G | P917R 2D  AIThe SynGAP1 missense variant P917R is listed in ClinVar with an “Uncertain” status and is present in gnomAD (gnomAD ID 6‑33443302‑C‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also as benign, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443302-C-G | 5 | 3.10e-6 | -4.475 | Likely Benign | 0.363 | Ambiguous | Likely Benign | 0.142 | Likely Benign | -1.70 | Neutral | 0.642 | Possibly Damaging | 0.316 | Benign | 2.68 | Benign | 0.00 | Affected | 3.77 | 5 | -2 | 0 | -2.9 | 59.07 | |||||||||||||||||||||||||||
| c.2753C>T | A918V 2D  AIThe SynGAP1 missense variant A918V is listed in ClinVar with an “Uncertain” status and is present in gnomAD (gnomAD ID 6‑33443305‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; a Foldetta stability prediction is not available. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 3 | 6-33443305-C-T | 2 | 1.24e-6 | -3.684 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.119 | Likely Benign | -1.61 | Neutral | 0.980 | Probably Damaging | 0.782 | Possibly Damaging | 2.61 | Benign | 0.03 | Affected | 4.32 | 4 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.2818G>A | G940S 2D  AIThe SynGAP1 missense variant G940S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443370‑G‑A). All available in silico predictors agree on a benign effect: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” High‑accuracy tools reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus likewise indicates a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no reported result for this variant, so its status is unavailable. Overall, the computational evidence strongly supports a benign classification, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443370-G-A | 1 | 6.20e-7 | -5.451 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.135 | Likely Benign | 0.45 | Neutral | 0.409 | Benign | 0.253 | Benign | 2.77 | Benign | 0.44 | Tolerated | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.2830G>A | G944S 2D  AIThe SynGAP1 missense variant G944S is listed in ClinVar as a benign alteration (ClinVar ID 833552.0) and is present in the gnomAD database (gnomAD ID 6‑33443382‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available for this variant. Overall, the majority of computational evidence supports a benign classification, which aligns with the ClinVar status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33443382-G-A | 13 | 8.05e-6 | -5.303 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.223 | Likely Benign | -0.75 | Neutral | 0.007 | Benign | 0.004 | Benign | 3.77 | Benign | 0.00 | Affected | 4.32 | 4 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.2835T>A | H945Q 2D  AIThe SynGAP1 missense variant H945Q is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33443387‑T‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The high‑accuracy consensus from AlphaMissense‑Optimized is benign, and the SGM consensus—derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—is also benign. Foldetta results are not available for this variant. Overall, the majority of computational evidence points to a benign impact, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 2 | 6-33443387-T-A | 3 | 1.86e-6 | -5.248 | Likely Benign | 0.091 | Likely Benign | Likely Benign | 0.343 | Likely Benign | -0.36 | Neutral | 0.995 | Probably Damaging | 0.939 | Probably Damaging | 5.03 | Benign | 0.06 | Tolerated | 4.32 | 4 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||
| c.2840G>C | G947A 2D  AIThe SynGAP1 missense variant G947A is listed in ClinVar (ID 1595137.0) as Benign and is present in gnomAD (6‑33443392‑G‑C). Prediction tools that assess sequence conservation and functional impact (REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM) all classify the variant as Benign. The AlphaMissense suite likewise reports Benign for both its Default and Optimized models. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates Likely Benign. No tool in the dataset predicts pathogenicity. High‑accuracy structural assessment via AlphaMissense‑Optimized confirms a Benign prediction, while the SGM‑Consensus result is consistent. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no reported result for this variant, so its status is unavailable. Overall, the computational evidence overwhelmingly supports a benign effect, aligning with the ClinVar designation and showing no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | 6-33443392-G-C | 28 | 1.73e-5 | -6.511 | Likely Benign | 0.080 | Likely Benign | Likely Benign | 0.156 | Likely Benign | -0.41 | Neutral | 0.224 | Benign | 0.131 | Benign | 4.97 | Benign | 0.10 | Tolerated | 4.32 | 4 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||
| c.2845G>A | G949S 2D  AIThe SynGAP1 missense variant G949S is listed in ClinVar as a benign alteration (ClinVar ID 212352.0) and is present in the gnomAD database (6‑33443397‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as likely benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar classification and indicating no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign/Likely benign | 4 | 6-33443397-G-A | 122 | 7.56e-5 | -5.693 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.321 | Likely Benign | 0.30 | Neutral | 0.611 | Possibly Damaging | 0.102 | Benign | 2.23 | Pathogenic | 0.00 | Affected | 4.32 | 4 | 1 | 0 | -0.4 | 30.03 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||
| c.2852A>G | H951R 2D 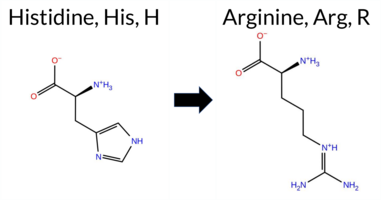 AIThe SynGAP1 missense variant H951R is listed in ClinVar as Pathogenic (ClinVar ID 1003739.0) and is not reported in gnomAD. Functional prediction tools uniformly classify the variant as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign. The Foldetta protein‑folding stability analysis is unavailable for this variant. Based on the collective predictions, the variant is most likely benign, which contradicts its ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Pathogenic | 1 | -4.964 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.185 | Likely Benign | -1.08 | Neutral | 0.048 | Benign | 0.029 | Benign | 5.46 | Benign | 0.24 | Tolerated | 3.77 | 5 | 2 | 0 | -1.3 | 19.05 | ||||||||||||||||||||||||||||||
| c.2854G>A | G952S 2D  AIThe SynGAP1 missense variant G952S is listed in ClinVar (ID 1325573.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33443406‑G‑A). All evaluated in‑silico predictors agree on a benign effect: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores. No tool predicts pathogenicity. High‑accuracy assessments reinforce this consensus: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so its status is unavailable. Overall, the computational evidence strongly supports a benign classification, which is consistent with the ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 2 | 6-33443406-G-A | 2 | 1.24e-6 | -6.190 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.167 | Likely Benign | 0.19 | Neutral | 0.000 | Benign | 0.002 | Benign | 3.31 | Benign | 0.07 | Tolerated | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.286G>A | G96S 2D  AIThe SynGAP1 missense variant G96S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33425894‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33425894-G-A | 5 | 3.10e-6 | -3.049 | Likely Benign | 0.065 | Likely Benign | Likely Benign | 0.071 | Likely Benign | -0.76 | Neutral | 0.364 | Benign | 0.008 | Benign | 4.25 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.2888A>G | H963R 2D  AIThe SynGAP1 missense variant H963R is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443440‑A‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Only ESM1b predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized reports benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) yields a benign consensus. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no reported result for this variant, so its status is unavailable. Overall, the preponderance of evidence indicates the variant is most likely benign, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443440-A-G | 8 | 4.96e-6 | -8.952 | Likely Pathogenic | 0.169 | Likely Benign | Likely Benign | 0.081 | Likely Benign | -1.28 | Neutral | 0.001 | Benign | 0.003 | Benign | 4.15 | Benign | 0.24 | Tolerated | 3.77 | 5 | 2 | 0 | -1.3 | 19.05 | |||||||||||||||||||||||||||
| c.2912C>A | P971H 2D  AIThe SynGAP1 missense variant P971H is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443464‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; a Foldetta stability prediction is not available. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443464-C-A | 1 | 6.20e-7 | -5.243 | Likely Benign | 0.086 | Likely Benign | Likely Benign | 0.039 | Likely Benign | -1.11 | Neutral | 0.898 | Possibly Damaging | 0.477 | Possibly Damaging | 3.89 | Benign | 0.00 | Affected | 4.32 | 2 | -2 | 0 | -1.6 | 40.02 | |||||||||||||||||||||||||||
| c.2924C>A | T975N 2D  AIThe SynGAP1 missense variant T975N is listed in ClinVar (ID 942242.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33443476‑C‑A). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report benign or tolerated outcomes. Only polyPhen‑2 HumDiv predicts a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as “Likely Benign.” High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact, which is consistent with the ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443476-C-A | 1 | 6.20e-7 | -4.671 | Likely Benign | 0.089 | Likely Benign | Likely Benign | 0.100 | Likely Benign | -0.58 | Neutral | 0.586 | Possibly Damaging | 0.302 | Benign | 4.13 | Benign | 0.07 | Tolerated | 4.32 | 2 | 0 | 0 | -2.8 | 13.00 | |||||||||||||||||||||||||||
| c.2928T>G | F976L 2D  AIThe SynGAP1 missense variant F976L is listed in ClinVar (ID 624245.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM. Only AlphaMissense‑Default predicts a pathogenic outcome. The high‑accuracy consensus predictor SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. AlphaMissense‑Optimized is inconclusive, and no Foldetta (FoldX‑MD/Rosetta stability) result is available. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar designation of uncertainty rather than a definitive pathogenic claim. Thus, the variant is most likely benign, and its predictions do not contradict the current ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.432 | Likely Benign | 0.825 | Likely Pathogenic | Ambiguous | 0.212 | Likely Benign | -0.87 | Neutral | 0.264 | Benign | 0.102 | Benign | 4.20 | Benign | 0.53 | Tolerated | 4.32 | 2 | 2 | 0 | 1.0 | -34.02 | ||||||||||||||||||||||||||||||
| c.2935T>C | F979L 2D  AIThe SynGAP1 missense variant F979L (ClinVar ID 1000410.0, status Uncertain, not found in gnomAD) has been evaluated by multiple in silico predictors. Benign predictions come from REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, and FATHMM, while pathogenic predictions are reported by polyPhen‑2 HumDiv and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is classified as Likely Benign. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain, whereas the SGM‑Consensus (majority vote) supports a benign outcome. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta, has no available result for this variant. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar status of Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.341 | Likely Benign | 0.870 | Likely Pathogenic | Ambiguous | 0.228 | Likely Benign | -1.00 | Neutral | 0.625 | Possibly Damaging | 0.430 | Benign | 4.22 | Benign | 0.73 | Tolerated | 4.32 | 2 | 2 | 0 | 1.0 | -34.02 | ||||||||||||||||||||||||||||||
| c.2962C>T | L988F 2D  AIThe SynGAP1 missense variant L988F is listed in ClinVar (ID 968833.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33443514‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic outcome are polyPhen‑2 (HumDiv and HumVar) and SIFT. AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is not in conflict with the ClinVar “Uncertain” classification. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443514-C-T | 1 | 6.20e-7 | -4.368 | Likely Benign | 0.356 | Ambiguous | Likely Benign | 0.135 | Likely Benign | -1.70 | Neutral | 0.977 | Probably Damaging | 0.900 | Possibly Damaging | 2.69 | Benign | 0.00 | Affected | 4.32 | 2 | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||
| c.2987C>G | P996R 2D  AIThe SynGAP1 missense variant P996R is listed in ClinVar (ID 2808854.0) as benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the consensus of the majority of tools, including the high‑accuracy methods, indicates a benign impact. This prediction aligns with the ClinVar benign classification and does not contradict the existing clinical annotation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | -4.457 | Likely Benign | 0.141 | Likely Benign | Likely Benign | 0.040 | Likely Benign | -1.04 | Neutral | 0.144 | Benign | 0.085 | Benign | 4.26 | Benign | 0.01 | Affected | 4.32 | 4 | -2 | 0 | -2.9 | 59.07 | ||||||||||||||||||||||||||||||
| c.2989G>A | A997T 2D  AIThe SynGAP1 missense variant A997T is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (derived from the four high‑accuracy predictors) is benign; Foldetta results are unavailable. Taken together, the majority of computational evidence indicates a benign effect, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -4.102 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.085 | Likely Benign | -0.62 | Neutral | 0.224 | Benign | 0.120 | Benign | 4.17 | Benign | 0.00 | Affected | 4.32 | 4 | 1 | 0 | -2.5 | 30.03 | ||||||||||||||||||||||||||||||
| c.303C>A | H101Q 2D  AIThe SynGAP1 missense variant H101Q is listed in ClinVar with an uncertain significance (ClinVar ID 1307533.0) and is present in gnomAD (ID 6‑33432168‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) also benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33432168-C-A | 1 | 6.20e-7 | -2.827 | Likely Benign | 0.124 | Likely Benign | Likely Benign | 0.147 | Likely Benign | -0.37 | Neutral | 0.824 | Possibly Damaging | 0.880 | Possibly Damaging | 4.24 | Benign | 0.00 | Affected | 4.32 | 1 | 3 | 0 | -0.3 | -9.01 | |||||||||||||||||||||||||||
| c.3053C>T | T1018I 2D  AIThe SynGAP1 missense variant T1018I is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443605‑C‑T). Prediction tools that agree on benign impact include REVEL, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Optimized, while those that predict pathogenicity are PROVEAN, polyPhen‑2 HumDiv, SIFT, and FATHMM; AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta results are unavailable. Overall, the predictions are split, with no clear majority leaning toward either benign or pathogenic. Thus, the variant’s impact remains inconclusive, and this uncertainty aligns with ClinVar’s current “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33443605-C-T | 4 | 2.48e-6 | -3.264 | Likely Benign | 0.524 | Ambiguous | Likely Benign | 0.076 | Likely Benign | -2.55 | Deleterious | 0.586 | Possibly Damaging | 0.304 | Benign | 2.24 | Pathogenic | 0.01 | Affected | 3.77 | 5 | -1 | 0 | 5.2 | 12.05 | ||||||||||||||||||||||||||||
| c.3056G>A | R1019H 2D  AIThe SynGAP1 missense variant R1019H is listed in ClinVar (ID 1195115.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33443608‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) also benign; Foldetta stability analysis is unavailable. Overall, the preponderance of evidence points to a benign impact for R1019H, and this conclusion does not contradict the current ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 2 | 6-33443608-G-A | 67 | 4.15e-5 | -4.610 | Likely Benign | 0.258 | Likely Benign | Likely Benign | 0.122 | Likely Benign | -1.95 | Neutral | 0.995 | Probably Damaging | 0.845 | Possibly Damaging | 2.39 | Pathogenic | 0.01 | Affected | 3.77 | 5 | 2 | 0 | 1.3 | -19.05 | |||||||||||||||||||||||||||
| c.3116T>C | I1039T 2D 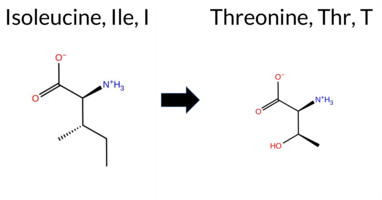 AIThe SynGAP1 missense variant I1039T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443668‑T‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM; the SGM‑Consensus score (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. Only AlphaMissense‑Default predicts a pathogenic effect. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM‑Consensus remains benign; a Foldetta stability analysis is not available. Overall, the majority of computational evidence supports a benign impact, and this is consistent with the ClinVar “Uncertain” classification, so there is no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443668-T-C | 12 | 7.43e-6 | -2.465 | Likely Benign | 0.645 | Likely Pathogenic | Likely Benign | 0.193 | Likely Benign | 0.45 | Neutral | 0.004 | Benign | 0.008 | Benign | 2.75 | Benign | 0.10 | Tolerated | 3.77 | 5 | -1 | 0 | -5.2 | -12.05 | |||||||||||||||||||||||||||
| c.3134C>G | A1045G 2D  AIThe SynGAP1 missense variant A1045G is reported in ClinVar as Benign (ClinVar ID 416778.0) and is present in the gnomAD database (gnomAD ID 6‑33443686‑C‑G). Prediction tools that assess pathogenicity all converge on a benign outcome: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all classify the variant as benign. No tool in the dataset predicts pathogenicity. High‑accuracy assessments reinforce this consensus: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are not available. Overall, the variant is most likely benign, and this prediction aligns with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign/Likely benign | 7 | 6-33443686-C-G | 1407 | 8.72e-4 | -3.246 | Likely Benign | 0.075 | Likely Benign | Likely Benign | 0.024 | Likely Benign | -1.21 | Neutral | 0.224 | Benign | 0.066 | Benign | 2.64 | Benign | 0.33 | Tolerated | 3.77 | 5 | 1 | 0 | -2.2 | -14.03 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||
| c.3160G>A | G1054S 2D  AIThe SynGAP1 missense variant G1054S is listed in ClinVar (ID 699126.0) as Benign and is present in gnomAD (variant ID 6‑33443712‑G‑A). All evaluated in‑silico predictors classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool reports a pathogenic or likely pathogenic outcome. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts Benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) yields Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the computational evidence strongly supports a benign effect, consistent with the ClinVar designation and not contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33443712-G-A | 32 | 1.99e-5 | -5.294 | Likely Benign | 0.075 | Likely Benign | Likely Benign | 0.160 | Likely Benign | 0.21 | Neutral | 0.121 | Benign | 0.013 | Benign | 4.04 | Benign | 0.63 | Tolerated | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.3172G>A | G1058S 2D  AIThe SynGAP1 missense variant G1058S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6-33443724-G-A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also benign. Foldetta results are unavailable. Overall, the majority of computational evidence indicates that the variant is most likely benign, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | 6-33443724-G-A | 114 | 7.08e-5 | -5.178 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.108 | Likely Benign | 0.26 | Neutral | 0.001 | Benign | 0.001 | Benign | 5.38 | Benign | 0.04 | Affected | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.3176G>C | G1059A 2D  AIThe SynGAP1 missense variant G1059A is listed in ClinVar (ID 1420036.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33443728‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign effect. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of computational evidence supports a benign impact for G1059A, which does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443728-G-C | 4 | 2.49e-6 | -6.754 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.329 | Likely Benign | -0.17 | Neutral | 0.001 | Benign | 0.002 | Benign | 2.56 | Benign | 0.00 | Affected | 4.32 | 2 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||
| c.3178G>A | G1060S 2D  AIThe SynGAP1 missense variant G1060S is listed in ClinVar with an uncertain significance (ClinVar ID 1512003.0) and is present in gnomAD (variant ID 6‑33443730‑G‑A). All evaluated in‑silico predictors classify the change as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity. High‑accuracy assessments reinforce this benign view: AlphaMissense‑Optimized reports benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the computational evidence overwhelmingly supports a benign effect, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443730-G-A | -4.759 | Likely Benign | 0.082 | Likely Benign | Likely Benign | 0.376 | Likely Benign | -0.08 | Neutral | 0.271 | Benign | 0.054 | Benign | 2.69 | Benign | 0.49 | Tolerated | 4.32 | 2 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||||
| c.3181G>A | G1061S 2D  AIThe SynGAP1 missense variant G1061S is listed in ClinVar (ID 3571724.0) with an uncertain significance designation and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while only SIFT indicates a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign and the SGM‑Consensus is likely benign; Foldetta stability analysis is unavailable. Overall, the consensus of the majority of prediction tools and the high‑accuracy methods supports a benign classification for G1061S, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -4.891 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.283 | Likely Benign | -0.68 | Neutral | 0.004 | Benign | 0.004 | Benign | 4.00 | Benign | 0.00 | Affected | 1 | 0 | -0.4 | 30.03 | ||||||||||||||||||||||||||||||||
| c.3192G>C | Q1064H 2D  AIThe SynGAP1 missense variant Q1064H is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT all predict a pathogenic impact. The SGM‑Consensus, which aggregates the majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote) also indicates benign. The Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence—including high‑accuracy tools—points to a benign effect, and this conclusion does not contradict the current ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -4.576 | Likely Benign | 0.162 | Likely Benign | Likely Benign | 0.063 | Likely Benign | -0.66 | Neutral | 0.938 | Possibly Damaging | 0.596 | Possibly Damaging | 4.15 | Benign | 0.05 | Affected | 3 | 0 | 0.3 | 9.01 | ||||||||||||||||||||||||||||||||
| c.3238G>A | A1080T 2D  AIThe SynGAP1 missense variant A1080T (ClinVar ID 1473274.0) is listed as “Uncertain” in ClinVar and is present in gnomAD (ID 6‑33443790‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool in the dataset predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the computational evidence strongly suggests the variant is most likely benign, which does not contradict the current ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 2 | 6-33443790-G-A | 17 | 1.06e-5 | -3.928 | Likely Benign | 0.133 | Likely Benign | Likely Benign | 0.144 | Likely Benign | -0.19 | Neutral | 0.253 | Benign | 0.042 | Benign | 4.10 | Benign | 0.60 | Tolerated | 3.77 | 5 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.3251C>A | P1084H 2D  AIThe SynGAP1 missense variant P1084H is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443803‑C‑A). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (which reports “Likely Benign”). Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar designation of uncertainty rather than a definitive pathogenic claim. Thus, the variant is most likely benign, and its prediction profile does not contradict the current ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443803-C-A | 1 | 6.31e-7 | -4.125 | Likely Benign | 0.323 | Likely Benign | Likely Benign | 0.134 | Likely Benign | -3.16 | Deleterious | 0.997 | Probably Damaging | 0.840 | Possibly Damaging | 3.96 | Benign | 0.00 | Affected | 3.77 | 5 | -2 | 0 | -1.6 | 40.02 | |||||||||||||||||||||||||||
| c.3287A>C | E1096A 2D  AIThe SynGAP1 missense variant E1096A is listed in ClinVar (ID 2579889.0) with an uncertain significance annotation and is not reported in gnomAD. Consensus from multiple in‑silico predictors shows a predominance of benign calls: REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only polyPhen‑2 HumDiv assigns a pathogenic label, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus also indicates likely benign; Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the aggregate evidence points to a benign effect, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -4.504 | Likely Benign | 0.510 | Ambiguous | Likely Benign | 0.164 | Likely Benign | -1.37 | Neutral | 0.626 | Possibly Damaging | 0.184 | Benign | 2.77 | Benign | 0.16 | Tolerated | 3.77 | 5 | -1 | 0 | 5.3 | -58.04 | ||||||||||||||||||||||||||||||
| c.3304G>A | A1102T 2D  AIThe SynGAP1 missense variant A1102T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443856‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only FATHMM predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443856-G-A | 11 | 7.17e-6 | -3.540 | Likely Benign | 0.070 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -0.30 | Neutral | 0.001 | Benign | 0.001 | Benign | 2.32 | Pathogenic | 0.95 | Tolerated | 3.77 | 5 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.3305C>T | A1102V 2D  AIThe SynGAP1 missense variant A1102V is listed in ClinVar (ID 2846719.0) as Benign and is present in gnomAD (variant ID 6‑33443857‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion aligns with the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33443857-C-T | -2.440 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.081 | Likely Benign | -1.27 | Neutral | 0.017 | Benign | 0.028 | Benign | 2.29 | Pathogenic | 0.12 | Tolerated | 3.77 | 5 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||||
| c.3308G>A | R1103H 2D  AIThe SynGAP1 missense variant R1103H is listed in ClinVar (ID 577408.0) as benign and is present in gnomAD (variant ID 6‑33443860‑G‑A). Prediction tools that classify the variant as benign include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM Consensus. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as benign, while Foldetta results are unavailable. Overall, the majority of predictions support a benign impact, and this conclusion aligns with the ClinVar benign classification, indicating no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign/Likely benign | 3 | 6-33443860-G-A | 31 | 2.03e-5 | -3.622 | Likely Benign | 0.156 | Likely Benign | Likely Benign | 0.116 | Likely Benign | -1.97 | Neutral | 0.996 | Probably Damaging | 0.733 | Possibly Damaging | 2.49 | Pathogenic | 0.01 | Affected | 3.77 | 5 | 2 | 0 | 1.3 | -19.05 | |||||||||||||||||||||||||||
| c.3328A>G | S1110G 2D  AIThe SynGAP1 missense variant S1110G is listed in ClinVar (ID 1722210.0) as benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only FATHMM predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion aligns with the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | -4.674 | Likely Benign | 0.079 | Likely Benign | Likely Benign | 0.035 | Likely Benign | -2.26 | Neutral | 0.036 | Benign | 0.026 | Benign | 2.19 | Pathogenic | 0.08 | Tolerated | 4.32 | 2 | 1 | 0 | 0.4 | -30.03 | ||||||||||||||||||||||||||||||
| c.3354C>A | S1118R 2D  AIThe SynGAP1 missense variant S1118R (ClinVar ID 2656489.0) is listed as ClinVar status Uncertain and is not present in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only SIFT predicts a pathogenic effect, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar Uncertain designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.670 | Likely Benign | 0.553 | Ambiguous | Likely Benign | 0.166 | Likely Benign | -0.74 | Neutral | 0.034 | Benign | 0.023 | Benign | 5.17 | Benign | 0.05 | Affected | 4.32 | 2 | -1 | 0 | -3.7 | 69.11 | ||||||||||||||||||||||||||||||
| c.335G>C | G112A 2D  AIThe SynGAP1 missense variant G112A is listed in ClinVar with an uncertain significance (ClinVar ID 1425533.0) and is present in gnomAD (6‑33432200‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also benign. Foldetta results are unavailable. Overall, the majority of computational evidence indicates that the variant is most likely benign, which is consistent with its ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33432200-G-C | 15 | 9.30e-6 | -2.456 | Likely Benign | 0.119 | Likely Benign | Likely Benign | 0.114 | Likely Benign | -2.34 | Neutral | 0.231 | Benign | 0.054 | Benign | 4.07 | Benign | 0.00 | Affected | 3.61 | 5 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||
| c.3364G>A | G1122S 2D  AIThe SynGAP1 missense variant G1122S is listed in ClinVar as a benign alteration (ClinVar ID 643187.0) and is present in gnomAD (gnomAD ID 6‑33443916‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool in the dataset predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates likely benign, while Foldetta’s protein‑folding stability result is unavailable. Overall, the variant is most likely benign, and this assessment is consistent with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign/Likely benign | 2 | 6-33443916-G-A | 27 | 1.79e-5 | -4.880 | Likely Benign | 0.072 | Likely Benign | Likely Benign | 0.189 | Likely Benign | -0.08 | Neutral | 0.022 | Benign | 0.006 | Benign | 4.89 | Benign | 0.92 | Tolerated | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.3374G>C | G1125A 2D  AIThe SynGAP1 missense variant G1125A is listed in ClinVar with an “Uncertain” status and is present in gnomAD (6‑33443926‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized; the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic effect. High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (derived from the four high‑accuracy tools) is benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443926-G-C | 1 | 6.68e-7 | -6.569 | Likely Benign | 0.083 | Likely Benign | Likely Benign | 0.232 | Likely Benign | -0.60 | Neutral | 0.999 | Probably Damaging | 0.995 | Probably Damaging | 4.60 | Benign | 0.11 | Tolerated | 3.77 | 5 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||
| c.3380G>C | G1127A 2D  AIThe SynGAP1 missense variant G1127A is listed in ClinVar (ID 426748.0) with an uncertain significance status and is present in gnomAD (variant ID 6‑33443932‑G‑C). Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all score the variant as benign. No tool predicts pathogenicity. The high‑accuracy consensus methods corroborate this: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta, a protein‑folding stability predictor combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant, so its status is unavailable. Overall, the evidence strongly supports a benign classification, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 4 | 6-33443932-G-C | 4 | 2.68e-6 | -5.949 | Likely Benign | 0.080 | Likely Benign | Likely Benign | 0.164 | Likely Benign | -0.43 | Neutral | 0.001 | Benign | 0.002 | Benign | 4.83 | Benign | 1.00 | Tolerated | 4.32 | 4 | 1 | 0 | 2.2 | 14.03 | |||||||||||||||||||||||||||
| c.3405G>C | K1135N 2D  AIThe SynGAP1 missense variant K1135N is listed in ClinVar (ID 633521.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and FATHMM. In contrast, AlphaMissense‑Default and AlphaMissense‑Optimized both predict a pathogenic outcome. High‑accuracy assessments further show AlphaMissense‑Optimized as pathogenic, while the SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) remains Likely Benign. No Foldetta (FoldX‑MD/Rosetta stability) result is available for this variant. Overall, the majority of predictions support a benign classification, which does not contradict the current ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -5.715 | Likely Benign | 0.960 | Likely Pathogenic | Likely Pathogenic | 0.166 | Likely Benign | -0.97 | Neutral | 0.411 | Benign | 0.321 | Benign | 5.43 | Benign | 0.07 | Tolerated | 4.32 | 2 | 1 | 0 | 0.4 | -14.07 | ||||||||||||||||||||||||||||||
| c.3410A>C | H1137P 2D  AIThe SynGAP1 missense variant H1137P is listed in ClinVar as a benign alteration (ClinVar ID 3685596.0) and is present in the gnomAD database (gnomAD ID 6‑33444445‑A‑C). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). In contrast, polyPhen‑2 (HumDiv and HumVar) and SIFT predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (derived from the same four high‑accuracy predictors) also indicates benign. No Foldetta (FoldX‑MD/Rosetta stability) result is available for this variant. Overall, the majority of predictions, including the most reliable tools, support a benign classification, which is consistent with the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33444445-A-C | 12 | 7.44e-6 | -2.098 | Likely Benign | 0.054 | Likely Benign | Likely Benign | 0.419 | Likely Benign | -1.93 | Neutral | 0.925 | Possibly Damaging | 0.703 | Possibly Damaging | 5.29 | Benign | 0.00 | Affected | 4.32 | 4 | -2 | 0 | 1.6 | -40.02 | |||||||||||||||||||||||||||
| c.3449C>T | A1150V 2D  AIThe SynGAP1 missense variant A1150V is listed in ClinVar (ID 589625.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33444484‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are SIFT and FATHMM. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is also benign. Foldetta, a protein‑folding stability method, did not provide a result for this variant. Overall, the majority of computational evidence indicates that A1150V is most likely benign, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33444484-C-T | 3 | 1.86e-6 | -3.648 | Likely Benign | 0.192 | Likely Benign | Likely Benign | 0.066 | Likely Benign | -2.22 | Neutral | 0.114 | Benign | 0.055 | Benign | 2.32 | Pathogenic | 0.04 | Affected | 3.77 | 5 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.3508A>G | S1170G 2D  AIThe SynGAP1 missense variant S1170G is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on benign impact include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool in the dataset predicts pathogenicity, so the pathogenic group is empty. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also predicts benign, while Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | -4.288 | Likely Benign | 0.221 | Likely Benign | Likely Benign | 0.349 | Likely Benign | -0.81 | Neutral | 0.241 | Benign | 0.229 | Benign | 5.31 | Benign | 0.54 | Tolerated | 4.32 | 4 | 1 | 0 | 0.4 | -30.03 | |||||||||||||||||||||||||||||
| c.3511G>A | A1171T 2D  AIThe SynGAP1 missense variant A1171T is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. All available in‑silico predictors classify the change as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports a “Likely Benign” outcome. No tool predicts pathogenicity. High‑accuracy assessments confirm the benign prediction: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | -3.658 | Likely Benign | 0.149 | Likely Benign | Likely Benign | 0.201 | Likely Benign | -0.48 | Neutral | 0.245 | Benign | 0.138 | Benign | 5.45 | Benign | 0.07 | Tolerated | 4.32 | 4 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||||
| c.3511_3512delinsTG | A1171C 2D  AIThe SynGAP1 missense variant A1171C (ClinVar ID 1723483.0) is listed as “Uncertain” in ClinVar and is not present in gnomAD. Prediction tools that converge on a benign outcome include PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized, all of which report a benign or neutral effect. In contrast, PolyPhen‑2 (HumDiv and HumVar) and SIFT uniformly predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign,” reinforcing the benign signal. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus (majority vote) as benign, and Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | -5.363 | Likely Benign | 0.496 | Ambiguous | Likely Benign | -1.16 | Neutral | 0.978 | Probably Damaging | 0.825 | Possibly Damaging | 5.32 | Benign | 0.02 | Affected | 4.32 | 4 | -2 | 0 | 0.7 | 32.06 | |||||||||||||||||||||||||||||||
| c.3607C>T | H1203Y 2D  AIThe SynGAP1 missense variant H1203Y is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (ID 6‑33446599‑C‑T). Functional prediction tools uniformly indicate a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all score the variant as benign. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized is benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also yields a benign prediction. Foldetta results are unavailable. Overall, the evidence strongly supports a benign impact for H1203Y, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Uncertain | 1 | 6-33446599-C-T | 2 | 1.24e-6 | -6.834 | Likely Benign | 0.149 | Likely Benign | Likely Benign | 0.233 | Likely Benign | -1.52 | Neutral | 0.006 | Benign | 0.011 | Benign | 5.55 | Benign | 0.10 | Tolerated | 3.77 | 5 | 2 | 0 | 1.9 | 26.03 | ||||||||||||||||||||||||||
| c.3638A>C | N1213T 2D  AIThe SynGAP1 missense variant N1213T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33446630‑A‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support benignity: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, while Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Coiled-coil | Conflicting | 2 | 6-33446630-A-C | 46 | 2.85e-5 | -5.428 | Likely Benign | 0.266 | Likely Benign | Likely Benign | 0.097 | Likely Benign | -1.08 | Neutral | 0.959 | Probably Damaging | 0.721 | Possibly Damaging | 2.74 | Benign | 1.00 | Tolerated | 3.77 | 5 | 0 | 0 | 2.8 | -13.00 | ||||||||||||||||||||||||||
| c.371C>T | A124V 2D  AIThe SynGAP1 A124V missense variant is listed in ClinVar (ID 1040523.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33432236‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of computational evidence indicates a benign effect, and this consensus does not contradict the ClinVar “Uncertain” designation. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 2 | 6-33432236-C-T | 9 | 5.58e-6 | -4.259 | Likely Benign | 0.138 | Likely Benign | Likely Benign | 0.073 | Likely Benign | -1.52 | Neutral | 0.173 | Benign | 0.009 | Benign | 4.07 | Benign | 0.03 | Affected | 3.61 | 5 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.382C>A | P128T 2D  AIThe SynGAP1 missense variant P128T is listed in ClinVar with an “Uncertain” status (ClinVar ID 2801315.0) and is present in gnomAD (ID 6‑33432247‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33432247-C-A | 1 | 6.20e-7 | -4.217 | Likely Benign | 0.267 | Likely Benign | Likely Benign | 0.075 | Likely Benign | -0.96 | Neutral | 0.952 | Possibly Damaging | 0.500 | Possibly Damaging | 4.19 | Benign | 0.35 | Tolerated | 3.74 | 4 | -1 | 0 | 0.9 | 3.99 | |||||||||||||||||||||||||||
| c.3835G>A | A1279T 2D  AIThe SynGAP1 missense variant A1279T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33447883‑G‑A). All available in silico predictors report a benign effect: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized are benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” No tool predicts pathogenicity. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is benign; Foldetta results are not available. Overall, the computational evidence strongly supports a benign classification, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33447883-G-A | 2 | 1.29e-6 | -4.871 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.178 | Likely Benign | -0.30 | Neutral | 0.001 | Benign | 0.000 | Benign | 2.71 | Benign | 0.09 | Tolerated | 3.77 | 5 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.3859C>A | P1287T 2D  AIThe SynGAP1 missense variant P1287T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33447907‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33447907-C-A | -3.940 | Likely Benign | 0.077 | Likely Benign | Likely Benign | 0.044 | Likely Benign | -0.22 | Neutral | 0.126 | Benign | 0.041 | Benign | 2.78 | Benign | 0.04 | Affected | 3.77 | 5 | -1 | 0 | 0.9 | 3.99 | |||||||||||||||||||||||||||||
| c.3862A>G | K1288E 2D  AISynGAP1 missense variant K1288E is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33447910‑A‑G). Functional prediction tools cluster into two groups: benign predictions come from REVEL, ESM1b, and AlphaMissense‑Optimized, while pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, whereas the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—labels the variant pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this change. Overall, the preponderance of evidence points to a pathogenic effect, which is consistent with the ClinVar designation of uncertain significance rather than a clear benign classification. Thus, the variant is most likely pathogenic, and this does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33447910-A-G | 5 | 3.22e-6 | -2.751 | Likely Benign | 0.407 | Ambiguous | Likely Benign | 0.185 | Likely Benign | -3.27 | Deleterious | 0.979 | Probably Damaging | 0.973 | Probably Damaging | 2.13 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 1 | 0 | 0.4 | 0.94 | ||||||||||||||||||||||||||||
| c.3906G>C | L1302F 2D  AISynGAP1 missense variant L1302F is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized, while pathogenic calls are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments indicate AlphaMissense‑Optimized predicts benign, whereas the SGM consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is inconclusive due to a 2‑to‑2 split; Foldetta stability analysis is unavailable. Overall, the majority of predictions (five pathogenic versus four benign) lean toward a pathogenic effect. Thus, the variant is most likely pathogenic, a conclusion that contrasts with its current ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | -5.674 | Likely Benign | 0.148 | Likely Benign | Likely Benign | 0.211 | Likely Benign | -2.70 | Deleterious | 0.960 | Probably Damaging | 0.657 | Possibly Damaging | 1.53 | Pathogenic | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | |||||||||||||||||||||||||||||||||
| c.3907G>A | G1303S 2D  AIThe SynGAP1 missense variant G1303S is listed in ClinVar (ID 1736068.0) with an “Uncertain” clinical significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Only polyPhen‑2 HumDiv predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; a Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign impact, and this is consistent with the ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.271 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.155 | Likely Benign | -0.19 | Neutral | 0.649 | Possibly Damaging | 0.433 | Benign | 2.84 | Benign | 0.18 | Tolerated | 1 | 0 | -0.4 | 30.03 | ||||||||||||||||||||||||||||||||
| c.3913A>G | T1305A 2D  AIThe SynGAP1 missense variant T1305A is listed in ClinVar (ID 411587.0) with an “Uncertain” clinical significance and is present in the gnomAD database (variant ID 6‑33451787‑A‑G). All evaluated in‑silico predictors classify the change as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool reports a pathogenic or likely pathogenic outcome. High‑accuracy assessments reinforce this benign prediction: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are unavailable. Overall, the computational evidence overwhelmingly supports a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 4 | 6-33451787-A-G | 30 | 1.86e-5 | -2.692 | Likely Benign | 0.055 | Likely Benign | Likely Benign | 0.069 | Likely Benign | 1.74 | Neutral | 0.000 | Benign | 0.001 | Benign | 3.24 | Benign | 1.00 | Tolerated | 3.77 | 5 | 1 | 0 | 2.5 | -30.03 | |||||||||||||||||||||||||||
| c.3923G>A | R1308H 2D  AIThe SynGAP1 missense variant R1308H (ClinVar ID 1996244.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33451797‑G‑A). Prediction tools that agree on a benign effect include REVEL, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessment shows AlphaMissense‑Optimized predicts a benign outcome; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive, and Foldetta results are unavailable. Consequently, the overall computational evidence leans toward a pathogenic interpretation, but the presence of a single high‑accuracy benign prediction and the inconclusive SGM Consensus leave the variant’s effect uncertain. This computational assessment does not contradict the ClinVar status, which remains Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33451797-G-A | 3 | 1.86e-6 | -3.586 | Likely Benign | 0.201 | Likely Benign | Likely Benign | 0.319 | Likely Benign | -3.12 | Deleterious | 0.998 | Probably Damaging | 0.991 | Probably Damaging | 2.33 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||||||||
| c.3949G>A | G1317S 2D  AIThe SynGAP1 missense variant G1317S is listed in ClinVar with an uncertain significance and is present in the gnomAD database. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized returns a benign prediction, and the SGM‑Consensus (derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates benign. Foldetta, a protein‑folding stability method, has no available result for this variant. Overall, the computational evidence overwhelmingly points to a benign effect, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | 6-33451823-G-A | 1 | 6.26e-7 | -3.522 | Likely Benign | 0.145 | Likely Benign | Likely Benign | 0.092 | Likely Benign | -2.45 | Neutral | 0.127 | Benign | 0.045 | Benign | 4.08 | Benign | 0.00 | Affected | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.3956C>G | A1319G 2D  AIThe SynGAP1 missense variant A1319G is listed in ClinVar (ID 1690510.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451830‑C‑G). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic impact. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this is consistent with the ClinVar “Uncertain” status rather than contradicting it. Thus, the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33451830-C-G | -3.927 | Likely Benign | 0.084 | Likely Benign | Likely Benign | 0.128 | Likely Benign | -0.74 | Neutral | 0.819 | Possibly Damaging | 0.581 | Possibly Damaging | 4.07 | Benign | 0.06 | Tolerated | 3.77 | 5 | 1 | 0 | -2.2 | -14.03 | |||||||||||||||||||||||||||||
| c.3977C>A | P1326Q 2D  AIThe SynGAP1 missense variant P1326Q is listed in ClinVar (ID 2806103.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33451851‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This conclusion does not contradict the ClinVar “Uncertain” classification, which remains inconclusive. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33451851-C-A | 1 | 6.40e-7 | -5.422 | Likely Benign | 0.128 | Likely Benign | Likely Benign | 0.138 | Likely Benign | -0.86 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 3.62 | Benign | 0.00 | Affected | 3.77 | 5 | -1 | 0 | -1.9 | 31.01 | |||||||||||||||||||||||||||
| c.4003G>A | G1335S 2D  AIThe SynGAP1 missense variant G1335S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33451877‑G‑A). Prediction tools that agree on a benign effect include REVEL and ESM1b, whereas the remaining tools (PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized) all predict a pathogenic outcome. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized classifies the variant as pathogenic, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) reports it as Likely Pathogenic. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence indicates that G1335S is most likely pathogenic, which is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Conflicting | 2 | 6-33451877-G-A | 3 | 2.37e-6 | -4.495 | Likely Benign | 0.986 | Likely Pathogenic | Likely Pathogenic | 0.362 | Likely Benign | -3.79 | Deleterious | 1.000 | Probably Damaging | 0.997 | Probably Damaging | 2.04 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 1 | 0 | -0.4 | 30.03 | |||||||||||||||||||||||||||
| c.4021G>A | A1341T 2D  AIThe SynGAP1 missense variant A1341T is listed in ClinVar (ID 837815.0) with an “Uncertain” clinical significance and is present in gnomAD (variant ID 6‑33451895‑G‑A). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all report benign or likely benign. Only SIFT predicts a pathogenic effect. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates a likely benign outcome. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact for A1341T, which is consistent with the ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | 6-33451895-G-A | 45 | 3.44e-5 | -3.224 | Likely Benign | 0.081 | Likely Benign | Likely Benign | 0.099 | Likely Benign | -0.58 | Neutral | 0.000 | Benign | 0.000 | Benign | 4.09 | Benign | 0.03 | Affected | 3.77 | 5 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.43G>A | A15T 2D  AIThe SynGAP1 missense variant A15T is listed in ClinVar (ID 1925632.0) with an “Uncertain” clinical significance and is present in gnomAD (6‑33420307‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as “Likely Benign”; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33420307-G-A | 4 | 2.60e-6 | -3.720 | Likely Benign | 0.125 | Likely Benign | Likely Benign | 0.086 | Likely Benign | -0.08 | Neutral | 0.602 | Possibly Damaging | 0.017 | Benign | 4.16 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 0 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.44C>T | A15V 2D  AIThe SynGAP1 missense variant A15V is listed in ClinVar (ID 1801174.0) with an “Uncertain” status and is present in gnomAD (6‑33420308‑C‑T). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized, while polyPhen‑2 HumDiv and SIFT predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign effect, and this does not contradict the ClinVar designation, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33420308-C-T | 1 | 6.49e-7 | -3.560 | Likely Benign | 0.161 | Likely Benign | Likely Benign | 0.105 | Likely Benign | 0.20 | Neutral | 0.602 | Possibly Damaging | 0.015 | Benign | 4.19 | Benign | 0.00 | Affected | 4.32 | 1 | 0 | 0 | 2.4 | 28.05 | |||||||||||||||||||||||||||
| c.458C>A | T153N 2D  AIThe SynGAP1 missense variant T153N is listed in ClinVar (ID 984906.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict benign, while the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also indicates a likely benign outcome. In contrast, polyPhen‑2 (HumDiv and HumVar) predict pathogenic. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence supports a benign classification, and this is consistent with the ClinVar “Uncertain” designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | -0.739 | Likely Benign | 0.226 | Likely Benign | Likely Benign | 0.161 | Likely Benign | 0.88 | Neutral | 0.888 | Possibly Damaging | 0.537 | Possibly Damaging | 4.23 | Benign | 0.81 | Tolerated | 3.61 | 5 | 0 | 0 | -2.8 | 13.00 | ||||||||||||||||||||||||||||||
| c.470G>A | R157H 2D  AIThe SynGAP1 missense variant R157H (ClinVar ID 2065231.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33432767‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—yields a tie and is therefore inconclusive. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no reported result for this variant. Overall, the balance of predictions leans toward pathogenic, but the high‑accuracy tools do not provide a definitive verdict. This assessment does not contradict the ClinVar status, which remains Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33432767-G-A | 1 | 6.20e-7 | -10.235 | Likely Pathogenic | 0.604 | Likely Pathogenic | Likely Benign | 0.254 | Likely Benign | -2.23 | Neutral | 0.999 | Probably Damaging | 0.987 | Probably Damaging | 3.80 | Benign | 0.00 | Affected | 3.74 | 4 | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||||||||
| c.485G>A | R162H 2D  AIThe SynGAP1 missense variant R162H is listed in ClinVar with an uncertain significance and is present in the gnomAD database (variant ID 6‑33432782‑G‑A). Functional prediction tools cluster into two groups: benign calls are made by REVEL, PROVEAN, SIFT, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic calls come from polyPhen‑2 (HumDiv and HumVar) and ESM1b; AlphaMissense‑Default remains uncertain. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—also yields a benign verdict. Foldetta, a protein‑folding stability method that integrates FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the preponderance of evidence points to a benign effect, which does not contradict the ClinVar uncertain classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33432782-G-A | 2 | 1.24e-6 | -9.730 | Likely Pathogenic | 0.480 | Ambiguous | Likely Benign | 0.167 | Likely Benign | -1.13 | Neutral | 0.957 | Probably Damaging | 0.513 | Possibly Damaging | 4.03 | Benign | 0.12 | Tolerated | 3.74 | 4 | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||||||||
| c.558G>C | L186F 2D  AIThe SynGAP1 missense variant L186F is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, polyPhen‑2 (HumDiv and HumVar), and FATHMM. In contrast, tools that predict a pathogenic effect are PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Pathogenic.” High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized predicts pathogenicity, and the SGM‑Consensus (majority vote) also indicates likely pathogenic. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of computational evidence points to a pathogenic effect, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Uncertain | 1 | -11.861 | Likely Pathogenic | 0.993 | Likely Pathogenic | Likely Pathogenic | 0.132 | Likely Benign | -3.03 | Deleterious | 0.009 | Benign | 0.012 | Benign | 3.50 | Benign | 0.00 | Affected | 2 | 0 | -1.0 | 34.02 | ||||||||||||||||||||||||||||||||
| c.597C>A | N199K 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant N199K is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Optimized all classify the substitution as benign. Only ESM1b and AlphaMissense‑Default predict pathogenicity. High‑accuracy assessments show AlphaMissense‑Optimized as benign, Foldetta as benign, while the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split. Overall, the preponderance of evidence supports a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Uncertain | 1 | -8.198 | Likely Pathogenic | 0.686 | Likely Pathogenic | Likely Benign | 0.024 | Likely Benign | -0.19 | Likely Benign | 0.1 | 0.03 | Likely Benign | -0.08 | Likely Benign | 0.33 | Likely Benign | -1.48 | Neutral | 0.276 | Benign | 0.083 | Benign | 4.27 | Benign | 0.13 | Tolerated | 3.47 | 9 | 1 | 0 | -0.4 | 14.07 | 207.8 | 21.5 | -0.1 | 1.5 | 0.1 | 0.0 | X | Uncertain | Asn199, located in the N-terminal loop before the first anti-parallel β sheet strand (res. Ile205-Pro208), is replaced by a positively charged lysine. On the protein surface, both the carboxamide group of Asn199 and the amino group of Lys199 side chains can form hydrogen bonds with the backbone carbonyl groups of residues (e.g., Ala249) at the end of an α helix (res. Ala236-Lys251). However, since the model ends abruptly at the N-terminus, no definite conclusions can be drawn from the simulations. | ||||||||||||
| c.600G>C | L200F 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant L200F is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33435242‑G‑C). Functional prediction tools that agree on a benign effect include REVEL, premPS, PROVEAN, SIFT, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. Predictions that are inconclusive are FoldX, Rosetta, Foldetta, and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as benign, and Foldetta as uncertain. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Uncertain | 1 | 6-33435242-G-C | 2 | 1.24e-6 | -7.606 | In-Between | 0.592 | Likely Pathogenic | Likely Benign | 0.094 | Likely Benign | 1.00 | Ambiguous | 0.5 | 1.45 | Ambiguous | 1.23 | Ambiguous | 0.43 | Likely Benign | -1.97 | Neutral | 0.997 | Probably Damaging | 0.916 | Probably Damaging | 4.02 | Benign | 0.17 | Tolerated | 3.46 | 9 | 2 | 0 | -1.0 | 34.02 | 250.4 | -15.1 | 0.6 | 0.2 | 0.5 | 0.0 | X | Uncertain | Leu200, a hydrophobic residue located in the N-terminal loop before the first anti-parallel β sheet strand (res. Ile205-Pro208), is replaced by another hydrophobic residue, phenylalanine. Both the phenyl group of Phe200 and the branched iso-butyl hydrocarbon sidechain of Leu200 occupy an inward hydrophobic niche (e.g., Leu246, Val222, Phe231) during the simulations. However, since the model ends abruptly at the N-terminus, no definite conclusions can be drawn from the simulations. | |||||||||
| c.667A>G | T223A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T223A is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (ID 6‑33435518‑A‑G). Functional prediction tools that agree on a benign effect include FoldX, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are REVEL and PROVEAN. Predictions that are inconclusive are Rosetta, Foldetta, premPS, and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also resolves to benign; and Foldetta, which integrates FoldX‑MD and Rosetta outputs, remains uncertain. Overall, the majority of evidence points to a benign impact, which does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Uncertain | 1 | 6-33435518-A-G | 3 | 1.86e-6 | -7.076 | In-Between | 0.316 | Likely Benign | Likely Benign | 0.574 | Likely Pathogenic | 0.30 | Likely Benign | 0.1 | 0.77 | Ambiguous | 0.54 | Ambiguous | 0.74 | Ambiguous | -3.36 | Deleterious | 0.231 | Benign | 0.058 | Benign | 5.74 | Benign | 0.09 | Tolerated | 3.41 | 13 | 1 | 0 | 2.5 | -30.03 | 186.4 | 44.0 | 0.0 | 0.0 | 0.0 | 0.0 | X | X | Uncertain | The introduced residue Ala223 is located on the outer surface of an anti-parallel β sheet strand (res. Cys219-Thr224). Unlike the hydroxyl group of the Thr223 side chain in the WT protein, the methyl side chain of Ala223 cannot form hydrogen bonds with nearby residues Thr228 and Lys207. Without these hydrogen-bonding interactions at the β sheet surface, the secondary structure element becomes unstable and partially unfolds in the variant simulations. However, since the model ends abruptly at the N-terminus, no definite conclusions can be drawn from the simulations. | ||||||||
| c.670A>G | T224A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T224A is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33435521‑A‑G). Prediction tools that agree on a benign effect include REVEL, FoldX, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN and AlphaMissense‑Default. The remaining tools (Rosetta, Foldetta, premPS, ESM1b) return uncertain or inconclusive results. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta as uncertain. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Uncertain | 3 | 6-33435521-A-G | 2 | 1.24e-6 | -7.379 | In-Between | 0.651 | Likely Pathogenic | Likely Benign | 0.464 | Likely Benign | 0.33 | Likely Benign | 0.1 | 1.05 | Ambiguous | 0.69 | Ambiguous | 0.91 | Ambiguous | -2.96 | Deleterious | 0.243 | Benign | 0.079 | Benign | 5.57 | Benign | 0.57 | Tolerated | 3.41 | 13 | 1 | 0 | 2.5 | -30.03 | 169.0 | 41.4 | -0.5 | 1.1 | -0.4 | 0.0 | X | X | Uncertain | The introduced residue Ala224 is located on the outer surface of an anti-parallel β sheet strand (res. Cys219-Thr224). Unlike the hydroxyl group of the Thr224 side chain in the WT model, the methyl side chain of Ala224 cannot form hydrogen bonds with nearby residues Ser204, Ser226, and Gly227. Without these hydrogen-bonding interactions at the β sheet surface, the secondary structure element becomes unstable and unfolds during the variant simulations. However, since the model ends abruptly at the N-terminus, no definite conclusions can be drawn from the simulations. | ||||||||
| c.694G>A | A232T 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant A232T is listed in ClinVar as Benign (ClinVar ID 1165963.0) and is present in gnomAD (ID 6‑33435545‑G‑A). Prediction tools that agree on a benign effect include REVEL, FoldX, Rosetta, Foldetta, PROVEAN, polyPhen‑2 HumVar, SIFT, and FATHMM. Those that predict a pathogenic effect are polyPhen‑2 HumDiv and AlphaMissense‑Default. Predictions that are inconclusive are premPS, ESM1b, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as Uncertain; the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) resolves to Benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) also reports Benign. Overall, the majority of evidence supports a benign impact, which is consistent with the ClinVar classification and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Benign | 1 | 6-33435545-G-A | 1 | 6.20e-7 | -7.655 | In-Between | 0.874 | Likely Pathogenic | Ambiguous | 0.469 | Likely Benign | 0.47 | Likely Benign | 0.1 | -0.04 | Likely Benign | 0.22 | Likely Benign | 0.61 | Ambiguous | -1.42 | Neutral | 0.608 | Possibly Damaging | 0.240 | Benign | 5.80 | Benign | 0.09 | Tolerated | 3.40 | 14 | 1 | 0 | -2.5 | 30.03 | 210.8 | -42.0 | 0.5 | 0.1 | 0.4 | 0.5 | X | Uncertain | The hydroxyl group of Thr232, located at the end of an anti-parallel β sheet strand (res. Thr228-Ala232), forms hydrogen bonds with nearby residues Glu217, Cys233, and Cys219 in the variant simulations. These hydrogen-bonding interactions at the β sheet surface contribute to the stability of the secondary structure element and prevent it from unfolding. The new hydrogen bond interactions may be more favorable for structural stability than the steric interactions of the methyl side chain of Ala with the side chains of Gln216 and Cys219 in the WT. However, since the model ends abruptly at the N-terminus, no definite conclusions can be drawn from the simulations. | |||||||||
| c.707C>T | A236V 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant A236V is listed in ClinVar as Benign (ID 469162.0) and is present in gnomAD (6‑33435558‑C‑T). Prediction tools that report benign include polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict pathogenicity are REVEL, PROVEAN, polyPhen‑2 HumDiv, SIFT, and ESM1b. Four tools give uncertain or inconclusive results: FoldX, Rosetta, Foldetta, and premPS. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive because the votes are evenly split. High‑accuracy assessments show AlphaMissense‑Optimized as benign, Foldetta as uncertain, and the SGM Consensus as unavailable. Consequently, the overall prediction profile is mixed, but the most reliable high‑accuracy evidence points toward a benign effect. Therefore, the variant is most likely benign, which aligns with its ClinVar classification and does not contradict the reported status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | PH | Benign/Likely benign | 2 | 6-33435558-C-T | 6 | 3.72e-6 | -8.752 | Likely Pathogenic | 0.267 | Likely Benign | Likely Benign | 0.777 | Likely Pathogenic | 0.61 | Ambiguous | 0.2 | 1.08 | Ambiguous | 0.85 | Ambiguous | 0.64 | Ambiguous | -3.55 | Deleterious | 0.981 | Probably Damaging | 0.446 | Benign | 5.79 | Benign | 0.03 | Affected | 3.40 | 14 | 0 | 0 | 2.4 | 28.05 | 213.8 | -44.7 | 0.0 | 0.0 | -0.2 | 0.2 | X | Potentially Benign | The methyl side chain of Ala236, located on an α helix (residues Ala236-Val250) facing an anti-parallel β sheet strand (residues Ile205-Val209), interacts hydrophobically with nearby residues such as Arg239 and Phe218. In the variant simulations, the isopropyl branched hydrocarbon side chain of Val236 maintains similar hydrophobic interactions as alanine in the WT, with an overall arrangement remarkably similar to Ala236. The residue swap does not affect the protein structure based on the simulations. | |||||||||
| c.745G>A | A249T 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant A249T is listed in ClinVar (ID 1031675.0) with an uncertain significance annotation and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, premPS, PROVEAN, SIFT, ESM1b, and FATHMM, whereas polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default predict a pathogenic outcome. Predictions that are inconclusive are FoldX, Rosetta, Foldetta, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as likely benign, and Foldetta (combining FoldX‑MD and Rosetta) as uncertain. Overall, the balance of evidence favors a benign interpretation, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | PH | Uncertain | 1 | -3.564 | Likely Benign | 0.805 | Likely Pathogenic | Ambiguous | 0.487 | Likely Benign | 1.50 | Ambiguous | 0.6 | 1.39 | Ambiguous | 1.45 | Ambiguous | 0.30 | Likely Benign | -0.96 | Neutral | 0.990 | Probably Damaging | 0.815 | Possibly Damaging | 5.65 | Benign | 0.40 | Tolerated | 3.39 | 15 | 1 | 0 | -2.5 | 30.03 | 214.5 | -43.3 | 0.0 | 0.0 | 0.5 | 0.2 | X | Potentially Benign | The methyl group of Ala249, located on the surface of an α helix (res. Ala236-Val250) facing an anti-parallel β sheet strand (res. Ile205-Val209), packs against nearby hydrophobic residues such as Leu200, Leu246, and Val250. In the variant simulations, the hydroxyl group of Thr249, which is not suitable for hydrophobic packing, forms a stable hydrogen bond with the backbone carbonyl of Asn245 in the same helix. Although this interaction could theoretically weaken the structural integrity of the α helix, this destabilizing effect is not observed in the variant simulations. | |||||||||||
| c.762G>C | K254N 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant K254N is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools that classify the variant as benign include polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. The majority of other in silico predictors—REVEL, premPS, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—indicate a pathogenic effect. Stability‑based methods FoldX, Rosetta, and Foldetta returned uncertain results and are therefore not considered evidence for or against pathogenicity. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, the SGM‑Consensus as likely pathogenic, and Foldetta as unavailable. Overall, the preponderance of evidence supports a pathogenic classification, which contradicts the current ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | PH | Uncertain | 1 | -13.306 | Likely Pathogenic | 0.999 | Likely Pathogenic | Likely Pathogenic | 0.757 | Likely Pathogenic | 0.73 | Ambiguous | 0.2 | 1.87 | Ambiguous | 1.30 | Ambiguous | 1.19 | Destabilizing | -4.23 | Deleterious | 0.384 | Benign | 0.070 | Benign | 5.93 | Benign | 0.01 | Affected | 3.39 | 15 | 1 | 0 | 0.4 | -14.07 | 215.3 | -21.0 | -1.0 | 1.7 | 0.2 | 0.3 | X | Potentially Pathogenic | The amino group of Lys254, located in an α-β loop connecting the PH and C2 domains (res. Lys251-Arg258), forms salt bridges with the carboxylate groups of Glu244 and Asp684. Since the neutral carboxamide group of the Asn254 side chain cannot form salt bridges with acidic residues, the residue swap potentially weakens the tertiary structure assembly and/or influences the loop positioning. Regardless, in both the variant and WT simulations, all hydrogen bonds formed by the residue’s side chain were broken, and the residue rotated outwards. The partially α helical conformation of the loop, which extends to a nearby α helix (res. Met414-Asn426), is dynamic, making it unclear if the mutation affects it. | |||||||||||
| c.773G>A | R258H 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R258H is listed as Benign in ClinVar (ID 949697.0) and is present in gnomAD (6‑33437678‑G‑A). Prediction tools that agree on a benign effect include FATHMM and AlphaMissense‑Optimized. Those that predict a pathogenic effect are REVEL, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and ESM1b. Uncertain calls come from FoldX, Rosetta, Foldetta, and AlphaMissense‑Default. The high‑accuracy consensus (SGM) derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN yields a pathogenic verdict. AlphaMissense‑Optimized remains benign, while Foldetta is inconclusive. Overall, the majority of evidence points to a pathogenic impact, which contradicts the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | C2 | Benign/Likely benign | 3 | 6-33437678-G-A | 10 | 6.20e-6 | -10.533 | Likely Pathogenic | 0.525 | Ambiguous | Likely Benign | 0.830 | Likely Pathogenic | 1.60 | Ambiguous | 0.6 | 1.00 | Ambiguous | 1.30 | Ambiguous | 1.47 | Destabilizing | -4.06 | Deleterious | 1.000 | Probably Damaging | 0.991 | Probably Damaging | 5.77 | Benign | 0.01 | Affected | 3.39 | 15 | 2 | 0 | 1.3 | -19.05 | 212.5 | 81.8 | 0.1 | 0.0 | -0.5 | 0.2 | X | Potentially Pathogenic | The guanidinium group of Arg258, located at the end of an α-β loop connecting the PH domain to the C2 domain (res. Lys251-Arg258), forms hydrogen bonds with the carboxamide groups of Asn727 and Asn729 side chains, as well as with the backbone carbonyl groups of Ala724, Leu725, and Asn727 in the WT simulations. Although the imidazole group of His258 can act as a hydrogen bond donor/acceptor, the swapped residue is unable to maintain an equally well-coordinated hydrogen bond network for linking the C2 and GAP domains in the variant simulations. | |||||||||
| c.860A>C | D287A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant D287A is listed in ClinVar with an Uncertain significance status and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions include REVEL, FoldX, Rosetta, Foldetta, and premPS, whereas pathogenic predictions are made by PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Pathogenic, and Foldetta (integrating FoldX‑MD and Rosetta) as benign. The overall tally favors pathogenicity (8 tools vs 5 benign), but the conflicting high‑accuracy results leave uncertainty. Thus, the variant is most likely pathogenic according to the majority of predictions, which does not contradict its ClinVar Uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | -14.686 | Likely Pathogenic | 0.996 | Likely Pathogenic | Likely Pathogenic | 0.484 | Likely Benign | 0.30 | Likely Benign | 0.1 | -0.04 | Likely Benign | 0.13 | Likely Benign | 0.40 | Likely Benign | -7.35 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.58 | Pathogenic | 0.01 | Affected | 3.38 | 23 | -2 | 0 | 5.3 | -44.01 | ||||||||||||||||||||
| c.878G>A | R293H 2D  AISynGAP1 missense variant R293H is listed in ClinVar with an uncertain significance (ClinVar ID 3901513.0) and is not reported in gnomAD. Prediction tools that indicate a benign effect include REVEL and premPS, whereas the remaining 13 tools—FoldX, Rosetta, Foldetta, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus—predict a pathogenic outcome. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized scores the variant as pathogenic; the SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also reports pathogenic; and Foldetta, which integrates FoldX‑MD and Rosetta stability predictions, classifies the variant as pathogenic. Overall, the preponderance of evidence indicates that R293H is most likely pathogenic, a conclusion that does not contradict the current ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | -13.009 | Likely Pathogenic | 0.973 | Likely Pathogenic | Likely Pathogenic | 0.438 | Likely Benign | 4.45 | Destabilizing | 2.3 | 2.12 | Destabilizing | 3.29 | Destabilizing | 0.32 | Likely Benign | -4.60 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.45 | Pathogenic | 0.04 | Affected | 2 | 0 | 1.3 | -19.05 | ||||||||||||||||||||||
| c.896G>A | R299H 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R299H is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33437801‑G‑A). Functional prediction tools cluster into two groups: benign predictions from REVEL and AlphaMissense‑Optimized, and pathogenic predictions from FoldX, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; Rosetta, ESM1b, and AlphaMissense‑Default are inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as benign, Foldetta as pathogenic, and the SGM consensus (derived from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a tie between pathogenic and uncertain calls. Overall, the majority of evidence points to a pathogenic effect, which is consistent with the ClinVar uncertain designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | C2 | Conflicting | 2 | 6-33437801-G-A | 10 | 6.20e-6 | -7.731 | In-Between | 0.388 | Ambiguous | Likely Benign | 0.238 | Likely Benign | 3.97 | Destabilizing | 1.0 | 0.94 | Ambiguous | 2.46 | Destabilizing | 1.41 | Destabilizing | -3.35 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.69 | Pathogenic | 0.02 | Affected | 3.39 | 19 | 2 | 0 | 1.3 | -19.05 | 211.2 | 72.5 | -0.1 | 0.2 | -0.2 | 0.3 | X | Potentially Pathogenic | The guanidinium group of Arg299, located in a β hairpin loop linking two anti-parallel β sheet strands (res. Met289-Pro298, res. Thr305-Asn315), forms hydrogen bonds that stabilize the tight turn. In the WT simulations, the Arg299 side chain hydrogen bonds with the loop backbone carbonyl groups (e.g., Ser302, Thr305, Leu274, Gly303), the hydroxyl group of Ser300, and even forms a salt bridge with the carboxylate group of Asp304.In the variant simulations, the imidazole ring of His299 (epsilon protonated state) hydrogen bonds with the carbonyl group of Asp304 and the hydroxyl group of Ser300. However, it does not form as many or as strong interactions as arginine, which could affect the initial formation of the secondary hairpin loop during folding. β hairpins are potential nucleation sites during the initial stages of protein folding, so even minor changes in them could be significant.Additionally, His299 prefers to hydrophobically interact with other hydrophobic residues inside the C2 domain core (e.g., Val306, Leu274), which destabilizes the C2 domain. Indeed, the β strand partially unfolds during the second simulation. Moreover, the positively charged Arg299 side chain faces the polar head group region of the inner leaflet membrane and could directly anchor the C2 domain to the membrane. In short, the residue swap could negatively affect both protein folding and the stability of the SynGAP-membrane association. | |||||||||
| c.901G>A | A301T 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant A301T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33437806‑G‑A). Prediction tools that uniformly indicate a benign effect include REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only the two polyPhen‑2 implementations (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is “Likely Benign”; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, is benign. Overall, the majority of evidence points to a benign effect, and this is not in conflict with the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Uncertain | 5 | 6-33437806-G-A | 2 | 1.24e-6 | -3.448 | Likely Benign | 0.070 | Likely Benign | Likely Benign | 0.150 | Likely Benign | 0.36 | Likely Benign | 0.2 | -0.33 | Likely Benign | 0.02 | Likely Benign | 0.03 | Likely Benign | -0.25 | Neutral | 0.997 | Probably Damaging | 0.989 | Probably Damaging | 4.15 | Benign | 0.22 | Tolerated | 4.32 | 14 | 1 | 0 | -2.5 | 30.03 | 219.8 | -42.8 | -0.1 | 0.0 | -0.5 | 0.2 | Uncertain | The methyl group of Ala301, located in a β hairpin loop linking two anti-parallel β sheet strands (res. Met289-Pro298, res. Thr305-Asn315), points outward from the β hairpin loop, and its backbone atoms do not participate in the loop formation in the WT simulations. In the variant simulations, the hydroxyl group of the Thr301 side chain also mostly points outward; however, the guanidinium group of Arg299 is moved away from its central hairpin loop position.β hairpins are potential nucleation sites during the initial stages of protein folding, so even minor changes in them could be significant. Due to its location near the membrane surface, the residue swap could also affect the C2 loop dynamics and SynGAP-membrane association. However, this is beyond the scope of the solvent-only simulations to unravel. | |||||||||
| c.913A>G | T305A 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 T305A variant is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33437818‑A‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus as benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) as uncertain. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Conflicting | 2 | 6-33437818-A-G | 13 | 8.05e-6 | -4.307 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.144 | Likely Benign | 1.30 | Ambiguous | 0.6 | 1.55 | Ambiguous | 1.43 | Ambiguous | 0.77 | Ambiguous | -2.10 | Neutral | 0.939 | Possibly Damaging | 0.645 | Possibly Damaging | 1.76 | Pathogenic | 0.12 | Tolerated | 3.40 | 20 | 1 | 0 | 2.5 | -30.03 | 177.9 | 43.5 | -0.2 | 0.1 | 0.4 | 0.0 | Uncertain | The hydroxyl group of Thr305, located at the beginning of an anti-parallel β strand (res. Thr305-Asn315), hydrogen bonds with the carboxylate groups of Glu270 and Asp304 in the anti-parallel β strand and the adjacent β hairpin loop, respectively. In the variant simulations, the methyl group of the Ala305 side chain cannot hydrogen bond with either of the acidic residues, which could weaken the integrity of the tertiary structure and the β hairpin loop. Indeed, the guanidinium group of Arg299 does not acquire its central hairpin loop position due to the residue swap.β hairpins are potential nucleation sites during the initial stages of protein folding, so even minor changes in them could be significant. Due to its location near the membrane surface, the residue swap could also affect the C2 loop dynamics and SynGAP-membrane association. However, this is beyond the scope of the solvent-only simulations to unravel. | |||||||||
| c.929A>G | E310G 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant E310G is listed in ClinVar as Pathogenic (ClinVar ID 2732842.0) and is not reported in gnomAD. Prediction tools that assess the variant’s effect largely agree on a deleterious outcome: REVEL, FoldX, Rosetta, Foldetta, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all predict pathogenicity, while only premPS predicts a benign effect. High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized is pathogenic; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is pathogenic; and Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, also predicts pathogenicity. Consequently, the variant is most likely pathogenic, and this prediction is consistent with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Pathogenic | 1 | -14.132 | Likely Pathogenic | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.848 | Likely Pathogenic | 2.38 | Destabilizing | 0.7 | 3.56 | Destabilizing | 2.97 | Destabilizing | 0.36 | Likely Benign | -6.43 | Deleterious | 1.000 | Probably Damaging | 0.996 | Probably Damaging | 1.12 | Pathogenic | 0.00 | Affected | 3.38 | 19 | -2 | 0 | 3.1 | -72.06 | ||||||||||||||||||||
| c.962G>A | R321H 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R321H is listed in ClinVar with an uncertain significance and is present in gnomAD (variant ID 6‑33437867‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, FoldX, Rosetta, Foldetta, PROVEAN, SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic outcome are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM, while premPS remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as benign, Foldetta as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split. Overall, the majority of predictions support a benign impact, and this consensus does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | C2 | Uncertain | 1 | 6-33437867-G-A | 8 | 4.96e-6 | -8.751 | Likely Pathogenic | 0.136 | Likely Benign | Likely Benign | 0.323 | Likely Benign | 0.48 | Likely Benign | 0.1 | -0.36 | Likely Benign | 0.06 | Likely Benign | 0.59 | Ambiguous | -1.43 | Neutral | 1.000 | Probably Damaging | 0.998 | Probably Damaging | 1.92 | Pathogenic | 0.25 | Tolerated | 3.38 | 23 | 2 | 0 | 1.3 | -19.05 | 218.5 | 86.9 | 1.1 | 0.0 | 0.3 | 0.0 | X | Potentially Benign | The guanidinium group of Arg321, located in a β hairpin loop linking two anti-parallel β sheet strands (res. Thr305-Asn315, res. Ala322-Asp330), faces outward without forming any stable interactions in the WT simulations. Similarly, in the variant simulations, the imidazole ring of His321 also points outward without making any stable intra-protein interactions. Thus, the residue swap does not seem to cause adverse effects on the protein structure based on the simulations. However, β hairpins are potential nucleation sites during the initial stages of protein folding, so even minor changes in them could be significant. | |||||||||
| c.986G>A | R329H 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R329H is listed in ClinVar with an uncertain significance (ClinVar ID 2074400.0) and is present in gnomAD (ID 6‑33437891‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, FATHMM, and AlphaMissense‑Optimized. Tools that agree on a pathogenic effect include FoldX, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized predicts benign, while the SGM Consensus—derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN—predicts pathogenic. Foldetta, which integrates FoldX‑MD and Rosetta outputs, yields an uncertain result and is treated as unavailable evidence. Overall, the balance of predictions favors a pathogenic impact, which does not contradict the ClinVar uncertain status but suggests the variant is more likely deleterious. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | 6-33437891-G-A | 2 | 1.24e-6 | -10.154 | Likely Pathogenic | 0.769 | Likely Pathogenic | Likely Benign | 0.155 | Likely Benign | 2.53 | Destabilizing | 0.7 | 0.71 | Ambiguous | 1.62 | Ambiguous | 0.82 | Ambiguous | -3.17 | Deleterious | 0.995 | Probably Damaging | 0.778 | Possibly Damaging | 4.04 | Benign | 0.05 | Affected | 3.41 | 15 | 2 | 0 | 1.3 | -19.05 | 220.4 | 81.4 | 0.1 | 0.1 | 0.2 | 0.3 | Uncertain | The guanidinium group of Arg329, located at the end of an anti-parallel β sheet strand (res. Ala322-Asp330), faces the negatively charged lipid bilayer surface. While the residue swap does not cause any apparent negative effects on the protein structure in the variant simulations, it could adversely affect the SynGAP-membrane association in reality. The positively charged Arg329 side chain forms hydrogen bonds with other loop residues (e.g., Ser371, Asp338) that are expected to dynamically interact with the membrane head group region. However, this phenomenon is beyond the scope of the solvent-only simulations to unravel. Notably, histidine can also be double protonated and positively charged, but this alternative protonation state was not considered in the variant simulations. | |||||||||
| c.1042G>A | V348M 2D  3DClick to see structure in 3D Viewer AISynGAP1 variant V348M is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools that report a clear outcome fall into two groups: benign calls come from REVEL, Foldetta, PROVEAN, and AlphaMissense‑Optimized; pathogenic calls come from polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. The remaining tools (FoldX, Rosetta, premPS, AlphaMissense‑Default, ESM1b) give uncertain results, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is unavailable due to no majority. High‑accuracy methods specifically show AlphaMissense‑Optimized as benign, Foldetta as benign, and the SGM Consensus is not available. With four benign and four pathogenic predictions, the evidence is evenly split, providing no definitive direction. Therefore, the variant is not clearly benign or pathogenic based on current predictions, and this lack of consensus does not contradict its ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | C2 | Uncertain | 1 | -7.076 | In-Between | 0.546 | Ambiguous | Likely Benign | 0.191 | Likely Benign | -1.19 | Ambiguous | 0.1 | 0.72 | Ambiguous | -0.24 | Likely Benign | 0.76 | Ambiguous | -1.62 | Neutral | 0.966 | Probably Damaging | 0.564 | Possibly Damaging | 1.58 | Pathogenic | 0.03 | Affected | 3.37 | 25 | 2 | 1 | -2.3 | 32.06 | 253.8 | -47.4 | -0.3 | 0.1 | 0.2 | 0.1 | X | Potentially Benign | The iso-propyl side chain of Val348, located in an anti-parallel β sheet strand (res. Gly341-Pro349), packs against multiple hydrophobic C2 domain residues (e.g., Leu353, Leu323, Leu402). In the variant simulations, the thioether side chain of Met348 can form similar interactions as valine due to its comparable hydrophobic profile. In fact, the thioether group of methionine can even stack favorably with the phenol ring of Tyr363 in the anti-parallel β sheet strand (res. Ala399-Ile411). Overall, the residue swap does not appear to cause negative effects on the protein structure based on the simulations. | ||||||||||||
| c.1198G>C | V400L 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant V400L is listed in ClinVar as Benign (ClinVar ID 1166313.0) and is present in gnomAD (variant ID 6‑33438103‑G‑C). Prediction tools that agree on a benign effect include REVEL, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts a pathogenic outcome; the only inconclusive result is from FoldX, which is treated as unavailable. High‑accuracy assessments confirm benignity: AlphaMissense‑Optimized is benign; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is benign; and Foldetta, which integrates FoldX‑MD and Rosetta outputs, is benign. Overall, the computational evidence strongly supports a benign classification, consistent with the ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | C2 | Benign | 1 | 6-33438103-G-C | 22 | 1.36e-5 | -1.000 | Likely Benign | 0.137 | Likely Benign | Likely Benign | 0.325 | Likely Benign | -0.71 | Ambiguous | 0.2 | 0.39 | Likely Benign | -0.16 | Likely Benign | -0.29 | Likely Benign | -0.60 | Neutral | 0.001 | Benign | 0.001 | Benign | 5.33 | Benign | 0.64 | Tolerated | 3.38 | 27 | 2 | 1 | -0.4 | 14.03 | 251.0 | -30.1 | 0.0 | 0.0 | 0.7 | 0.1 | X | Potentially Benign | The iso-propyl side chain of Val400, located in an anti-parallel β sheet strand (res. Ala399-Ile411), hydrophobically packs against hydrophobic residues within the anti-parallel β sheet of the C2 domain (e.g., Ile268, Ala404, Leu325, Leu402). Val400 is swapped for another hydrophobic residue, leucine, whose branched hydrocarbon side chain is of a comparable size and thus packs favorably within the C2 domain. In short, the residue swap has no apparent negative effect on the structure based on the variant simulations. | 10.1016/j.ajhg.2020.11.011 | |||||||
| c.1202G>A | R401Q 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant R401Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33438107‑G‑A). Prediction tools that agree on a benign effect are limited to FATHMM, whereas the majority of algorithms (REVEL, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) predict a pathogenic impact. Uncertain results are reported by FoldX, Rosetta, and Foldetta. High‑accuracy methods reinforce the pathogenic prediction: AlphaMissense‑Optimized scores the variant as pathogenic, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Pathogenic,” and Foldetta’s stability assessment is inconclusive. Overall, the preponderance of evidence points to a pathogenic effect, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | C2 | Uncertain | 1 | 6-33438107-G-A | -11.213 | Likely Pathogenic | 0.969 | Likely Pathogenic | Likely Pathogenic | 0.780 | Likely Pathogenic | 0.96 | Ambiguous | 0.1 | 1.50 | Ambiguous | 1.23 | Ambiguous | 1.20 | Destabilizing | -3.69 | Deleterious | 0.999 | Probably Damaging | 0.978 | Probably Damaging | 5.47 | Benign | 0.04 | Affected | 3.38 | 27 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||
| c.1240A>G | M414V 2D  AISynGAP1 M414V is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: benign calls come from REVEL, SIFT, FATHMM, and AlphaMissense‑Optimized; pathogenic calls come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), and ESM1b; the remaining tools (FoldX, Rosetta, Foldetta, premPS, AlphaMissense‑Default) are inconclusive. The SGM consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, yields a pathogenic majority. High‑accuracy assessments give AlphaMissense‑Optimized benign, SGM consensus pathogenic, and Foldetta uncertain. Because the high‑accuracy predictions are divided and the overall tool set is evenly split, there is no definitive evidence for pathogenicity or benignity. Thus, the variant is most likely inconclusive, and this lack of consensus does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 1 | -8.003 | Likely Pathogenic | 0.541 | Ambiguous | Likely Benign | 0.261 | Likely Benign | 1.81 | Ambiguous | 0.4 | 1.73 | Ambiguous | 1.77 | Ambiguous | 0.95 | Ambiguous | -2.95 | Deleterious | 0.999 | Probably Damaging | 0.987 | Probably Damaging | 3.43 | Benign | 0.24 | Tolerated | 2 | 1 | 2.3 | -32.06 | |||||||||||||||||||||||
| c.1286G>A | R429Q 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant R429Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33438191‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, FoldX, Rosetta, Foldetta, PROVEAN, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and ESM1b, while premPS is inconclusive. The high‑accuracy consensus (SGM Consensus) – a majority vote among AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN – yields a “Likely Benign” result. AlphaMissense‑Optimized itself predicts benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) also predicts benign. Taken together, the preponderance of evidence points to a benign impact for R429Q, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 2 | 6-33438191-G-A | 10 | 6.20e-6 | -8.227 | Likely Pathogenic | 0.143 | Likely Benign | Likely Benign | 0.156 | Likely Benign | 0.45 | Likely Benign | 0.1 | 0.36 | Likely Benign | 0.41 | Likely Benign | 0.98 | Ambiguous | -1.25 | Neutral | 1.000 | Probably Damaging | 0.979 | Probably Damaging | 3.47 | Benign | 0.58 | Tolerated | 3.38 | 25 | 1 | 1 | 1.0 | -28.06 | 235.8 | 59.5 | 0.0 | 0.0 | -0.3 | 0.4 | X | Potentially Pathogenic | The guanidinium group of the Arg429 side chain, located in an α helix (res. Met414-Glu436), either forms a salt bridge with the carboxylate group of an acidic residue (Asp474, Asp467) or an H-bond with the hydroxyl group of Ser471 in an opposing α helix (res. Ala461-Phe476). In the variant simulations, Gln429 cannot form ionic interactions with the acidic residues; however, the carboxamide group can form multiple H-bonds. The H-bonding coordination of the Asn429 side chain varied between the replica simulations. In one simulation, three H-bonds were formed simultaneously with the Asp467 side chain, the backbone carbonyl group of Asn426, and the amide group of Met430 at the end of the same α helix. The residue swap could affect the tertiary structure assembly during folding due to weaker bond formation, but no large-scale negative effects were seen during the simulations. | ||||||||
| c.1306G>A | E436K 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant E436K is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include only FATHMM, whereas the remaining evaluated algorithms (REVEL, Rosetta, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus) uniformly predict a pathogenic impact; FoldX, Foldetta, and premPS are inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as pathogenic, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as likely pathogenic, and Foldetta as uncertain. Overall, the preponderance of evidence points to a pathogenic effect for E436K, which does not conflict with the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -13.869 | Likely Pathogenic | 0.997 | Likely Pathogenic | Likely Pathogenic | 0.829 | Likely Pathogenic | 0.56 | Ambiguous | 0.1 | 2.86 | Destabilizing | 1.71 | Ambiguous | 0.82 | Ambiguous | -3.77 | Deleterious | 0.994 | Probably Damaging | 0.951 | Probably Damaging | 4.71 | Benign | 0.02 | Affected | 3.37 | 29 | 0 | 1 | -0.4 | -0.94 | 186.8 | 39.8 | 0.0 | 0.0 | -0.2 | 0.0 | X | X | X | Potentially Pathogenic | The carboxylate group of Glu436, located on the α helix (res. Met414-Glu436), forms a salt bridge with the amino group of the Lys444 side chain on an opposing α helix (res. Val441-Ser457). The backbone carbonyl of Glu436 also H-bonds with the Lys444 side chain, which helps keep the ends of the two α helices tightly connected. In contrast, in the variant simulations, the salt bridge formation with Lys444 is not possible. Instead, the repelled Lys436 side chain rotates outward, causing a change in the α helix backbone H-bonding: the amide group of Lys444 H-bonds with the carbonyl of Ala433 instead of the carbonyl of Cys432. | |||||||||
| c.1345A>G | S449G 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S449G is listed in ClinVar with an “Uncertain” status and is present in the gnomAD database (variant ID 6‑33438250‑A‑G). Prediction tools that agree on a benign effect include REVEL, FoldX, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only polyPhen‑2 HumDiv predicts a pathogenic outcome, while Rosetta, Foldetta, and premPS are inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as “Likely Benign,” and Foldetta as uncertain. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | 6-33438250-A-G | 3 | 1.86e-6 | -5.936 | Likely Benign | 0.071 | Likely Benign | Likely Benign | 0.116 | Likely Benign | 0.47 | Likely Benign | 0.0 | 0.55 | Ambiguous | 0.51 | Ambiguous | 0.85 | Ambiguous | -2.32 | Neutral | 0.948 | Possibly Damaging | 0.124 | Benign | 3.35 | Benign | 0.13 | Tolerated | 3.37 | 32 | 0 | 1 | 0.4 | -30.03 | |||||||||||||||||
| c.1370G>A | S457N 2D  AIThe SynGAP1 missense variant S457N is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools show a split: benign predictions come from REVEL, FoldX, Rosetta, SIFT, and FATHMM, while pathogenic predictions arise from PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Default. The high‑accuracy consensus methods give a mixed picture: AlphaMissense‑Optimized is inconclusive, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) favors pathogenicity, and Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, predicts a benign effect. Overall, the majority of individual predictors lean toward pathogenicity, but the high‑accuracy Foldetta result suggests a benign impact. Thus, the variant is most likely pathogenic based on the preponderance of predictions, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -10.221 | Likely Pathogenic | 0.949 | Likely Pathogenic | Ambiguous | 0.241 | Likely Benign | 0.19 | Likely Benign | 0.0 | -0.22 | Likely Benign | -0.02 | Likely Benign | 0.67 | Ambiguous | -2.76 | Deleterious | 0.940 | Possibly Damaging | 0.843 | Possibly Damaging | 3.28 | Benign | 0.06 | Tolerated | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||
| c.1393C>G | L465V 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant L465V is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools cluster into two groups: benign predictions come from REVEL and SIFT, while the remaining tools—FoldX, Rosetta, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Default—indicate pathogenicity. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized is uncertain; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports likely pathogenic; and Foldetta, which combines FoldX‑MD and Rosetta stability outputs, predicts pathogenic. Overall, the majority of evidence points to a pathogenic impact, which is consistent with the ClinVar uncertain status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -9.893 | Likely Pathogenic | 0.838 | Likely Pathogenic | Ambiguous | 0.276 | Likely Benign | 2.46 | Destabilizing | 0.1 | 2.66 | Destabilizing | 2.56 | Destabilizing | 1.21 | Destabilizing | -2.98 | Deleterious | 0.996 | Probably Damaging | 0.992 | Probably Damaging | 2.44 | Pathogenic | 0.10 | Tolerated | 3.37 | 34 | 2 | 1 | 0.4 | -14.03 | 204.3 | 30.9 | 0.0 | 0.0 | -0.4 | 0.6 | X | Potentially Benign | The iso-butyl side chain of Leu465, located in the middle of an α helix (res. Ala461–Phe476), packs with hydrophobic residues (e.g., Phe464, Met468, Tyr497, Ile494) in an inter-helix space formed with two other α helices (res. Ala461–Phe476 and res. Thr488-Gly502). In the variant simulations, the iso-propyl side chain of Val465 is equally sized and similarly hydrophobic as the original side chain of Leu465. Hence, the mutation does not exert any negative effects on the protein structure based on the variant simulations. | |||||||||||
| c.140G>A | R47Q 2D  AIThe SynGAP1 missense variant R47Q is listed in ClinVar (ID 436920.0) as Benign and is present in gnomAD (6‑33423549‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. AlphaMissense‑Default is uncertain, and Foldetta results are unavailable. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, the SGM‑Consensus as Benign, and no Foldetta data to influence the conclusion. Overall, the majority of evidence points to a benign impact, consistent with the ClinVar classification; there is no contradiction with the reported ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | 6-33423549-G-A | 4 | 2.48e-6 | -4.989 | Likely Benign | 0.347 | Ambiguous | Likely Benign | 0.096 | Likely Benign | -0.57 | Neutral | 0.829 | Possibly Damaging | 0.614 | Possibly Damaging | 4.12 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | 1.0 | -28.06 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||
| c.1424G>A | R475Q 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant R475Q is listed in ClinVar with an uncertain significance and is present in gnomAD (variant ID 6-33438456‑G‑A). Prediction tools that indicate a benign effect include AlphaMissense‑Optimized, Foldetta, and Rosetta. Those that predict a pathogenic effect comprise SGM Consensus, SIFT, PolyPhen‑2 (HumDiv and HumVar), REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Default; FoldX and premPS are inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta as benign. Overall, the majority of evidence points toward a pathogenic impact, which contrasts with the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 2 | 6-33438456-G-A | 5 | 3.10e-6 | -12.087 | Likely Pathogenic | 0.721 | Likely Pathogenic | Likely Benign | 0.632 | Likely Pathogenic | 0.71 | Ambiguous | 0.1 | 0.12 | Likely Benign | 0.42 | Likely Benign | 0.82 | Ambiguous | -3.65 | Deleterious | 1.000 | Probably Damaging | 0.991 | Probably Damaging | -1.32 | Pathogenic | 0.01 | Affected | 3.39 | 28 | 1 | 1 | 1.0 | -28.06 | 253.6 | 52.7 | 0.0 | 0.0 | -0.8 | 0.0 | X | X | X | Potentially Pathogenic | In the WT simulations, the guanidinium group of Arg475, located near the end of an α-helix (res. Ala461-Phe476), stacks with the phenyl ring of Phe476 and forms a salt bridge with Glu472. Additionally, Arg475 occasionally forms another salt bridge with the carboxylate group of Glu486 on the α-α loop connecting the two α-helices (res. Ala461-Phe476 and Leu489-Glu519) at the GAP-Ras interface. Therefore, Arg475 potentially plays a key role in positioning the loop by interacting with Glu486, which is necessary for the positioning of the “arginine finger” (Arg485) and, ultimately, for RasGTPase activation. In the variant simulations, Asn475 forms a hydrogen bond with Arg479 on the proceeding α-α loop. The absence of Phe476/Arg475 stacking and the Arg475-Glu472 salt bridge weakens the integrity of the terminal end of the α-helix during the variant simulations. Lastly, the potential effect of the residue swap on the SynGAP-Ras complex formation or GTPase activation cannot be fully addressed using the SynGAP solvent-only simulations. | ||||||
| c.1436G>A | R479Q 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant R479Q is listed in ClinVar with an “Uncertain” significance and is present in gnomAD (variant ID 6‑33438468‑G‑A). Prediction tools that agree on a benign effect include REVEL, premPS, PROVEAN, SIFT, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 HumDiv and HumVar both predict a pathogenic impact. Uncertain or inconclusive results come from FoldX, Rosetta, Foldetta, and ESM1b. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM‑Consensus also as likely benign, while Foldetta remains uncertain. Overall, the majority of evidence points to a benign effect, and this consensus does not contradict the ClinVar “Uncertain” status; thus the variant is most likely benign based on current predictions. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | 6-33438468-G-A | 7 | 4.34e-6 | -7.109 | In-Between | 0.259 | Likely Benign | Likely Benign | 0.191 | Likely Benign | 0.54 | Ambiguous | 0.1 | 0.57 | Ambiguous | 0.56 | Ambiguous | 0.49 | Likely Benign | -1.16 | Neutral | 1.000 | Probably Damaging | 0.991 | Probably Damaging | 3.42 | Benign | 0.31 | Tolerated | 3.39 | 32 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||
| c.1456G>A | E486K 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant E486K is listed in ClinVar with an uncertain significance status and is not reported in gnomAD. Functional prediction tools show mixed results: benign predictions come from REVEL, FoldX, Rosetta, Foldetta, premPS, SIFT, and FATHMM, whereas pathogenic predictions are reported by PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and AlphaMissense‑Default. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenic, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) indicates likely pathogenic, and the Foldetta stability analysis (combining FoldX‑MD and Rosetta) predicts benign. Overall, the majority of high‑confidence tools and the consensus analysis favor a pathogenic interpretation, which contrasts with the ClinVar uncertain designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -14.545 | Likely Pathogenic | 0.988 | Likely Pathogenic | Likely Pathogenic | 0.435 | Likely Benign | 0.06 | Likely Benign | 0.1 | 0.37 | Likely Benign | 0.22 | Likely Benign | 0.41 | Likely Benign | -3.58 | Deleterious | 1.000 | Probably Damaging | 0.988 | Probably Damaging | 3.40 | Benign | 0.12 | Tolerated | 3.37 | 35 | 0 | 1 | -0.4 | -0.94 | 206.8 | 52.1 | -0.3 | 0.1 | 0.2 | 0.0 | X | X | Uncertain | Glu486 is located in an α-α loop connecting the two α-helices (res. Ala461-Phe476 and Leu489-Glu519) at the GAP-Ras interface. It is adjacent to the arginine finger (Arg485) and is expected to closely interact with Ras. The residue swap could affect complex formation with the GTPase and its activation. In the WT simulations, the carboxylate group of Glu486 forms salt bridges with Arg485 and Arg475 on the preceding α-helix (res. Ala461-Phe476). In the variant simulations, Lys486 does not form any specific interactions. Although the amino group of the Lys486 side chain cannot form these salt bridges, no negative effects on the protein structure are observed. Nevertheless, the potential role of Glu486 in SynGAP-Ras complex formation or GTPase activation cannot be fully addressed using the SynGAP solvent-only simulations, and no definite conclusions can be drawn. | ||||||||||
| c.1468G>C | A490P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant A490P is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Among the available in‑silico predictors, 10 tools (REVEL, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus) uniformly predict a pathogenic effect, whereas only Foldetta predicts a benign outcome; FoldX, Rosetta, and AlphaMissense‑Optimized are inconclusive. High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is uncertain, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is pathogenic, and Foldetta (combining FoldX‑MD and Rosetta stability outputs) is benign. Overall, the preponderance of evidence indicates that the variant is most likely pathogenic, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -12.905 | Likely Pathogenic | 0.941 | Likely Pathogenic | Ambiguous | 0.878 | Likely Pathogenic | -1.27 | Ambiguous | 0.1 | 1.31 | Ambiguous | 0.02 | Likely Benign | 1.07 | Destabilizing | -4.81 | Deleterious | 1.000 | Probably Damaging | 0.998 | Probably Damaging | -1.42 | Pathogenic | 0.01 | Affected | 3.37 | 35 | -1 | 1 | -3.4 | 26.04 | ||||||||||||||||||||
| c.1540A>T | I514F 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant I514F is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that classify the variant as benign include only FATHMM. All other evaluated algorithms—REVEL, FoldX, Rosetta, Foldetta, premPS (uncertain), PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized—report it as pathogenic or likely pathogenic. High‑accuracy assessments further support a deleterious effect: AlphaMissense‑Optimized predicts pathogenicity; the SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, also indicates pathogenicity; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, classifies the variant as pathogenic. Based on the overwhelming consensus of pathogenic predictions, the variant is most likely pathogenic, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -13.383 | Likely Pathogenic | 0.962 | Likely Pathogenic | Likely Pathogenic | 0.601 | Likely Pathogenic | 2.35 | Destabilizing | 0.3 | 3.74 | Destabilizing | 3.05 | Destabilizing | 0.93 | Ambiguous | -3.98 | Deleterious | 0.997 | Probably Damaging | 0.993 | Probably Damaging | 2.89 | Benign | 0.00 | Affected | 3.37 | 35 | 0 | 1 | -1.7 | 34.02 | ||||||||||||||||||||
| c.1600T>C | S534P 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S534P is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33438843‑T‑C). Functional prediction tools that report a benign effect include REVEL, FoldX, Rosetta, Foldetta, premPS, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The high‑accuracy assessments are consistent with a benign outcome: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign,” and Foldetta (combining FoldX‑MD and Rosetta outputs) also predicts benign. Based on the aggregate predictions, the variant is most likely benign, which does not contradict the ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | 6-33438843-T-C | 3 | 1.86e-6 | -5.056 | Likely Benign | 0.265 | Likely Benign | Likely Benign | 0.203 | Likely Benign | -0.40 | Likely Benign | 0.2 | 0.35 | Likely Benign | -0.03 | Likely Benign | 0.47 | Likely Benign | -3.81 | Deleterious | 0.993 | Probably Damaging | 0.993 | Probably Damaging | 3.32 | Benign | 0.05 | Affected | 3.37 | 35 | -1 | 1 | -0.8 | 10.04 | |||||||||||||||||
| c.1604G>C | S535T 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S535T is catalogued in ClinVar as benign (ClinVar ID 537005.0) and is observed in gnomAD (variant ID 6‑33438847‑G‑C). In silico prediction tools largely agree on a benign effect: REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores. Only FATHMM predicts a pathogenic outcome. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, classifies the variant as likely benign. High‑accuracy assessments confirm this: AlphaMissense‑Optimized is benign; the SGM Consensus is likely benign; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, also reports a benign effect. Overall, the consensus of predictive tools and high‑accuracy methods indicates that the variant is most likely benign, consistent with its ClinVar classification and presence in gnomAD. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign | 1 | 6-33438847-G-C | 14 | 8.67e-6 | -3.886 | Likely Benign | 0.069 | Likely Benign | Likely Benign | 0.177 | Likely Benign | 0.45 | Likely Benign | 0.1 | -0.27 | Likely Benign | 0.09 | Likely Benign | 0.17 | Likely Benign | -0.81 | Neutral | 0.000 | Benign | 0.001 | Benign | -1.25 | Pathogenic | 0.25 | Tolerated | 3.37 | 35 | 1 | 1 | 0.1 | 14.03 | 201.3 | -17.3 | -0.1 | 0.7 | -0.2 | 0.1 | X | Potentially Benign | Ser535 is located near the terminal end of an α-helix (res. Ala533-Val560) close to the membrane interface. In the WT simulations, the hydroxyl side chain of Ser535 forms hydrogen bonds with nearby residues (e.g., His539, Glu538) without any specific interactions. These hydrogen bonds disrupt the structure of the terminal end of the α-helix (Ala533-Ser535), causing it to weaken or unfold during the WT simulations. In the variant simulations, Thr535, a hydrophilic residue with a hydroxyl group of almost the same size as Ser, interacts more frequently with the preceding loop residues (e.g., Thr532, Cys531) due to its longer side chain. Regardless, the residue swap is tolerated in the simulations with no negative effects. However, due to its location near the SynGAP-membrane interface, the effect of the residue swap cannot be fully addressed using the SynGAP solvent-only simulations. | 10.1016/j.ajhg.2020.11.011 | |||||||
| c.1606T>G | L536V 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant L536V is listed in ClinVar (ID 1690714.0) with an uncertain significance designation and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from SIFT, AlphaMissense‑Default, and AlphaMissense‑Optimized; pathogenic predictions arise from REVEL, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, and FATHMM. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely pathogenic verdict. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) remains uncertain. No evidence from FoldX or Rosetta alone is available. Overall, the majority of evidence points toward a pathogenic effect, which does not contradict the ClinVar uncertain status but suggests a higher likelihood of pathogenicity. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -9.014 | Likely Pathogenic | 0.269 | Likely Benign | Likely Benign | 0.586 | Likely Pathogenic | 1.25 | Ambiguous | 0.3 | 1.22 | Ambiguous | 1.24 | Ambiguous | 1.20 | Destabilizing | -2.81 | Deleterious | 0.998 | Probably Damaging | 0.992 | Probably Damaging | -1.34 | Pathogenic | 0.09 | Tolerated | 3.37 | 34 | 2 | 1 | 0.4 | -14.03 | 204.7 | 26.4 | 0.2 | 0.0 | -0.2 | 0.2 | X | Potentially Benign | Leu536 is located on an α-helix (res. Ala533-Val560) at the membrane interface. The iso-butyl group of Leu536 interacts with nearby hydrophobic residues in the preceding loop (e.g., Val526, Pro528, Cys531). In the variant simulations, the iso-propyl side chain of Val536 forms similar hydrophobic interactions as Leu536 in the WT, causing no negative structural effects. | |||||||||||
| c.1625A>G | N542S 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant N542S is listed in ClinVar as benign (ClinVar ID 833567.0) and is not reported in gnomAD. Prediction tools that classify the variant as benign include SIFT and AlphaMissense‑Optimized, whereas the majority of tools predict pathogenicity: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (Likely Pathogenic). High‑accuracy assessments show AlphaMissense‑Optimized predicting benign, SGM‑Consensus predicting likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) yielding an uncertain result. Overall, the preponderance of evidence points to a pathogenic effect, which is in conflict with the ClinVar benign designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Benign | 1 | -9.675 | Likely Pathogenic | 0.767 | Likely Pathogenic | Likely Benign | 0.752 | Likely Pathogenic | 0.98 | Ambiguous | 0.1 | 0.99 | Ambiguous | 0.99 | Ambiguous | 0.91 | Ambiguous | -4.40 | Deleterious | 1.000 | Probably Damaging | 0.989 | Probably Damaging | -1.36 | Pathogenic | 0.13 | Tolerated | 3.37 | 35 | 1 | 1 | 2.7 | -27.03 | 212.5 | 32.1 | 0.0 | 0.0 | -0.6 | 0.3 | X | Potentially Pathogenic | Asn542 is located in an α-helix (res. Ala533-Val560) next to an α-α loop between two α-helices (res. Gly502-Tyr518 and Ala533-Val560). In the WT simulations, the carboxamide group of the Asn542 side chain forms a hydrogen bond with the backbone carbonyl group of Asn523 and packs favourably against Glu522 from the loop. In contrast, in the variant simulations, the hydroxyl group of the Ser542 side chain is unable to maintain either the hydrogen bond with Asn523 or the packing against the Glu522 side chain. Instead, the hydroxyl group of Ser542 occasionally forms a hydrogen bond with the backbone carbonyl group of Glu538.Altogether, the residue swap results in a looser helix-loop association, which is especially evident in the third replica simulation, where Asn523 moves away from its initial placement next to the α-helix. In short, based on the simulations, the residue swap weakens the GAP domain tertiary structure assembly, which in turn could negatively affect protein folding. | |||||||||||
| c.1631G>A | R544Q 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant R544Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33438874‑G‑A). Prediction tools that classify the change as benign include FoldX, PROVEAN, SIFT, and AlphaMissense‑Optimized. Those that predict pathogenicity are REVEL, premPS, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, and AlphaMissense‑Default. Foldetta and Rosetta give uncertain results. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta remains uncertain. Overall, the majority of evidence points toward a pathogenic effect, which is not contradictory to the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | 6-33438874-G-A | 1 | 6.20e-7 | -10.281 | Likely Pathogenic | 0.596 | Likely Pathogenic | Likely Benign | 0.542 | Likely Pathogenic | 0.19 | Likely Benign | 0.2 | 0.87 | Ambiguous | 0.53 | Ambiguous | 1.40 | Destabilizing | -2.41 | Neutral | 1.000 | Probably Damaging | 0.997 | Probably Damaging | -1.40 | Pathogenic | 0.09 | Tolerated | 3.37 | 35 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||
| c.163C>A | Q55K 2D 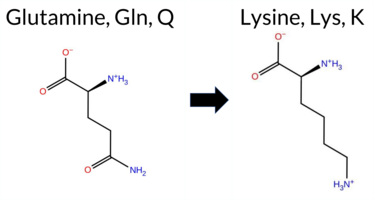 AIThe SynGAP1 missense variant Q55K is listed in ClinVar (ID 520688.0) with an “Uncertain” status and is present in gnomAD (variant ID 6‑33423572‑C‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are SIFT and AlphaMissense‑Default. The SGM‑Consensus, which aggregates AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, and this is not in conflict with the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33423572-C-A | 24 | 1.49e-5 | -5.840 | Likely Benign | 0.612 | Likely Pathogenic | Likely Benign | 0.085 | Likely Benign | -1.21 | Neutral | 0.140 | Benign | 0.184 | Benign | 3.91 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | -0.4 | 0.04 | |||||||||||||||||||||||||||
| c.1667A>G | N556S 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant N556S (ClinVar ID 941099.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33438910‑A‑G). Functional prediction tools that agree on a benign effect include REVEL, Rosetta, Foldetta, premPS, SIFT, ESM1b, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM. The high‑accuracy AlphaMissense‑Optimized score is benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑vs‑2 split, and Foldetta predicts a benign effect. No other high‑accuracy or folding‑stability methods provide additional evidence. Overall, the majority of predictions support a benign impact, which does not contradict the ClinVar Uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 1 | 6-33438910-A-G | 3 | 1.86e-6 | -6.576 | Likely Benign | 0.197 | Likely Benign | Likely Benign | 0.449 | Likely Benign | 0.52 | Ambiguous | 0.1 | 0.14 | Likely Benign | 0.33 | Likely Benign | 0.16 | Likely Benign | -3.60 | Deleterious | 1.000 | Probably Damaging | 0.989 | Probably Damaging | -1.22 | Pathogenic | 0.14 | Tolerated | 3.37 | 35 | 1 | 1 | 2.7 | -27.03 | 198.8 | 31.0 | 0.0 | 0.0 | -0.5 | 0.2 | X | Potentially Benign | Asn556 is located on the outer surface of an α-helix (res. Ala533-Val560). The carboxamide group of Asn556 forms hydrogen bonds with nearby residues such as Lys553 and Cys552. It also forms a hydrogen bond with the backbone carbonyl group of Cys552, which weakens the α-helix integrity. In the variant simulations, the hydroxyl group of Ser556 forms a more stable hydrogen bond with the backbone carbonyl oxygen of the same helix residue, Cys552, compared to Asn556 in the WT. Serine has a slightly lower propensity to reside in an α-helix than asparagine, which may exacerbate the negative effect on the α-helix integrity. However, the residue swap does not cause negative structural effects during the simulations. | |||||||||
| c.1678G>A | V560M 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 V560M missense variant is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6-33440730-G-A). Functional prediction tools that agree on a benign effect include FoldX, Foldetta, PROVEAN, SIFT, and AlphaMissense‑Optimized. Those that agree on a pathogenic effect are REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. Uncertain predictions come from Rosetta, premPS, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta as benign. Overall, the majority of high‑confidence tools predict a benign impact, with only one consensus pathogenic prediction. Therefore, the variant is most likely benign based on current computational evidence, and this does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 2 | 6-33440730-G-A | 15 | 9.50e-6 | -9.598 | Likely Pathogenic | 0.517 | Ambiguous | Likely Benign | 0.520 | Likely Pathogenic | -0.33 | Likely Benign | 0.1 | 0.88 | Ambiguous | 0.28 | Likely Benign | 0.72 | Ambiguous | -2.42 | Neutral | 0.999 | Probably Damaging | 0.863 | Possibly Damaging | -1.25 | Pathogenic | 0.14 | Tolerated | 3.37 | 35 | 2 | 1 | -2.3 | 32.06 | 234.9 | -52.6 | 0.0 | 0.0 | -0.1 | 0.1 | X | Potentially Benign | Val560 is located on the surface at the end of an α-helix (res. Ala533-Val560). The iso-propyl group of Val560 favorably packs against Asp508 of the opposing α-helix (res. Gln503-Glu519). However, in the variant simulations, the bulkier thioether side chain of Met560 does not form equally favorable inter-helix interactions. Regardless, no negative structural effects are observed during the simulations. | |||||||||
| c.1718G>A | R573Q 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R573Q is reported in ClinVar as Pathogenic (ClinVar ID 1176819.0) and is not present in gnomAD. Functional prediction tools largely agree on a deleterious effect: pathogenic predictions come from SGM‑Consensus, REVEL, FoldX, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, and AlphaMissense‑Default, while only SIFT predicts a benign outcome. Two tools give inconclusive results: Rosetta (Uncertain) and AlphaMissense‑Optimized (Uncertain). High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized remains uncertain, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is Pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is Pathogenic. Overall, the preponderance of evidence indicates the variant is most likely pathogenic, consistent with its ClinVar classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Pathogenic | 1 | -9.900 | Likely Pathogenic | 0.923 | Likely Pathogenic | Ambiguous | 0.733 | Likely Pathogenic | 2.28 | Destabilizing | 0.8 | 1.94 | Ambiguous | 2.11 | Destabilizing | 1.08 | Destabilizing | -3.16 | Deleterious | 1.000 | Probably Damaging | 0.995 | Probably Damaging | -1.31 | Pathogenic | 0.12 | Tolerated | 3.37 | 35 | 1 | 1 | 1.0 | -28.06 | 230.1 | 49.9 | 0.0 | 0.0 | -0.6 | 0.0 | X | X | Potentially Pathogenic | The guanidinium group of Arg573, located in an α-helix (res. Arg563-Glu578), forms a salt bridge with the carboxylate groups of Glu582 and/or Asp586 from a nearby α-helix (res. Glu582-Met603) in the WT simulations. Additionally, the Arg573 side chain stacks planarly with the aromatic phenol ring of Tyr665 and hydrogen bonds with the hydroxyl group of Ser668 from another α-helix (res. Ser641-Ser668). In the variant simulations, although the carboxamide group of the Gln573 side chain can hydrogen bond with the carboxylate group of Glu582 or the hydroxyl group of Ser668, these interactions are not as coordinated, stable, or strong as those of the positively charged Arg573. Consequently, the integrity of the opposing α-helix end (res. Glu582-Met603) is weakened. Overall, the residue swap has the potential to substantially affect the tertiary structure assembly during the protein folding process. | ||||||||||
| c.1736G>A | R579Q 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R579Q is listed in ClinVar with an uncertain significance (ClinVar ID 3964539.0) and is present in gnomAD (variant ID 6‑33440788‑G‑A). Prediction tools that classify the variant as benign include SIFT and AlphaMissense‑Optimized, whereas the majority of other in‑silico predictors (SGM‑Consensus, REVEL, premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default) predict it to be pathogenic. High‑accuracy assessments further support this view: AlphaMissense‑Optimized reports a benign effect, SGM‑Consensus (derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) predicts pathogenicity, and Foldetta, which integrates FoldX‑MD and Rosetta outputs, yields an inconclusive result. FoldX and Rosetta individually report uncertain effects. Overall, the preponderance of evidence from both general and high‑accuracy tools indicates that R579Q is most likely pathogenic, which is consistent with its ClinVar status of uncertain significance rather than a benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | 6-33440788-G-A | 18 | 1.12e-5 | -9.193 | Likely Pathogenic | 0.690 | Likely Pathogenic | Likely Benign | 0.673 | Likely Pathogenic | 0.65 | Ambiguous | 0.1 | 0.70 | Ambiguous | 0.68 | Ambiguous | 1.13 | Destabilizing | -3.31 | Deleterious | 1.000 | Probably Damaging | 0.995 | Probably Damaging | -1.34 | Pathogenic | 0.06 | Tolerated | 3.37 | 34 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||
| c.1742G>A | R581Q 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R581Q is reported in ClinVar as benign (ClinVar ID 1388591.0) and is present in gnomAD (ID 6‑33440794‑G‑A). Prediction tools that agree on a benign effect include REVEL, Rosetta, Foldetta, SIFT, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM‑Consensus as pathogenic, and Foldetta as benign. No other high‑confidence stability predictions are available. Overall, the predictions are mixed, with a slight bias toward benign outcomes from the majority of tools and the high‑accuracy AlphaMissense‑Optimized and Foldetta results. Therefore, the variant is most likely benign based on the current computational evidence, which is consistent with its ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Benign | 1 | 6-33440794-G-A | 8 | 4.96e-6 | -7.584 | In-Between | 0.673 | Likely Pathogenic | Likely Benign | 0.481 | Likely Benign | 1.31 | Ambiguous | 0.1 | -0.42 | Likely Benign | 0.45 | Likely Benign | 0.88 | Ambiguous | -2.77 | Deleterious | 1.000 | Probably Damaging | 0.995 | Probably Damaging | -1.21 | Pathogenic | 0.11 | Tolerated | 3.37 | 34 | 1 | 1 | 1.0 | -28.06 | 239.6 | 53.5 | -0.2 | 0.2 | -0.4 | 0.1 | X | Potentially Pathogenic | Arg581 is located on a short α-α loop between two α helices (res. Arg563-Glu578 and res. Glu582-Ser604). In the WT simulations, the guanidinium group of Arg581 forms salt bridges with the carboxylate groups of Asp583 within the same helix, as well as with Glu478 and/or Glu480 on a slightly α-helical loop (res. Glu478-Thr488) preceding another α helix (res. Ala461-Phe476).In the variant simulations, the neutral carboxamide group of the Gln581 side chain cannot form any of these salt bridges. Instead, it packs hydrophobically against Met477 and Ile587 or forms hydrogen bonds sporadically with nearby residues (e.g., Asp583, Arg587). Thus, although no drastic changes are observed in the variant simulations, the residue swap could weaken the tertiary structure assembly. | ||||||||
| c.1752C>G | I584M 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant I584M is listed in ClinVar with an uncertain significance (ClinVar ID 1301269.0) and is present in gnomAD (6‑33440804‑C‑G). Consensus from multiple in‑silico predictors shows a split: benign calls come from REVEL, FoldX, Rosetta, Foldetta, SIFT, and AlphaMissense‑Optimized, whereas pathogenic calls arise from premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, and FATHMM. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default (uncertain), ESM1b, FATHMM, and PROVEAN, is pathogenic. High‑accuracy assessments further support a pathogenic interpretation: AlphaMissense‑Optimized predicts benign, but SGM Consensus is pathogenic, and Foldetta (combining FoldX‑MD and Rosetta) predicts benign. Overall, the majority of tools favor pathogenicity, and the high‑accuracy consensus leans pathogenic, indicating the variant is most likely pathogenic, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 2 | 6-33440804-C-G | 1 | 6.20e-7 | -10.119 | Likely Pathogenic | 0.419 | Ambiguous | Likely Benign | 0.478 | Likely Benign | 0.11 | Likely Benign | 0.1 | 0.46 | Likely Benign | 0.29 | Likely Benign | 1.16 | Destabilizing | -2.62 | Deleterious | 0.983 | Probably Damaging | 0.925 | Probably Damaging | -1.25 | Pathogenic | 0.12 | Tolerated | 3.37 | 34 | 2 | 1 | -2.6 | 18.03 | 247.5 | -20.3 | -0.1 | 0.3 | -0.1 | 0.1 | X | Potentially Benign | A hydrophobic residue, Ile584, located in an α helix (res. Glu582-Met603), is swapped for another hydrophobic residue, Met584. The sec-butyl hydrocarbon side chain of Ile584 packs hydrophobically with residues in an inter-helix hydrophobic space (e.g., Leu588, Met477, Val473, and Ile483).In the variant simulations, the thioether hydrophobic side chain of Met584 maintains similar interactions as Ile584 in the WT, as it is roughly the same size and fits well within the hydrophobic space. Thus, the residue swap does not appear to cause any negative effects on the protein structure. | ||||||||
| c.1767C>G | I589M 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant I589M is listed in ClinVar with an uncertain significance (ClinVar ID 964298.0) and is not reported in gnomAD. Functional prediction tools that provide a definitive call overwhelmingly predict a deleterious effect: REVEL, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default all indicate pathogenicity, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also reports a likely pathogenic outcome. Tools that are inconclusive—FoldX, Rosetta, Foldetta, and AlphaMissense‑Optimized—are listed as uncertain and do not influence the overall assessment. High‑accuracy methods specifically show AlphaMissense‑Optimized as uncertain, SGM Consensus as likely pathogenic, and Foldetta as uncertain. Taken together, the majority of available predictions support a pathogenic effect, which is consistent with the ClinVar uncertain designation rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -12.225 | Likely Pathogenic | 0.926 | Likely Pathogenic | Ambiguous | 0.830 | Likely Pathogenic | 0.74 | Ambiguous | 0.2 | 1.54 | Ambiguous | 1.14 | Ambiguous | 1.33 | Destabilizing | -2.99 | Deleterious | 1.000 | Probably Damaging | 1.000 | Probably Damaging | -1.94 | Pathogenic | 0.00 | Affected | 3.37 | 35 | 2 | 1 | -2.6 | 18.03 | 267.6 | -24.5 | 0.0 | 0.0 | -0.1 | 0.1 | X | Potentially Benign | A hydrophobic residue, Ile589, located in an α helix (res. Glu582-Met603), is swapped for another hydrophobic residue, methionine. The sec-butyl hydrocarbon side chain of Ile589 packs favourably with multiple residues in the inter-helix hydrophobic space (e.g., Phe569, Ile667, and Leu664).Although the S-methyl thioether group of the Met589 side chain in the variant is longer than the branched side chain of isoleucine, it stacks favourably with the aromatic phenol ring. Additionally, the polar sulphur atom forms a weak hydrogen bond with the guanidinium group of Arg573, which in turn forms a salt bridge with the carboxylate group of Asp586.Overall, the hydrophobic packing in the inter-helix space does not appear to be disrupted in the variant simulations. | |||||||||||
| c.1792C>G | L598V 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant L598V is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that classify the variant as benign include REVEL, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic predictions are made by premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates a likely pathogenic effect. High‑accuracy assessments show AlphaMissense‑Optimized as benign, SGM Consensus as likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) as inconclusive. Overall, the majority of evidence points to a pathogenic impact, which contrasts with the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -10.002 | Likely Pathogenic | 0.578 | Likely Pathogenic | Likely Benign | 0.221 | Likely Benign | 1.89 | Ambiguous | 0.1 | 1.58 | Ambiguous | 1.74 | Ambiguous | 1.01 | Destabilizing | -2.92 | Deleterious | 0.944 | Possibly Damaging | 0.786 | Possibly Damaging | 3.21 | Benign | 0.02 | Affected | 3.37 | 35 | 2 | 1 | 0.4 | -14.03 | 218.4 | 29.6 | 0.0 | 0.0 | 0.8 | 0.0 | X | Potentially Benign | The iso-butyl side chain of Leu598, located on an α helix (res. Glu582-Met603), packs hydrophobically with other hydrophobic residues in the inter-helix space (e.g., Ile602, Phe594, Ile510).In the variant simulations, Val598, which has similar size and physicochemical properties to leucine, resides in the inter-helix hydrophobic space in a similar manner to Leu598 in the WT. This causes no negative effects on the protein structure. | |||||||||||
| c.1819C>G | L607V 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant L607V is listed in ClinVar with an uncertain significance (ClinVar ID 1450275.0) and is present in gnomAD (ID 6‑33440871‑C‑G). Prediction tools that agree on a benign effect include only AlphaMissense‑Optimized. All other evaluated algorithms—REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN)—predict a pathogenic impact. High‑accuracy assessments further support this: AlphaMissense‑Optimized reports benign, whereas the SGM‑Consensus, derived from the majority of pathogenic predictions, indicates pathogenic. Foldetta, which integrates FoldX‑MD and Rosetta outputs, is inconclusive and therefore not considered evidence. Overall, the preponderance of computational evidence points to a pathogenic effect for L607V, a conclusion that contrasts with the current ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 2 | 6-33440871-C-G | 2 | 1.24e-6 | -11.190 | Likely Pathogenic | 0.637 | Likely Pathogenic | Likely Benign | 0.715 | Likely Pathogenic | 1.04 | Ambiguous | 0.2 | 1.36 | Ambiguous | 1.20 | Ambiguous | 0.90 | Ambiguous | -2.99 | Deleterious | 0.985 | Probably Damaging | 0.992 | Probably Damaging | -1.50 | Pathogenic | 0.01 | Affected | 3.37 | 35 | 2 | 1 | 0.4 | -14.03 | 216.3 | 28.1 | 0.1 | 0.0 | 0.9 | 0.2 | X | Potentially Benign | Leu607 is located in a short helical region (res. Ser606-Phe608) within an α-α loop connecting two α helices (res. Glu582-Met603 and res. Glu617-Asn635). In the WT simulations, the iso-butyl side chain of Leu607 does not interact with any other residues, but it could potentially interact directly with Ras due to its location at the GAP domain.In the variant simulations, Val607, which has similar size and physicochemical properties to leucine, does not cause any negative effects on the protein structure. However, due to its location at the GAP-Ras interface, the residue swap could affect the complex formation with the GTPase, but this cannot be investigated using solvent-only simulations. | ||||||||
| c.1855A>T | T619S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T619S is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Prediction tools that agree on a benign effect include only AlphaMissense‑Optimized. All other evaluated algorithms—SGM‑Consensus (Likely Pathogenic), REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, and AlphaMissense‑Default—consistently predict a pathogenic impact. High‑accuracy assessments further support this view: AlphaMissense‑Optimized reports a benign outcome, whereas the SGM Consensus, derived from the majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, indicates pathogenicity. Foldetta, which integrates FoldX‑MD and Rosetta stability predictions, yields an uncertain result. Overall, the majority of evidence points to a pathogenic effect for T619S, and this conclusion does not contradict the ClinVar designation of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -8.608 | Likely Pathogenic | 0.677 | Likely Pathogenic | Likely Benign | 0.602 | Likely Pathogenic | 1.09 | Ambiguous | 0.2 | 1.35 | Ambiguous | 1.22 | Ambiguous | 0.85 | Ambiguous | -3.42 | Deleterious | 0.999 | Probably Damaging | 0.998 | Probably Damaging | -1.30 | Pathogenic | 0.05 | Affected | 3.37 | 35 | 1 | 1 | -0.1 | -14.03 | ||||||||||||||||||||
| c.1862G>A | R621Q 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R621Q is listed in ClinVar (ID 578137.0) as benign and is present in gnomAD (variant ID 6‑33440914‑G‑A). Functional prediction tools that agree on a benign effect include only FATHMM, whereas the remaining tools—REVEL, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, and AlphaMissense‑Default—consistently predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) as uncertain. No evidence from FoldX, Rosetta, or Foldetta supports a benign outcome. Overall, the preponderance of predictions indicates a likely pathogenic effect, which contradicts the benign classification reported in ClinVar. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Benign | 1 | 6-33440914-G-A | 19 | 1.18e-5 | -14.682 | Likely Pathogenic | 0.910 | Likely Pathogenic | Ambiguous | 0.621 | Likely Pathogenic | 0.81 | Ambiguous | 0.1 | 1.13 | Ambiguous | 0.97 | Ambiguous | 1.35 | Destabilizing | -3.98 | Deleterious | 1.000 | Probably Damaging | 0.997 | Probably Damaging | 2.82 | Benign | 0.01 | Affected | 3.37 | 35 | 1 | 1 | 1.0 | -28.06 | 243.7 | 54.3 | 0.0 | 0.0 | -0.4 | 0.2 | X | X | Potentially Pathogenic | The guanidinium group of Arg621, located in an α helix (res. Glu617-Asn635), forms a salt bridge with Glu525 in a nearby loop and stacks with Leu635. In the variant simulations, the carboxamide side chain of Gln621, which can act as both a hydrogen bond acceptor and donor, also stacks with Leu635 but can only sporadically hydrogen bond with Glu525.Accordingly, the residue swap could affect the tertiary structure integrity by disrupting the salt bridge formation. Additionally, due to its location at the GAP-Ras interface, the residue swap could impact the complex formation with the GTPase, but this cannot be investigated using solvent-only simulations. | |||||||
| c.1873C>G | L625V 2D  AISynGAP1 missense variant L625V is listed in ClinVar with an uncertain significance (ClinVar ID 3392716.0) and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL and FATHMM, while pathogenic predictions are made by premPS, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, and AlphaMissense‑Default. Four tools (FoldX, Rosetta, Foldetta, AlphaMissense‑Optimized) give inconclusive results. High‑accuracy assessments show AlphaMissense‑Optimized as uncertain, SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as likely pathogenic, and Foldetta as uncertain. Overall, the majority of evidence points toward a pathogenic effect, which does not contradict the ClinVar uncertain status but suggests a higher likelihood of pathogenicity. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -11.319 | Likely Pathogenic | 0.833 | Likely Pathogenic | Ambiguous | 0.480 | Likely Benign | 1.80 | Ambiguous | 0.7 | 1.69 | Ambiguous | 1.75 | Ambiguous | 1.42 | Destabilizing | -2.96 | Deleterious | 0.998 | Probably Damaging | 0.992 | Probably Damaging | 3.07 | Benign | 0.01 | Affected | 2 | 1 | 0.4 | -14.03 | ||||||||||||||||||||||
| c.1904A>G | N635S 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant N635S is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6-33440956-A-G). Functional prediction tools that agree on a benign effect include REVEL, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, SIFT, and ESM1b. Predictions that are inconclusive or unavailable are FoldX, Rosetta, Foldetta, and premPS. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive, and Foldetta is also inconclusive. Overall, the majority of available predictions lean toward a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Conflicting | 4 | 6-33440956-A-G | 10 | 6.20e-6 | -9.002 | Likely Pathogenic | 0.101 | Likely Benign | Likely Benign | 0.104 | Likely Benign | 0.80 | Ambiguous | 0.1 | 0.67 | Ambiguous | 0.74 | Ambiguous | 0.95 | Ambiguous | -4.45 | Deleterious | 0.261 | Benign | 0.044 | Benign | 3.06 | Benign | 0.05 | Affected | 3.37 | 34 | 1 | 1 | 2.7 | -27.03 | 196.0 | 30.9 | 0.1 | 0.0 | -0.3 | 0.2 | X | Uncertain | In the WT simulations, the carboxamide side chain of Asn635, located on the outer surface of an α helix (res. Glu617-Asn635), forms hydrogen bonds with Gln631 on the same α helix and with the hydroxyl side chain of Ser590 on an opposing α helix (res. Glu582-Met603).In the variant simulations, the side chain of Ser635 is shorter than asparagine and thus prefers to hydrogen bond with the carbonyl group of Gln631 on the same helix and, to a lesser extent, with Ser590 compared to Asn635 in the WT. Ser635 forms hydrogen bonds with the backbone atoms of the same helix, which may destabilize the helix, although this is not clearly evident in the simulations. The weakening of the hydrogen bond between Ser635 and Ser590 in the variant may also weaken the tertiary structure assembly between the helices.Additionally, Asn635 is at the GTPase interface. However, the implication of the residue swap on the complex formation with the GTPase cannot be investigated using solvent-only simulations. | |||||||||
| c.1918A>T | T640S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant T640S is listed in ClinVar as Benign (ClinVar ID 2980241.0) and is present in the gnomAD database (gnomAD ID 6‑33441177‑A‑T). Prediction tools that agree on a benign effect include REVEL, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts a pathogenic outcome; the only inconclusive result is from FoldX, which is treated as unavailable. High‑accuracy assessments confirm benignity: AlphaMissense‑Optimized is benign; the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is benign; and Foldetta, which integrates FoldX‑MD and Rosetta outputs, is benign. Overall, the variant is most likely benign, and this conclusion is consistent with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign | 1 | 6-33441177-A-T | 1 | 6.20e-7 | -2.371 | Likely Benign | 0.062 | Likely Benign | Likely Benign | 0.088 | Likely Benign | -0.78 | Ambiguous | 0.1 | 0.43 | Likely Benign | -0.18 | Likely Benign | -0.30 | Likely Benign | 0.92 | Neutral | 0.000 | Benign | 0.001 | Benign | 3.60 | Benign | 0.33 | Tolerated | 3.37 | 30 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||
| c.1947G>C | M649I 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant M649I is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that indicate a benign effect include REVEL, polyPhen‑2 HumVar, and FATHMM, whereas the majority of other in silico predictors (FoldX, Foldetta, premPS, PROVEAN, polyPhen‑2 HumDiv, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized) report a pathogenic outcome; Rosetta is inconclusive. High‑accuracy assessments further support a deleterious impact: AlphaMissense‑Optimized predicts pathogenicity, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is “Likely Pathogenic,” and Foldetta (combining FoldX‑MD and Rosetta outputs) also predicts pathogenicity. Overall, the preponderance of evidence points to a pathogenic effect for M649I, which is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -9.361 | Likely Pathogenic | 0.995 | Likely Pathogenic | Likely Pathogenic | 0.449 | Likely Benign | 2.42 | Destabilizing | 0.2 | 1.96 | Ambiguous | 2.19 | Destabilizing | 1.01 | Destabilizing | -3.99 | Deleterious | 0.672 | Possibly Damaging | 0.093 | Benign | 3.40 | Benign | 0.02 | Affected | 3.38 | 27 | 2 | 1 | 2.6 | -18.03 | 243.7 | 21.5 | 0.0 | 0.1 | 0.0 | 0.1 | X | Potentially Benign | The thioether side chain of Met649, located on an α helix (res. Ser641-Glu666), bridges Phe652, Phe648, and Phe639 in an inter-helix hydrophobic cavity in the WT simulations. In the variant simulations, the sec-butyl side chain of Ile649 maintains hydrophobic interactions with nearby residues, with no significant effects on the protein structure.However, methionine is known as a bridging motif for aromatic residues, and these Met-aromatic interactions are lost in the variant. Indeed, in the second variant simulation,the bridging of Phe652, Phe648 and Phe639 is completely lost. In reality, the effect could be more severe on the structure during the protein folding. | |||||||||||
| c.1957C>G | L653V 2D  AIThe SynGAP1 missense variant L653V is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that classify the variant as benign include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are FoldX, Rosetta, and premPS, while ESM1b is inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus (a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) as Likely Benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) as pathogenic. Overall, the majority of evidence points to a benign impact, and this does not contradict the ClinVar “Uncertain” classification. Thus, based on current predictions, the variant is most likely benign. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | -7.050 | In-Between | 0.301 | Likely Benign | Likely Benign | 0.146 | Likely Benign | 3.28 | Destabilizing | 0.3 | 2.18 | Destabilizing | 2.73 | Destabilizing | 1.32 | Destabilizing | -2.25 | Neutral | 0.227 | Benign | 0.039 | Benign | 3.28 | Benign | 0.08 | Tolerated | 2 | 1 | 0.4 | -14.03 | ||||||||||||||||||||||
| c.2050G>A | D684N 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant D684N is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that indicate a benign effect include REVEL, premPS, and FATHMM, whereas the majority of tools predict a pathogenic outcome: PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). High‑accuracy assessments reinforce this view: AlphaMissense‑Optimized classifies the variant as pathogenic, the SGM‑Consensus also reports it as likely pathogenic, and the Foldetta stability analysis is inconclusive. Protein‑stability predictors FoldX and Rosetta likewise return uncertain results. Overall, the preponderance of evidence points to a pathogenic effect, which contradicts the current ClinVar designation of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -13.155 | Likely Pathogenic | 0.985 | Likely Pathogenic | Likely Pathogenic | 0.382 | Likely Benign | 1.47 | Ambiguous | 0.8 | 1.76 | Ambiguous | 1.62 | Ambiguous | 0.37 | Likely Benign | -4.99 | Deleterious | 0.999 | Probably Damaging | 0.746 | Possibly Damaging | 3.39 | Benign | 0.01 | Affected | 2 | 1 | 0.0 | -0.98 | ||||||||||||||||||||||
| c.2050G>C | D684H 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant D684H is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include only FATHMM; all other evaluated algorithms (REVEL, FoldX, Rosetta, Foldetta, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized) predict a pathogenic impact, and the SGM‑Consensus score is “Likely Pathogenic.” High‑accuracy assessments further support pathogenicity: AlphaMissense‑Optimized is pathogenic, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is likely pathogenic, and Foldetta (combining FoldX‑MD and Rosetta outputs) is pathogenic. No predictions are inconclusive or missing. Based on the overwhelming majority of pathogenic predictions, the variant is most likely pathogenic, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Uncertain | 1 | -14.194 | Likely Pathogenic | 0.998 | Likely Pathogenic | Likely Pathogenic | 0.613 | Likely Pathogenic | 3.36 | Destabilizing | 1.0 | 2.95 | Destabilizing | 3.16 | Destabilizing | 0.55 | Ambiguous | -6.98 | Deleterious | 1.000 | Probably Damaging | 0.972 | Probably Damaging | 3.36 | Benign | 0.00 | Affected | 3.42 | 17 | -1 | 1 | 0.3 | 22.05 | ||||||||||||||||||||
| c.2060G>A | R687Q 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R687Q is annotated in ClinVar as benign (ClinVar ID 2693600.0) and is not reported in gnomAD. Functional prediction tools cluster into two groups: benign predictions come from REVEL, Rosetta, Foldetta, FATHMM, and AlphaMissense‑Optimized, while pathogenic predictions arise from premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). High‑accuracy assessments show AlphaMissense‑Optimized labeling the variant as benign, SGM‑Consensus indicating pathogenicity, and Foldetta (integrating FoldX‑MD and Rosetta outputs) classifying it as benign. With three high‑accuracy tools giving benign or uncertain results and only one (SGM‑Consensus) suggesting pathogenicity, the overall evidence leans toward a benign effect. This prediction aligns with the ClinVar benign classification, indicating no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | GAP | Likely Benign | 1 | -10.002 | Likely Pathogenic | 0.575 | Likely Pathogenic | Likely Benign | 0.401 | Likely Benign | 0.92 | Ambiguous | 0.1 | -0.37 | Likely Benign | 0.28 | Likely Benign | 1.55 | Destabilizing | -3.37 | Deleterious | 1.000 | Probably Damaging | 0.844 | Possibly Damaging | 3.91 | Benign | 0.03 | Affected | 3.42 | 17 | 1 | 1 | 1.0 | -28.06 | ||||||||||||||||||||
| c.2095G>A | V699M 2D  3DClick to see structure in 3D Viewer AISynGAP1 variant V699M is listed in ClinVar with an uncertain significance and is present in gnomAD (ID 6‑33441354‑G‑A). Across in silico predictors, benign calls are made by REVEL, Rosetta, Foldetta, PROVEAN, FATHMM, and AlphaMissense‑Optimized, whereas pathogenic calls come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and ESM1b. Predictions that are inconclusive (FoldX, premPS, AlphaMissense‑Default) are noted but not used as evidence. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also yields benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) reports benign stability. Overall, the preponderance of evidence indicates the variant is most likely benign, which does not contradict the ClinVar uncertain status but provides a stronger leaning toward benignity. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 2 | 6-33441354-G-A | 8 | 4.96e-6 | -8.869 | Likely Pathogenic | 0.484 | Ambiguous | Likely Benign | 0.276 | Likely Benign | -0.58 | Ambiguous | 0.1 | 0.29 | Likely Benign | -0.15 | Likely Benign | 0.96 | Ambiguous | -2.18 | Neutral | 0.994 | Probably Damaging | 0.806 | Possibly Damaging | 3.37 | Benign | 0.03 | Affected | 3.47 | 10 | 2 | 1 | -2.3 | 32.06 | 257.8 | -47.2 | 0.0 | 0.0 | 0.9 | 0.1 | X | Potentially Benign | The isopropyl side chain of Val699, located on an α-helix (res. Leu685-Gln702), packs against hydrophobic residues (e.g., Leu703, Leu696, Leu435, Leu439) in the inter-helix space. In the variant simulations, the thioether side chain of Met699 has similar physicochemical properties to Val699 in the WT, and thus, it is able to maintain similar interactions. Consequently, the mutation causes no apparent changes in the structure. | |||||||||
| c.2101C>T | P701S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant P701S (ClinVar ID 2995856.0) is listed as “Uncertain” in ClinVar and is present in gnomAD (ID 6‑33441360‑C‑T). Prediction tools that agree on a benign effect include REVEL, Rosetta, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). No tool in the dataset predicts a pathogenic outcome; all remaining predictions are either benign or uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as Benign, the SGM‑Consensus as Likely Benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) as Uncertain. Based on the collective evidence, the variant is most likely benign, and this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | 6-33441360-C-T | 3 | 1.86e-6 | -4.375 | Likely Benign | 0.221 | Likely Benign | Likely Benign | 0.132 | Likely Benign | 1.33 | Ambiguous | 0.0 | 0.12 | Likely Benign | 0.73 | Ambiguous | -0.36 | Likely Benign | 0.78 | Neutral | 0.044 | Benign | 0.025 | Benign | 3.48 | Benign | 1.00 | Tolerated | 3.47 | 10 | -1 | 1 | 0.8 | -10.04 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||
| c.2105A>G | Q702R 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant Q702R is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, FoldX, Foldetta, premPS, FATHMM, and AlphaMissense‑Optimized. Those that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. Predictions that remain inconclusive are Rosetta, ESM1b, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized as benign, Foldetta as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to equal benign and pathogenic signals. Overall, the majority of available predictions lean toward a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 1 | -7.894 | In-Between | 0.348 | Ambiguous | Likely Benign | 0.294 | Likely Benign | -0.31 | Likely Benign | 0.1 | 0.63 | Ambiguous | 0.16 | Likely Benign | 0.13 | Likely Benign | -3.14 | Deleterious | 0.909 | Possibly Damaging | 0.889 | Possibly Damaging | 3.43 | Benign | 0.02 | Affected | 3.47 | 10 | 1 | 1 | -1.0 | 28.06 | 270.3 | -52.9 | 0.0 | 0.0 | 0.0 | 0.1 | X | Potentially Pathogenic | The carboxamide side chain of Gln702 is located at the end and outer surface of an α-helix (res. Leu685-Gln702), where it does not directly form hydrogen bonds with any residues in the WT simulations. In the variant simulations, the positively charged guanidinium group of Arg702 forms a salt bridge with the negatively charged carboxylate group of Glu698 on the same helix and/or hydrogen bonds with the backbone carbonyl group of Ala438 on an opposite α-helix (res. Tyr428-Glu436). Consequently, the residue swap could strengthen the tertiary structure assembly, which could have either positive or negative effects on its function. | ||||||||||||
| c.2111G>A | S704N 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S704N is listed in ClinVar as benign (ClinVar ID 962301.0) and is present in gnomAD (ID 6‑33441370‑G‑A). Functional prediction tools largely agree on a benign effect: REVEL, FoldX, Rosetta, Foldetta, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus all report benign or likely benign. Only polyPhen‑2 HumDiv predicts a pathogenic outcome, while premPS and AlphaMissense‑Default are uncertain. High‑accuracy assessments reinforce the benign consensus: AlphaMissense‑Optimized scores benign, the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is likely benign, and Foldetta (combining FoldX‑MD and Rosetta outputs) indicates benign stability. Overall, the predictions support a benign classification, consistent with the ClinVar status and with no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Benign/Likely benign | 3 | 6-33441370-G-A | 27 | 1.67e-5 | -5.917 | Likely Benign | 0.421 | Ambiguous | Likely Benign | 0.058 | Likely Benign | 0.48 | Likely Benign | 0.1 | -0.12 | Likely Benign | 0.18 | Likely Benign | 0.54 | Ambiguous | -0.49 | Neutral | 0.771 | Possibly Damaging | 0.275 | Benign | 3.39 | Benign | 0.08 | Tolerated | 3.47 | 10 | 1 | 1 | -2.7 | 27.03 | 233.2 | -29.1 | -0.1 | 0.0 | -0.1 | 0.1 | X | Potentially Benign | Ser704 is located at the end and outer surface of an α-helix (res. Thr704-Gly712), which is connected via a tight turn or loop to another α-helix (res. Asp684-Gln702). The hydroxyl side chain of Ser704 occasionally forms a hydrogen bond with the amide group of Ala707. However, in the variant simulations, the carboxamide side chain of Asn704 achieves more lasting and numerous hydrogen-bonding interactions with the residues at the helix end, such as Glu706, Ala707, and Leu708. Consequently, the residue swap could strengthen the α-helix secondary structure integrity at the helix end, which could have either positive or negative effects on its function. | ||||||||
| c.2111G>C | S704T 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant S704T is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Consensus from multiple in‑silico predictors shows a predominance of benign calls: REVEL, Rosetta, Foldetta, premPS, PROVEAN, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus. Only polyPhen‑2 HumDiv predicts a pathogenic effect, while FoldX remains inconclusive. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized is benign; the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is benign; and Foldetta, which integrates FoldX‑MD and Rosetta stability calculations, also predicts benign. Overall, the aggregate evidence indicates that S704T is most likely benign, which is consistent with its ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | -4.930 | Likely Benign | 0.265 | Likely Benign | Likely Benign | 0.071 | Likely Benign | 0.80 | Ambiguous | 0.0 | 0.15 | Likely Benign | 0.48 | Likely Benign | 0.29 | Likely Benign | -1.72 | Neutral | 0.525 | Possibly Damaging | 0.107 | Benign | 3.45 | Benign | 0.07 | Tolerated | 3.47 | 10 | 1 | 1 | 0.1 | 14.03 | 201.7 | -18.0 | 0.0 | 0.0 | -0.2 | 0.7 | X | Potentially Benign | Ser704 is located at the end and outer surface of an α-helix (res. Thr704-Gly712), which is connected via a tight turn or loop to another α-helix (res. Asp684-Gln702). The hydroxyl side chain of Ser704 occasionally forms a hydrogen bond with the amide group of Ala707. Similarly, in the variant simulations, the hydroxyl side chain of Thr704 forms hydrogen bonds with the amide groups of Ala707 and Leu708. Thus, the residue swap does not cause any apparent structural change. | |||||||||||
| c.2113A>C | K705Q 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 K705Q missense variant (ClinVar ID 3699560.0) is listed as “Uncertain” in ClinVar and is present in gnomAD (variant ID 6‑33441372‑A‑C). Prediction tools that uniformly indicate a benign effect include REVEL, FoldX, Rosetta, Foldetta, premPS, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign; the SGM Consensus—derived from a majority vote of AlphaMissense‑Default (uncertain), ESM1b (benign), FATHMM (benign), and PROVEAN (benign)—also yields a benign classification; Foldetta, which integrates FoldX‑MD and Rosetta stability outputs, predicts benign. Overall, the preponderance of evidence supports a benign impact for K705Q, and this conclusion does not contradict the ClinVar “Uncertain” status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | 6-33441372-A-C | 1 | 6.20e-7 | -5.787 | Likely Benign | 0.436 | Ambiguous | Likely Benign | 0.142 | Likely Benign | -0.10 | Likely Benign | 0.0 | 0.33 | Likely Benign | 0.12 | Likely Benign | -0.02 | Likely Benign | -0.24 | Neutral | 0.997 | Probably Damaging | 0.969 | Probably Damaging | 3.42 | Benign | 0.78 | Tolerated | 3.47 | 10 | 1 | 1 | 0.4 | -0.04 | |||||||||||||||||
| c.2116G>A | E706K 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant E706K is listed in ClinVar with an uncertain significance and is not reported in gnomAD. Functional prediction tools largely agree on a benign effect: REVEL, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM all classify the change as benign. In contrast, ESM1b and AlphaMissense‑Default predict a pathogenic impact. Tools that return uncertain results—FoldX, Rosetta, Foldetta, and AlphaMissense‑Optimized—do not provide decisive evidence. The SGM Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is inconclusive (two pathogenic versus two benign calls). High‑accuracy assessments are likewise ambiguous: AlphaMissense‑Optimized is uncertain, Foldetta is uncertain, and the SGM Consensus remains inconclusive. Overall, the preponderance of evidence points to a benign effect, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Uncertain | 1 | -10.519 | Likely Pathogenic | 0.833 | Likely Pathogenic | Ambiguous | 0.080 | Likely Benign | 1.17 | Ambiguous | 0.1 | 0.51 | Ambiguous | 0.84 | Ambiguous | 0.08 | Likely Benign | -1.51 | Neutral | 0.345 | Benign | 0.028 | Benign | 4.15 | Benign | 0.52 | Tolerated | 3.47 | 10 | 0 | 1 | -0.4 | -0.94 | 187.1 | 49.2 | 0.0 | 0.0 | 0.4 | 0.1 | X | Uncertain | The carboxylate side chain of Glu706, located at the end and outer surface of an α-helix (res. Thr704-Gly712), forms a salt bridge with Lys710 and a hydrogen bond with its own backbone amino group at the helix end in the WT simulations. Although Lys706 is unable to make these transient interactions in the variant simulations, there is no apparent negative effect on the protein structure due to the residue swap. However, because the model ends abruptly at the C-terminus, no definite conclusions can be drawn based on the simulations. | ||||||||||||
| c.2147G>A | R716Q 2D  3DClick to see structure in 3D Viewer AISynGAP1 missense variant R716Q is listed in ClinVar with an uncertain significance (ClinVar ID 411585.0) and is present in gnomAD (ID 6‑33441612‑G‑A). Functional prediction tools that report a benign effect include REVEL, FoldX, Rosetta, Foldetta, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and ESM1b, while premPS is inconclusive. High‑accuracy assessments show AlphaMissense‑Optimized as benign, Foldetta as benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑vs‑2 split. Overall, the balance of evidence leans toward a benign impact, which does not contradict the ClinVar uncertain status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | GAP | Conflicting | 2 | 6-33441612-G-A | 4 | 2.48e-6 | -8.338 | Likely Pathogenic | 0.308 | Likely Benign | Likely Benign | 0.210 | Likely Benign | -0.01 | Likely Benign | 0.0 | 0.47 | Likely Benign | 0.23 | Likely Benign | 0.58 | Ambiguous | -3.14 | Deleterious | 1.000 | Probably Damaging | 0.990 | Probably Damaging | 3.35 | Benign | 0.02 | Affected | 3.50 | 9 | 1 | 1 | 1.0 | -28.06 | 250.0 | 48.9 | 0.0 | 0.0 | -0.5 | 0.0 | X | Uncertain | The guanidinium group of Arg716, located on the outer surface of an α-helix (res. Leu714-Arg726), forms a salt bridge with the carboxylate group of Asp720. In the variant simulations, the carboxamide group of Gln716 also forms a hydrogen bond with the carboxylate group of Asp720, although this bond is weaker than the Arg716 salt bridge in the WT. Overall, no adverse effects on the protein structure are observed in the simulations. However, because the model ends abruptly at the C-terminus, no definite conclusions can be drawn based on the simulations. | |||||||||
| c.2186A>G | N729S 2D  3DClick to see structure in 3D Viewer AIThe SynGAP1 missense variant N729S is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, FoldX, premPS, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). No tool in the dataset predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign, the SGM Consensus also as benign, while Foldetta (combining FoldX‑MD and Rosetta outputs) is inconclusive. Based on the collective predictions, the variant is most likely benign, and this assessment does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | GAP | Uncertain | 1 | -1.578 | Likely Benign | 0.066 | Likely Benign | Likely Benign | 0.063 | Likely Benign | 0.14 | Likely Benign | 0.1 | 1.34 | Ambiguous | 0.74 | Ambiguous | -0.36 | Likely Benign | -0.42 | Neutral | 0.221 | Benign | 0.027 | Benign | 3.38 | Benign | 0.93 | Tolerated | 3.59 | 7 | 1 | 1 | 2.7 | -27.03 | ||||||||||||||||||||
| c.2219G>A | R740Q 2D  AIThe SynGAP1 missense variant R740Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33441684‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic outcome. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is also benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no reported result for this variant, so it does not influence the assessment. Overall, the majority of predictions indicate that R740Q is most likely benign, which is consistent with the ClinVar “Uncertain” classification and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33441684-G-A | 4 | 2.48e-6 | -5.195 | Likely Benign | 0.078 | Likely Benign | Likely Benign | 0.102 | Likely Benign | -0.67 | Neutral | 0.999 | Probably Damaging | 0.881 | Possibly Damaging | 2.60 | Benign | 0.08 | Tolerated | 4.32 | 2 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.221G>A | S74N 2D  AIThe SynGAP1 missense variant S74N is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33425829‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” No Foldetta stability result is available. Overall, the majority of computational evidence indicates that the variant is most likely benign, which does not contradict the current ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33425829-G-A | 5 | 3.10e-6 | -5.156 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.031 | Likely Benign | -0.89 | Neutral | 0.043 | Benign | 0.007 | Benign | 4.09 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||
| c.2225G>A | R742Q 2D  AIThe SynGAP1 missense variant R742Q is listed in ClinVar (ID 928481.0) with an uncertain significance annotation and is observed in gnomAD (variant ID 6‑33441690‑G‑A). Consensus from multiple in‑silico predictors—REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized—uniformly classify the change as benign. No tool in the dataset reports a pathogenic prediction. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign effect. A protein‑folding stability analysis via Foldetta is not available for this variant. Overall, the computational evidence strongly favors a benign interpretation, which is consistent with the ClinVar uncertain status rather than contradicting it. The variant is most likely benign, and this assessment does not contradict its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33441690-G-A | 24 | 1.49e-5 | -4.090 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.054 | Likely Benign | -0.19 | Neutral | 0.032 | Benign | 0.007 | Benign | 2.73 | Benign | 0.07 | Tolerated | 4.32 | 2 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.2239G>C | V747L 2D  AIThe SynGAP1 missense variant V747L (ClinVar ID 1985039.0) is listed as ClinVar status Uncertain and is present in gnomAD (6‑33441704‑G‑C). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta stability analysis is unavailable. Overall, the majority of computational evidence supports a benign classification, which is consistent with the ClinVar Uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33441704-G-C | 2 | 1.24e-6 | -2.790 | Likely Benign | 0.096 | Likely Benign | Likely Benign | 0.047 | Likely Benign | -0.52 | Neutral | 0.065 | Benign | 0.033 | Benign | 2.67 | Benign | 0.00 | Affected | 4.32 | 2 | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||
| c.223G>A | E75K 2D  AIThe SynGAP1 missense variant E75K is listed in ClinVar as Benign (ClinVar ID 3360083.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 HumVar, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and SIFT, while AlphaMissense‑Default remains uncertain. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar classification and not contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign/Likely benign | 2 | -4.020 | Likely Benign | 0.358 | Ambiguous | Likely Benign | 0.134 | Likely Benign | -1.12 | Neutral | 0.748 | Possibly Damaging | 0.017 | Benign | 4.07 | Benign | 0.00 | Affected | 0 | 1 | -0.4 | -0.94 | ||||||||||||||||||||||||||||||||
| c.2246G>A | R749Q 2D  AIThe SynGAP1 missense variant R749Q is listed in ClinVar (ID 793884.0) as Benign and is present in gnomAD (6‑33441711‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. The SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) also reports a Likely Benign outcome. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence—including high‑accuracy predictions—supports a benign classification, which is consistent with the ClinVar status and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | 6-33441711-G-A | 4 | 2.48e-6 | -3.069 | Likely Benign | 0.212 | Likely Benign | Likely Benign | 0.152 | Likely Benign | -1.00 | Neutral | 0.999 | Probably Damaging | 0.994 | Probably Damaging | 2.64 | Benign | 0.03 | Affected | 4.32 | 2 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.2282G>A | R761Q 2D  AIThe SynGAP1 missense variant R761Q is listed in ClinVar (ID 2882770.0) with an “Uncertain” status and is present in gnomAD (6‑33441747‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are not available. Overall, the majority of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33441747-G-A | 11 | 6.81e-6 | -4.187 | Likely Benign | 0.202 | Likely Benign | Likely Benign | 0.191 | Likely Benign | -0.63 | Neutral | 0.996 | Probably Damaging | 0.878 | Possibly Damaging | 2.75 | Benign | 0.40 | Tolerated | 3.99 | 5 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.2291A>G | N764S 2D  AIThe SynGAP1 missense variant N764S is listed in ClinVar as Benign (ClinVar ID 1948460.0) and is not reported in gnomAD. Prediction tools that agree on a benign effect include SGM‑Consensus (Likely Benign), REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. In contrast, polyPhen‑2 (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as Benign and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Benign; Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, consistent with the ClinVar classification, and there is no contradiction between the predictions and the reported ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | -3.149 | Likely Benign | 0.159 | Likely Benign | Likely Benign | 0.058 | Likely Benign | -0.84 | Neutral | 0.992 | Probably Damaging | 0.846 | Possibly Damaging | 2.65 | Benign | 0.61 | Tolerated | 3.64 | 6 | 1 | 1 | 2.7 | -27.03 | ||||||||||||||||||||||||||||||
| c.2294G>A | S765N 2D  AIThe SynGAP1 missense variant S765N (ClinVar ID 2979632.0) is listed as “Uncertain” in ClinVar and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). In contrast, PolyPhen‑2 (HumDiv and HumVar) predict a pathogenic impact. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is also benign. No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect, which is consistent with the ClinVar “Uncertain” classification and does not contradict it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -5.098 | Likely Benign | 0.378 | Ambiguous | Likely Benign | 0.094 | Likely Benign | -0.94 | Neutral | 0.985 | Probably Damaging | 0.950 | Probably Damaging | 4.11 | Benign | 0.06 | Tolerated | 3.64 | 6 | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||
| c.2324G>A | R775Q 2D  AIThe SynGAP1 missense variant R775Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33442482‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). In contrast, polyPhen‑2 (both HumDiv and HumVar models) predict a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores benign, and the SGM‑Consensus (derived from the same set of high‑confidence predictors) is “Likely Benign.” No Foldetta stability prediction is available, so it does not influence the assessment. Overall, the majority of evidence points to a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Conflicting | 3 | 6-33442482-G-A | 11 | 1.41e-5 | -4.476 | Likely Benign | 0.229 | Likely Benign | Likely Benign | 0.085 | Likely Benign | -0.63 | Neutral | 0.969 | Probably Damaging | 0.863 | Possibly Damaging | 4.17 | Benign | 0.16 | Tolerated | 3.64 | 6 | 1 | 1 | 1.0 | -28.06 | 10.1016/j.ajhg.2020.11.011 | ||||||||||||||||||||||||||
| c.2359C>T | P787S 2D  AIThe SynGAP1 P787S variant is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33442911‑C‑T). Prediction tools that agree on a benign effect include REVEL, ESM1b, and AlphaMissense‑Optimized, while those that predict a pathogenic outcome are PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM; AlphaMissense‑Default remains uncertain. The high‑accuracy consensus (SGM Consensus) derived from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN yields a pathogenic majority. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a pathogenic effect, and this assessment does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | SH3-binding motif | Uncertain | 1 | 6-33442911-C-T | 3 | 1.86e-6 | -4.203 | Likely Benign | 0.564 | Ambiguous | Likely Benign | 0.221 | Likely Benign | -3.81 | Deleterious | 1.000 | Probably Damaging | 0.999 | Probably Damaging | 2.48 | Pathogenic | 0.02 | Affected | 3.64 | 6 | -1 | 1 | 0.8 | -10.04 | |||||||||||||||||||||||||||
| c.2362T>A | S788T 2D  AIThe SynGAP1 missense variant S788T is listed in ClinVar with an uncertain significance (ClinVar ID 392728.0) and is present in the gnomAD database (gnomAD ID 6‑33442914‑T‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score, which is derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN. Tools that predict a pathogenic outcome are polyPhen‑2 (HumDiv and HumVar), SIFT, and FATHMM. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM‑Consensus (majority vote) also favors a benign interpretation. No Foldetta stability prediction is available for this variant. Overall, the majority of computational evidence points to a benign effect, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | Uncertain | 2 | 6-33442914-T-A | 4 | 2.49e-6 | -4.288 | Likely Benign | 0.288 | Likely Benign | Likely Benign | 0.092 | Likely Benign | -2.25 | Neutral | 0.979 | Probably Damaging | 0.982 | Probably Damaging | 1.55 | Pathogenic | 0.02 | Affected | 3.64 | 6 | 1 | 1 | 0.1 | 14.03 | ||||||||||||||||||||||||||
| c.2369C>G | T790S 2D  AIThe SynGAP1 missense variant T790S is listed in ClinVar (ID 1020340.0) with an “Uncertain” clinical significance and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). In contrast, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and FATHMM predict a pathogenic impact. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus also as likely benign; a Foldetta stability analysis is unavailable. Overall, the majority of evidence points to a benign effect, and this is consistent with the ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | Uncertain | 1 | -3.914 | Likely Benign | 0.123 | Likely Benign | Likely Benign | 0.134 | Likely Benign | -1.83 | Neutral | 0.997 | Probably Damaging | 0.989 | Probably Damaging | 2.39 | Pathogenic | 0.33 | Tolerated | 3.64 | 6 | 1 | 1 | -0.1 | -14.03 | |||||||||||||||||||||||||||||
| c.2401G>A | G801S 2D  AIThe SynGAP1 missense variant G801S is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. All available in‑silico predictors classify the change as benign: SGM‑Consensus (Likely Benign), REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity. High‑accuracy assessments reinforce this benign prediction: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is also Likely Benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no reported result for this variant. Overall, the evidence strongly supports a benign effect, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | SH3-binding motif | Uncertain | 1 | -3.665 | Likely Benign | 0.087 | Likely Benign | Likely Benign | 0.039 | Likely Benign | -0.41 | Neutral | 0.009 | Benign | 0.019 | Benign | 2.76 | Benign | 0.48 | Tolerated | 4.32 | 2 | 0 | 1 | -0.4 | 30.03 | |||||||||||||||||||||||||||||
| c.2485G>A | E829K 2D  AIThe SynGAP1 missense variant E829K is listed in ClinVar as Pathogenic (ClinVar ID 1721258.0) and is not reported in gnomAD. Functional prediction tools largely agree on a deleterious effect: pathogenic predictions come from PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, FATHMM, AlphaMissense‑Default, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Only REVEL predicts a benign outcome, while ESM1b and AlphaMissense‑Optimized are uncertain. High‑accuracy assessments show the SGM‑Consensus as Likely Pathogenic, AlphaMissense‑Optimized as uncertain, and Foldetta results are unavailable. Overall, the preponderance of evidence indicates that E829K is most likely pathogenic, and this conclusion aligns with the ClinVar pathogenic classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Pathogenic | Pathogenic | 1 | -7.527 | In-Between | 0.807 | Likely Pathogenic | Ambiguous | 0.194 | Likely Benign | -2.65 | Deleterious | 0.994 | Probably Damaging | 0.900 | Possibly Damaging | 2.27 | Pathogenic | 0.00 | Affected | 3.77 | 5 | 0 | 1 | -0.4 | -0.94 | ||||||||||||||||||||||||||||||
| c.2567A>G | N856S 2D  AIThe SynGAP1 missense variant N856S is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443119‑A‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Those that predict a pathogenic outcome are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign classification. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote) also benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign effect, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443119-A-G | 2 | 1.24e-6 | -2.104 | Likely Benign | 0.064 | Likely Benign | Likely Benign | 0.040 | Likely Benign | -1.54 | Neutral | 0.901 | Possibly Damaging | 0.535 | Possibly Damaging | 4.16 | Benign | 0.30 | Tolerated | 3.88 | 3 | 1 | 1 | 2.7 | -27.03 | |||||||||||||||||||||||||||
| c.2573G>A | S858N 2D  AIThe SynGAP1 missense variant S858N is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33443125‑G‑A). Functional prediction tools largely agree on a benign effect: REVEL, PROVEAN, polyPhen‑2 HumDiv, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) all indicate benign. In contrast, polyPhen‑2 HumVar and SIFT predict pathogenicity, but these two tools are in the minority. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta results are unavailable. Overall, the preponderance of evidence points to a benign impact, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443125-G-A | 2 | 1.24e-6 | -4.311 | Likely Benign | 0.121 | Likely Benign | Likely Benign | 0.107 | Likely Benign | -0.67 | Neutral | 0.448 | Benign | 0.846 | Possibly Damaging | 4.13 | Benign | 0.02 | Affected | 3.77 | 5 | 1 | 1 | -2.7 | 27.03 | |||||||||||||||||||||||||||
| c.2596G>T | V866L 2D  AIThe SynGAP1 missense variant V866L is listed in ClinVar (ID 469150.0) with an “Uncertain” clinical significance and is present in gnomAD (6‑33443148‑G‑T). All evaluated in‑silico predictors classify the substitution as benign: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool reports a pathogenic outcome. High‑accuracy assessments corroborate this benign prediction: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the computational evidence strongly supports a benign effect, and this conclusion does not contradict the current ClinVar status of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443148-G-T | 1 | 6.20e-7 | -3.352 | Likely Benign | 0.148 | Likely Benign | Likely Benign | 0.046 | Likely Benign | -0.97 | Neutral | 0.217 | Benign | 0.229 | Benign | 2.71 | Benign | 0.21 | Tolerated | 3.82 | 4 | 2 | 1 | -0.4 | 14.03 | |||||||||||||||||||||||||||
| c.2608C>G | L870V 2D  AIThe SynGAP1 missense variant L870V is listed in ClinVar (ID 946946.0) with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). In contrast, PolyPhen‑2 (HumDiv and HumVar) predict a pathogenic outcome. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; the Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the majority of evidence points to a benign impact, and this conclusion does not conflict with the ClinVar designation of uncertainty. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -4.123 | Likely Benign | 0.300 | Likely Benign | Likely Benign | 0.111 | Likely Benign | -1.19 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.64 | Benign | 0.12 | Tolerated | 3.88 | 3 | 2 | 1 | 0.4 | -14.03 | ||||||||||||||||||||||||||||||
| c.2623G>A | A875T 2D  AIThe SynGAP1 missense variant A875T is listed in ClinVar with an “Uncertain” status and is present in gnomAD (variant ID 6‑33443175‑G‑A). Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv and polyPhen‑2 HumVar. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as likely benign; Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence points to a benign impact, which is consistent with the ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443175-G-A | 1 | 6.20e-7 | -3.793 | Likely Benign | 0.179 | Likely Benign | Likely Benign | 0.110 | Likely Benign | -1.56 | Neutral | 0.972 | Probably Damaging | 0.864 | Possibly Damaging | 2.72 | Benign | 0.26 | Tolerated | 3.77 | 5 | 0 | 1 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.2651G>A | R884Q 2D  AIThe SynGAP1 missense variant R884Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33443203‑G‑A). All available in silico predictors agree on a benign effect: REVEL, PROVEAN, PolyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all return benign scores. No tool predicts pathogenicity. High‑accuracy assessments reinforce this consensus: AlphaMissense‑Optimized is benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) is “Likely Benign.” Foldetta results are not reported, so they are unavailable. Overall, the computational evidence strongly supports a benign classification, which does not contradict the ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33443203-G-A | 5 | 3.10e-6 | -3.785 | Likely Benign | 0.128 | Likely Benign | Likely Benign | 0.055 | Likely Benign | -0.42 | Neutral | 0.012 | Benign | 0.004 | Benign | 2.62 | Benign | 0.36 | Tolerated | 4.32 | 4 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.2684G>A | S895N 2D  AIThe SynGAP1 missense variant S895N is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, is “Likely Benign.” High‑accuracy assessments show AlphaMissense‑Optimized as benign, and the SGM‑Consensus (majority vote) also leans benign. No Foldetta (protein‑folding stability) result is available, so it does not influence the assessment. Overall, the preponderance of predictions indicates the variant is most likely benign, which is consistent with its ClinVar “Uncertain” classification rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -6.399 | Likely Benign | 0.604 | Likely Pathogenic | Likely Benign | 0.118 | Likely Benign | -0.85 | Neutral | 0.991 | Probably Damaging | 0.988 | Probably Damaging | 2.64 | Benign | 0.30 | Tolerated | 4.32 | 4 | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||
| c.2710A>G | M904V 2D  AIThe SynGAP1 missense variant M904V is reported in ClinVar (ID 833650.0) as benign and is present in gnomAD (variant ID 6‑33443262‑A‑G). All evaluated in‑silico predictors classify the change as benign: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity. High‑accuracy assessments corroborate this: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates a likely benign effect. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the computational evidence strongly supports a benign classification, which aligns with the ClinVar status and shows no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 2 | 6-33443262-A-G | 77 | 4.78e-5 | -2.907 | Likely Benign | 0.112 | Likely Benign | Likely Benign | 0.058 | Likely Benign | -0.33 | Neutral | 0.039 | Benign | 0.023 | Benign | 2.80 | Benign | 0.10 | Tolerated | 3.77 | 5 | 2 | 1 | 2.3 | -32.06 | |||||||||||||||||||||||||||
| c.2752G>A | A918T 2D  AISynGAP1 missense variant A918T is listed in ClinVar with an uncertain significance (ClinVar ID 3964538.0) and is present in gnomAD (6‑33443304‑G‑A). Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Pathogenic predictions are reported by polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized predicts benign, and the SGM‑Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) also indicates likely benign. Foldetta stability analysis is unavailable. Overall, the preponderance of evidence points to a benign effect, which is consistent with the ClinVar uncertain status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443304-G-A | 1 | 6.20e-7 | -4.139 | Likely Benign | 0.083 | Likely Benign | Likely Benign | 0.065 | Likely Benign | -1.09 | Neutral | 0.980 | Probably Damaging | 0.721 | Possibly Damaging | 2.64 | Benign | 0.03 | Affected | 4.32 | 4 | 0 | 1 | -2.5 | 30.03 | |||||||||||||||||||||||||||
| c.2765G>A | R922Q 2D  AIThe SynGAP1 missense variant R922Q is listed in ClinVar as Benign (ClinVar ID 2917638.0) and is present in gnomAD (ID 6‑33443317‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Tools that predict a pathogenic effect are PolyPhen‑2 HumDiv and PolyPhen‑2 HumVar. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (derived from the same set of high‑confidence predictors) also as benign. Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar classification and indicating no contradiction. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | 6-33443317-G-A | 7 | 4.34e-6 | -3.295 | Likely Benign | 0.189 | Likely Benign | Likely Benign | 0.085 | Likely Benign | -0.27 | Neutral | 0.992 | Probably Damaging | 0.736 | Possibly Damaging | 2.57 | Benign | 0.20 | Tolerated | 3.77 | 5 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.2809G>C | D937H 2D  AIThe SynGAP1 D937H missense variant (ClinVar ID 2825773.0) is listed as “Uncertain” and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic outcome are PolyPhen‑2 HumDiv, PolyPhen‑2 HumVar, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports the variant as “Likely Benign.” High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, the SGM‑Consensus (majority vote) is benign, and Foldetta (protein‑folding stability analysis combining FoldX‑MD and Rosetta) data are unavailable. Based on the preponderance of evidence from both general and high‑accuracy predictors, the variant is most likely benign, which is consistent with its ClinVar “Uncertain” status rather than contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -0.733 | Likely Benign | 0.677 | Likely Pathogenic | Likely Benign | 0.150 | Likely Benign | -1.74 | Neutral | 1.000 | Probably Damaging | 0.975 | Probably Damaging | 2.68 | Benign | 0.13 | Tolerated | 3.77 | 5 | -1 | 1 | 0.3 | 22.05 | ||||||||||||||||||||||||||||||
| c.2900G>A | R967Q 2D  AIThe SynGAP1 missense variant R967Q is listed in ClinVar as Benign (ClinVar ID 536992.0) and is present in gnomAD (6‑33443452‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus score (Likely Benign). Tools that predict a pathogenic effect are PolyPhen‑2 HumDiv and PolyPhen‑2 HumVar. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact, aligning with the ClinVar classification and not contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign/Likely benign | 2 | 6-33443452-G-A | 31 | 1.92e-5 | -3.057 | Likely Benign | 0.080 | Likely Benign | Likely Benign | 0.104 | Likely Benign | -0.01 | Neutral | 0.994 | Probably Damaging | 0.626 | Possibly Damaging | 4.21 | Benign | 0.36 | Tolerated | 4.32 | 2 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.2914C>G | P972A 2D  AIThe SynGAP1 missense variant P972A is listed in ClinVar with an uncertain significance (ClinVar ID 3172763.0) and is present in the gnomAD database (gnomAD ID 6‑33443466‑C‑G). All evaluated in‑silico predictors classify the substitution as benign: REVEL, PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool predicts pathogenicity. High‑accuracy assessments further support a benign outcome: AlphaMissense‑Optimized reports benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” The Foldetta protein‑folding stability analysis is unavailable for this variant. Overall, the computational evidence strongly suggests the variant is most likely benign, and this conclusion does not contradict the current ClinVar status of uncertain significance. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443466-C-G | 1 | 6.20e-7 | -0.167 | Likely Benign | 0.045 | Likely Benign | Likely Benign | 0.046 | Likely Benign | -0.89 | Neutral | 0.016 | Benign | 0.011 | Benign | 4.29 | Benign | 0.07 | Tolerated | 4.32 | 2 | -1 | 1 | 3.4 | -26.04 | |||||||||||||||||||||||||||
| c.2914C>T | P972S 2D  AIThe SynGAP1 missense variant P972S (ClinVar ID 3361353.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33443466‑C‑T). Consensus among most in silico predictors indicates a benign effect: REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized all score the substitution as benign. Only SIFT classifies it as pathogenic, representing the sole discordant prediction. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized predicts benign, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) reports “Likely Benign.” No Foldetta stability analysis is available for this residue. Overall, the preponderance of computational evidence points to a benign effect, which is consistent with the ClinVar designation of uncertainty rather than pathogenicity. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33443466-C-T | 4 | 2.48e-6 | -4.008 | Likely Benign | 0.058 | Likely Benign | Likely Benign | 0.074 | Likely Benign | -0.38 | Neutral | 0.001 | Benign | 0.002 | Benign | 4.28 | Benign | 0.05 | Affected | 4.32 | 2 | -1 | 1 | 0.8 | -10.04 | |||||||||||||||||||||||||||
| c.2924C>G | T975S 2D  AIThe SynGAP1 missense variant T975S is listed in ClinVar with an “Uncertain” status and is not reported in gnomAD. Functional prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), SIFT, ESM1b, FATHMM, AlphaMissense‑Default, and AlphaMissense‑Optimized. No tool in the dataset predicts a pathogenic outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized scores the variant as benign, and the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN) indicates “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, did not provide a result for this variant. Overall, the available predictions strongly suggest that T975S is most likely benign, and this conclusion does not contradict the ClinVar “Uncertain” classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -2.743 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.109 | Likely Benign | -0.57 | Neutral | 0.059 | Benign | 0.061 | Benign | 4.16 | Benign | 0.20 | Tolerated | 1 | 1 | -0.1 | -14.03 | ||||||||||||||||||||||||||||||||
| c.2948G>A | S983N 2D  AISynGAP1 missense variant S983N is listed as Benign in ClinVar (ID 469153) and is present in gnomAD (6‑33443500‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, and ESM1b, whereas tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, FATHMM, and AlphaMissense‑Default. The high‑accuracy AlphaMissense‑Optimized score is uncertain, and the SGM Consensus (majority vote of AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive; Foldetta results are not available. Overall, the majority of available predictions (five pathogenic vs. three benign) suggest a pathogenic impact, which contradicts the ClinVar benign classification. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | 1 | 6-33443500-G-A | 6 | 3.72e-6 | -5.604 | Likely Benign | 0.909 | Likely Pathogenic | Ambiguous | 0.136 | Likely Benign | -1.78 | Neutral | 0.991 | Probably Damaging | 0.988 | Probably Damaging | 2.04 | Pathogenic | 0.00 | Affected | 4.32 | 1 | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||
| c.2954G>A | S985N 2D  AIThe SynGAP1 missense variant S985N is listed in ClinVar (ID 2087879.0) with an “Uncertain” status and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, and FATHMM, while those that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, derived from a majority vote of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a “Likely Benign” classification. Separately, the high‑accuracy AlphaMissense‑Optimized result is “Uncertain,” and the Foldetta protein‑folding stability assessment is unavailable. Based on the overall distribution of predictions, the variant is most likely benign; this conclusion does not contradict the ClinVar status, which remains uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | -6.979 | Likely Benign | 0.845 | Likely Pathogenic | Ambiguous | 0.088 | Likely Benign | -1.68 | Neutral | 0.991 | Probably Damaging | 0.988 | Probably Damaging | 2.65 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||
| c.29G>A | R10Q 2D  AIThe SynGAP1 missense variant R10Q is listed in ClinVar with an “Uncertain” status and is present in gnomAD (ID 6‑33420293‑G‑A). Prediction tools that agree on a benign effect include REVEL, PROVEAN, polyPhen‑2 (HumDiv and HumVar), ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN). Only SIFT predicts a pathogenic outcome. High‑accuracy assessments further support a benign classification: AlphaMissense‑Optimized is benign, and the SGM‑Consensus is “Likely Benign.” Foldetta, a protein‑folding stability method combining FoldX‑MD and Rosetta outputs, has no available result for this variant. Overall, the preponderance of evidence indicates that R10Q is most likely benign, which does not contradict the current ClinVar “Uncertain” designation. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 2 | 6-33420293-G-A | 20 | 1.30e-5 | -4.438 | Likely Benign | 0.185 | Likely Benign | Likely Benign | 0.084 | Likely Benign | 0.03 | Neutral | 0.121 | Benign | 0.004 | Benign | 4.17 | Benign | 0.00 | Affected | 4.32 | 1 | 1 | 1 | 1.0 | -28.06 | |||||||||||||||||||||||||||
| c.3020G>A | S1007N 2D  AIThe SynGAP1 missense variant S1007N is listed in ClinVar (ID 2759915.0) as Benign and is not reported in gnomAD. Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, and the SGM‑Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. AlphaMissense‑Optimized is uncertain, and Foldetta (a protein‑folding stability method combining FoldX‑MD and Rosetta outputs) has no available result for this variant. Overall, the majority of high‑accuracy predictors (including the SGM‑Consensus) indicate a benign impact, and the single uncertain AlphaMissense‑Optimized result does not overturn this consensus. Therefore, the variant is most likely benign, and this conclusion is consistent with its ClinVar status. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Benign | 1 | -5.113 | Likely Benign | 0.803 | Likely Pathogenic | Ambiguous | 0.075 | Likely Benign | -1.54 | Neutral | 0.997 | Probably Damaging | 0.992 | Probably Damaging | 2.65 | Benign | 0.01 | Affected | 3.77 | 5 | 1 | 1 | -2.7 | 27.03 | ||||||||||||||||||||||||||||||
| c.3022G>A | D1008N 2D  AIThe SynGAP1 missense variant D1008N is listed in ClinVar (ID 1213097.0) as benign and is present in gnomAD (variant ID 6‑33443574‑G‑A). Functional prediction tools cluster into two groups: benign predictions come from REVEL, PROVEAN, ESM1b, FATHMM, and AlphaMissense‑Optimized; pathogenic predictions come from polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. The SGM‑Consensus, a majority‑vote model of AlphaMissense‑Default, ESM1b, FATHMM, and PROVEAN, reports a likely benign outcome. High‑accuracy assessments further support a benign interpretation: AlphaMissense‑Optimized is benign, SGM‑Consensus is likely benign, and Foldetta (combining FoldX‑MD and Rosetta) has no available result for this variant. Overall, the majority of evidence indicates a benign effect, consistent with the ClinVar classification and not contradicting it. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Likely Benign | 1 | 6-33443574-G-A | 3 | 1.86e-6 | -4.045 | Likely Benign | 0.714 | Likely Pathogenic | Likely Benign | 0.128 | Likely Benign | -2.15 | Neutral | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.75 | Benign | 0.01 | Affected | 3.77 | 5 | 2 | 1 | 0.0 | -0.98 | |||||||||||||||||||||||||||
| c.3023A>G | D1008G 2D  AIThe SynGAP1 D1008G missense variant (ClinVar ID 2963386.0) is listed as Uncertain in ClinVar and is present in gnomAD (ID 6‑33443575‑A‑G). Prediction tools that agree on a benign effect include REVEL, ESM1b, FATHMM, and AlphaMissense‑Optimized. Tools that predict a pathogenic effect are PROVEAN, polyPhen‑2 HumDiv, polyPhen‑2 HumVar, SIFT, and AlphaMissense‑Default. High‑accuracy assessments show AlphaMissense‑Optimized classifying the variant as benign, while the SGM Consensus (majority vote from AlphaMissense‑Default, ESM1b, FATHMM, PROVEAN) is inconclusive due to a 2‑to‑2 split, and Foldetta results are unavailable. Overall, the balance of evidence leans toward a pathogenic interpretation, which does not contradict the current ClinVar designation of Uncertain. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Uncertain | 1 | 6-33443575-A-G | 1 | 6.20e-7 | -3.213 | Likely Benign | 0.742 | Likely Pathogenic | Likely Benign | 0.203 | Likely Benign | -2.84 | Deleterious | 0.999 | Probably Damaging | 0.997 | Probably Damaging | 2.65 | Benign | 0.01 | Affected | 3.77 | 5 | -1 | 1 | 3.1 | -58.04 | ||||||||||||||||||||||||||||
| c.304T>G | L102V 2D  AIThe SynGAP1 missense variant L102V is listed in ClinVar (ID 1925749.0) with an “Uncertain” status and is present in gnomAD (6‑33432169‑T‑G). Prediction tools that agree on a benign effect include REVEL, PROVEAN, ESM1b, FATHMM, AlphaMissense‑Default, AlphaMissense‑Optimized, and the SGM‑Consensus (Likely Benign). Tools that predict a pathogenic effect are polyPhen‑2 HumDiv, polyPhen‑2 HumVar, and SIFT. High‑accuracy assessments show AlphaMissense‑Optimized as benign and the SGM‑Consensus as Likely Benign; Foldetta results are unavailable. Overall, the majority of evidence points to a benign impact. This consensus does not contradict the ClinVar “Uncertain” classification, which remains inconclusive. Disclaimer: This summary was generated using AI and should be interpreted alongside expert review. | Likely Benign | Uncertain | 1 | 6-33432169-T-G | 1 | 6.20e-7 | -4.316 | Likely Benign | 0.068 | Likely Benign | Likely Benign | 0.102 | Likely Benign | 0.32 | Neutral | 0.880 | Possibly Damaging | 0.899 | Possibly Damaging | 4.21 | Benign | 0.00 | Affected | 4.32 | 1 | 2 | 1 | 0.4 | -14.03 | |||||||||||||||||||||||||||
Found 757 rows. Show 200 rows per page. Page 3/4 « Previous | Next »